Abstract
The disproportionate increase in oropharyngeal candidiasis (OPC) compared with systemic and vaginal candidiasis in female patients with AIDS has been a paradox for almost three decades. New data now show that severe OPC develops in Th17-deficient mice, but not Th1-deficient mice, implicating Th17-induced effector molecules in resistance to oral disease. These findings clarify and extend our current thinking about how CD4 T cell deficiency influences susceptibility to OPC.
Commensalism and disease in humans
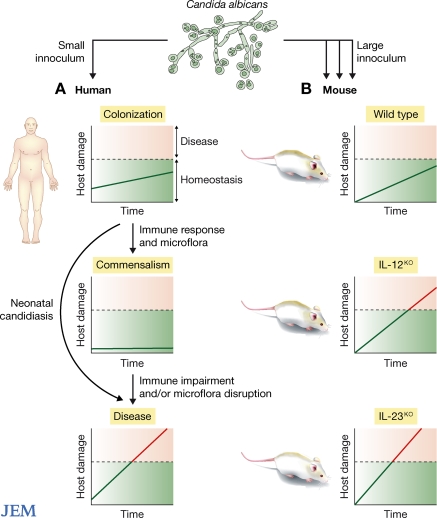
Candida albicans is unique among major pathogenic fungi in that it is intimately associated with human hosts (1). Primary infection with C. albicans occurs from acquisition of maternal flora in the perinatal period and is followed by a state of colonization, which evolves into a state of commensalism, except in rare cases of neonatal candidiasis (2). The state of commensalism, which does not result in damage to the host, is established as a result of host homeostasis, physiology, and development of the immune system (3, 4). Alterations in the immune status of the host or antibiotic-mediated disruption of bacterial microbiota can trigger a change from commensalism to colonization and/or disease—states that differ by the amount of host damage incurred (Fig. 1 A) (2). Because the microorganism remains the same, and the presence of C. albicans in tissues of immunocompetent individuals with intact mucosal surfaces and microbiota is not usually associated with inflammation or damage, the transition between commensalism and disease is almost certainly caused by the immune response. However, when inflammation and/or damage occur, resistance to disease is associated with the presence of immune effector cells that kill the fungus and clear infection (5). Patients with impaired cell-mediated immunity, including those with advanced HIV infection, are highly susceptible to OPC. On page 299 of this issue, Conti et al. show that defense against OPC is more dependent on Th17- than Th-1–type immunity (6). In this Commentary, we discuss this finding in light of unanswered questions about the pathogenesis of HIV-associated OPC.
Figure 1.
Schematic representation of the states of infection with C. albicans in humans and mice. (A) In humans, infection with C. albicans occurs during the perinatal period and likely reflects a small fungal inoculum. A period of colonization is followed by a state of commensalism. Neither colonization nor commensalism result in sufficient host damage to affect homeostasis such that it translates into disease (above dotted line), although impaired immunity or changes in microflora can lead to a change in this balance, leading to disease. Host damage can be caused directly by the fungus and/or by the resulting inflammatory response (B) In mice, infection is induced experimentally with a large fungal inoculum. The inoculum used by Conti et al. did not cause disease in normal mice, but induced host damage and disease in mice lacking IL-12p35 (impaired Th1 response) and more severe damage/disease in mice lacking IL-17p19 (impaired Th17 response).
Mouse models of candidiasis and relevance to human OPC
Mouse models of candidiasis, including models of OPC, vaginitis, and disseminated disease, have been invaluable in advancing our understanding of the immune response to C. albicans. However, because the relationship between humans and C. albicans is unique, animal models do not fully recapitulate the human disease. Consequently, observations from animal models must be viewed in light of several important limitations.
First, mice do not have C. albicans among their commensal flora. Thus, murine infection with C. albicans is a de novo event that generates an acute immune response in a naive host. And inducing disease in mice requires inoculation with large doses of the fungus (typically 105–7 yeasts), which is entirely different from the scenario in humans, where C. albicans is part of the normal flora. Hence, early snapshots of the mouse immune response to C. albicans most likely reflect an acute response, which is unlikely to occur in humans, in whom candidiasis occurs in a setting of prior infection/immunity. Although the number of organisms required to cause disease in humans is not known, human disease most likely reflects an inability to control increased fungal growth in a particular tissue rather than a sudden high-dose challenge (1). The cell type(s) that respond to a primary infection, and those that respond after a disturbance in commensalism, may or may not be the same.
In mice, Th17 responses have been implicated in mucosal immunity against fungal and bacterial infections in the lungs and gastrointestinal tract (7, 8). In a mouse model of systemic candidiasis, IL-17 protected against infection by enhancing neutrophil recruitment (9), whereas in gastric candidiasis, IL-17–induced neutrophil activation caused excessive inflammation (10). Hence, the outcome of Th17 responses to C. albicans depends on the model, tissue, and/or microbe, suggesting that the link between Th17 immunity and resistance to OPC, as suggested by Conti et al., may not be completely straightforward.
The morphological state of C. albicans cells could also influence whether a Th1- or Th17-type immune response dominates in mouse models of candidiasis. Evidence for this possibility is suggested by in vitro studies with human cells showing that C. albicans hyphae induced the production of IL-23, a cytokine that drives the expansion and function of Th17 cells, whereas yeasts induced the production of IL-12, the signature inducer of Th1 immunity (11). Although comparable evidence in mice is not available, this human precedent suggests that the state of the fungus influences the polarization of the CD4+ T cell response.
Helper T cell subsets and resistance to OPC
Until the identification of Th17 cells in 2005 (12), only two subsets of CD4+ Th cells were known: Th1 and Th2. The Th1/Th2 paradigm has largely been accepted and upheld, despite exceptions to the Th1/Th2 rule. Notably, detrimental effects of Th1-type immunity have been observed in infections in which Th1 cells were thought to be protective (10), and in certain models of Th1-driven autoimmunity, mice lacking IL-12 remained susceptible (13). Based on the Th1/Th2 paradigm and the well-known association between OPC and immunodeficiency in human and mouse studies, the risk for OPC has been thought to stem from an inadequate Th1 response (14, 15). However, the identification of the Th17 lineage opens up new avenues of investigation and provides an opportunity to dissect CD4+ T cell responses with greater precision.
In their study, Conti et al. compared the susceptibility to OPC in mice with impaired Th1 and/or Th17 responses. They found that fungal infections of the tongue were less severe in mice lacking IL-12p35 than in mice lacking IL-23p19, suggesting that Th17 responses play central role in control of infection. Compared with wild-type mice, however, the fungal burden in both IL-12p35– and IL-23p19–deficient mice was substantially higher (greater than three logs), demonstrating that the absence of either Th1 or Th17 cells compromised the ability of the mice to limit fungal growth (Fig. 1 B), albeit to a lesser extent than observed when mice were immunosuppressed with cortisone.
Consistent with the known ability of the Th17 response to induce neutrophil recruitment, more neutrophils were recruited into the oral cavity in wild-type and relatively resistant IL-12p35–deficient mice than in the more susceptible IL-23p19–deficient mice. This finding links neutrophil recruitment to IL-17/Th17 and fungal clearance. However, because more neutrophils were recruited in IL-12p35–deficient than in wild-type mice, and these mice had higher fungal burdens, neutrophil depletion studies in IL-12p35–deficient mice might help dissect the relative role of neutrophils in fungal clearance versus inflammation.
As mentioned, yeasts and hyphae preferentially induce human cells to produce IL-12 and IL-23, respectively (11). Given that large numbers of yeasts are used to induce infection in most murine models of candidiasis, including the study by Conti et al., Th1-mediated candidacidal mechanisms could dominate soon after infection, with Th17-mediated control coming into play after the yeasts germinate into hyphae. Because the tissue response to OPC in wild-type mice was characterized by more neutrophils and a lower fungal burden than in either IL-12p35– or IL-23p19–deficient mice, Th1 and Th17 effector mechanisms probably synergize to maximize fungal clearance and to minimize tissue damage that arises during clearance.
Synergy between Th1 and Th17 responses occurs in other infection models. For example, in a mouse model of Cryptococcus neoformans, IL-23 enhanced Th1-type immunity and fungal clearance (16). In OPC, neutrophils might enhance inflammatory pathology in IL-12p35–deficient mice, whereas their absence probably impairs fungal clearance in Th17-deficient mice. The benefit of IL-17–induced inflammation varies in different experimental models (10, 16-18). Clearly, more information is needed to fully understand the role that IL-17–mediated inflammation plays in resistance and susceptibility to OPC.
The mechanism by which Th17 immunity leads to resistance to OPC was suggested by Conti et al. to involve both IL-17–induced neutrophil recruitment and direct IL-17–induced antimicrobial effects. Indeed, the expression of Th17-inducible genes, including neutrophil activating factors, neutrophil-attracting CXC chemokines, and antimicrobial peptides, was greater in the oral mucosa of wild-type mice than of susceptible IL-17RA–deficient mice. Moreover, saliva from wild-type mice could kill the fungus in vitro, whereas the killing capacity of saliva from IL-23p19– and IL-17RA–deficient mice was impaired. These data are consistent with previous studies showing that IL-23, in concert with IL-17, induced the production of antimicrobial peptides, including β defensins, from mouse keratinocytes (19) and that patients with OPC had reduced expression of β defensins (20).
However, association is not causation, and the reported differences in the cytokines that drive Th17 expansion and differentiation in mice and humans suggest the need for caution in directly extrapolating these mouse findings to human OPC. The ability of Th17-polarized cells to trigger the production of β defensins in OPC could be a function of microbial factors (antigenic determinants, phase transition, and gene expression) and/or host factors (receptors, cytokines/chemokines, and genetics). Indeed, the importance of host genetics in governing Th17 responses was recently demonstrated by the discovery that mutations in STAT3 and IL12RB1 impair the production of IL-17 by T cells (21).
OPC in HIV infection
OPC was historically recognized as one of the earliest manifestations of AIDS. Although studies of Th17 expression in the setting of HIV infection are in their infancy, the findings of Conti et al. are provocative in light of longstanding questions about the relationship between HIV-associated CD4 T cell deficiency and the pathogenesis of OPC. CD4+ T cells from HIV-infected individuals at early stages of infection express IL-17; however, in one study, CD4+ T cells from chronically infected individuals, nonprogressors, and patients on highly active antiretroviral therapy (HAART) produced less IL-17 (22). HAART reduces the frequency of OPC without reducing fungal burden (23), and the development of OPC in patients receiving HAART has been linked to a failure of immune reconstitution (24, 25). On the other hand, oral candidiasis has been observed in association with immune reconstitution (26, 27). Hence, the relationship between OPC and HIV-associated CD4+ T cell levels is not straightforward. In mice, depletion of CD4 T cells alone did not increase experimental OPC (28). However, depletion of neutrophils or macrophages in addition to CD4+ T cells enhanced the severity of disease (28). In another mouse model, IL-12p40–deficient mice were susceptible to OPC, but not systemic candidiasis, a finding that the authors attributed to the ability of innate immune mechanisms to clear systemic candidiasis, but not oral candidiasis, which requires the generation of an adaptive immune response (29).
IL-17–producing CD4+ T cells were found to be preferentially depleted from the gastrointestinal tract of HIV-infected individuals (30). This link between HIV infection and reduced IL-17 expression suggests the possibility of divergent roles for IL-17 in the pathogenesis of HIV-associated OPC. In early HIV infection, when Th17 cells are more prevalent, IL-17 signaling could provide protection against OPC by attracting neutrophils. In some instances, however, excessive IL-17–mediated inflammation could be detrimental, particularly if Th1 immunity is impaired. Alternatively, depletion of IL-17–producing CD4+ T cells from the gastrointestinal tract or during chronic HIV infection could be associated with a higher mucosal fungal burden, including in the oral cavity.
Ultimately, the balance between Th1 and Th17 immunity could influence the balance between inflammation and fungal clearance and could determine whether OPC develops (10, 16). The same concept may apply to vaginal candidiasis. Several studies have demonstrated that vaginal colonization with C. albicans is more prevalent in HIV-infected women than in HIV-uninfected women and that higher viral loads are associated with increased colonization (31–34). In most studies, however, neither disease nor disease severity is increased in the setting of HIV infection, and disease does not correlate with CD4+ T cell levels (31, 32, 35), suggesting that other factors contribute more to disease pathogenesis and severity than defects in CD4+ T cell responses (36). Differences in immune control of C. albicans proliferation and/or inflammation-mediated host damage are likely to differ in the oral and vaginal milieus, which have markedly different microbiota. For example, in the vagina, local control of bacteria is affected primarily by innate immunity, including epithelial cells, and by the fungal-inhibiting effects of the bacterial flora, whereas adaptive immunity has a less prominent role (36).
Paradigms for resistance to OPC: Th cell redux
By design, experimental models hold either the microbe or host constant while varying the other. At present, the degree to which mucosal damage in OPC results from microbial factors (yeasts, hyphae, or fungal invasion) or host factors (inflammatory response, genetics, or both) is unknown. In murine vaginal candidiasis, mucosal damage results from an exuberant host inflammatory response to fungal proliferation (37).
Th17-deficient mice in the study by Conti et al. had impaired neutrophil recruitment and high fungal burdens. More work is needed to understand whether Th17 responses also govern the response to vaginal candidiasis in mice, although data suggest that neutrophils contribute more to the symptoms associated with vaginitis than they provide protection against disease (36). Ultimately, human studies of OPC are needed, because it is unclear whether mouse and human diseases have the same etiology. In humans, OPC has diverse etiologies ranging from antimicrobial use, to immune dysregulation associated with advanced HIV infection, to mutations in autoimmune regulator genes. Among patients with chronic mucocutaneous candidiasis, there are profound differences in cytokine production, including IL-23, depending on the etiology of the disorder (38). The extent to which Th17 polarization is antigen-driven is also not known (30). Although HIV infection and host genetics are likely to be important variables in Th17 expression, environmental factors, particularly those that affect the microbiota, could also influence Th cell polarization. Finally, although microbe-specific motifs could drive Th polarization, common microbial motifs that induce Th1 or Th17 may also be present. Hence, a diverse group of microbial ligands could induce Th1 or Th2, or Th17, not unlike the diverse ligands that trigger signaling from different TLRs, and microbial immunity could be less specific than previously thought.
In light of the many unknowns discussed here, the mechanism/s underlying the predilection for HIV-infected persons to develop OPC, rather than other forms of candidiasis, remains unexplained. Nonetheless, the article by Conti et al. suggests a new twist to understanding the pathogenesis of OPC that is likely to fuel new hypotheses and experimental approaches that could help unravel this mystery.
Acknowledgments
We thank Dr. Paul Fidel for reading our manuscript.
This article was supported by National Institutes of Health grants R01AI035370 and R01AI04545 to L. Pirofski and grants AI033774, HL059843, and AI033142 to A. Casadevall.
References
- Casadevall A., Pirofski L.A. 2007. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes.Eukaryot. Cell. 6:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Pirofski L. 2003. The damage-response framework of microbial pathogenesis.Nat. Rev. Microbiol. 1:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Medzhitov R. 2006. Role of the innate immune system and host-commensal mutualism.Curr. Top. Microbiol. Immunol. 308:1–18 [DOI] [PubMed] [Google Scholar]
- Bauer E., Williams B.A., Smidt H., Verstegen M.W., Mosenthin R. 2006. Influence of the gastrointestinal microbiota on development of the immune system in young animals.Curr. Issues Intest. Microbiol. 7:35–51 [PubMed] [Google Scholar]
- Fidel P.L., Jr. 2002. Immunity to Candida.Oral Dis. 8(Suppl 2):69–75 [DOI] [PubMed] [Google Scholar]
- Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Filler S.G., et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis.J. Exp. Med. 205:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel K.I., Zheng M., Young E., Quinton L.J., Lockhart E., Ramsay A.J., Shellito J.E., Schurr J.R., Bagby G.J., Nelson S., Kolls J.K. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection.J. Immunol. 170:4432–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez I., Haneda T., Raffatellu M., George M.D., Paixao T.A., Rolan H.G., Santos R.L., Dandekar S., Tsolis R.M., Baumler A.J. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa.Infect. Immun. 76:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Na L., Fidel P.L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice.J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- Zelante T., De L.A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M.L., Vacca C., Conte C., Mosci P., et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance.Eur. J. Immunol. 37:2695–2706 [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells.Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.Nat. Immunol. 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation.J. Exp. Med. 201:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P.L., Jr. 2006. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis.Adv. Dent. Res. 19:80–84 [DOI] [PubMed] [Google Scholar]
- Farah C.S., Elahi S., Drysdale K., Pang G., Gotjamanos T., Seymour G.J., Clancy R.L., Ashman R.B. 2002. Primary role for CD4(+) T lymphocytes in recovery from oropharyngeal candidiasis.Infect. Immun. 70:724–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek M.A., Muller U., Brodie S.J., Stenzel W., Kohler G., Blumenschein W.M., Straubinger R.K., McClanahan T., Kastelein R.A., Alber G. 2006. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12.J. Immunol. 176:1098–1106 [DOI] [PubMed] [Google Scholar]
- Happel K.I., Dubin P.J., Zheng M., Ghilardi N., Lockhart C., Quinton L.J., Odden A.R., Shellito J.E., Bagby G.J., Nelson S., Kolls J.K. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae.J. Exp. Med. 202:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin P.J., Kolls J.K. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice.Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L519–L528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides.J. Exp. Med. 203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida T., Okamoto T., Okamoto A., Wang H., Hamada T., Ueta E., Osaki T. 2003. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis.J. Oral Pathol. Med. 32:586–594 [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Janniere L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17–producing T cells.J. Exp. Med. 205:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F.Y., Merchant A., Kovacs C.M., Loutfy M., Persad D., Ostrowski M.A. 2008. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection.J. Virol. 82:6767–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Lo H.J., Hung C.C., Li Y. 2006. Effect of prolonged HAART on oral colonization with Candida and candidiasis.BMC Infect. Dis. 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan-Cepeda L.A., Martinez-Gonzalez M., Ceballos-Salobrena A. 2005. Oral candidosis as a clinical marker of immune failure in patients with HIV/AIDS on HAART.AIDS Patient Care STDS. 19:70–77 [DOI] [PubMed] [Google Scholar]
- Miziara I.D., Weber R. 2006. Oral candidosis and oral hairy leukoplakia as predictors of HAART failure in Brazilian HIV-infected patients.Oral Dis. 12:402–407 [DOI] [PubMed] [Google Scholar]
- Gaitan Cepeda L.A., Ceballos S.A., Lopez O.K., Arzate M.N., Jimenez S.Y. 2008. Oral lesions and immune reconstitution syndrome in HIV+/AIDS patients receiving highly active antiretroviral therapy. Epidemiological evidence.Med. Oral Patol. Oral Cir. Bucal. 13:E85–E93 [PubMed] [Google Scholar]
- Nacher M., Vantilcke V., Mahamat A., El G.M., Vaz T., Randrianjohany A., Clyti E., Aznar C., Carme B., Couppie P. 2007. Increased incidence of cutaneous mycoses after HAART initiation: a benign form of immune reconstitution disease? AIDS. 21:2248–2250 [DOI] [PubMed] [Google Scholar]
- Farah C.S., Elahi S., Pang G., Gotjamanos T., Seymour G.J., Clancy R.L., Ashman R.B. 2001. T cells augment monocyte and neutrophil function in host resistance against oropharyngeal candidiasis.Infect. Immun. 69:6110–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah C.S., Hu Y., Riminton S., Ashman R.B. 2006. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting.Oral Microbiol. Immunol. 21:252–255 [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Paiardini M., Knox K.S., Asher A.I., Cervasi B., Asher T.E., Scheinberg P., Price D.A., Hage C.A., Kohli L.M., et al. 2008. Differential Th-17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections.Blood. 112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr A., Heilig C.M., Meikle S.F., Cu-Uvin S., Klein R.S., Rompalo A., Sobel J.D. 2003. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus-infected women: risk factors and severity.Obstet. Gynecol. 101:548–556 [DOI] [PubMed] [Google Scholar]
- Sobel J.D. 2002. Vulvovaginal candidiasis: a comparison of HIV-positive and -negative women.Int. J. STD AIDS. 13:358–362 [DOI] [PubMed] [Google Scholar]
- Beltrame A., Matteelli A., Carvalho A.C., Saleri N., Casalini C., Capone S., Patroni A., Manfrin M., Carosi G. 2006. Vaginal colonization with Candida spp. in human immunodeficiency virus-infected women: a cohort study.Int. J. STD AIDS. 17:260–266 [DOI] [PubMed] [Google Scholar]
- Ohmit S.E., Sobel J.D., Schuman P., Duerr A., Mayer K., Rompalo A., Klein R.S. 2003. Longitudinal study of mucosal Candida species colonization and candidiasis among human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women.J. Infect. Dis. 188:118–127 [DOI] [PubMed] [Google Scholar]
- Leigh J.E., Barousse M., Swoboda R.K., Myers T., Hager S., Wolf N.A., Cutright J.L., Thompson J., Sobel J.D., Fidel P.L., Jr 2001. Candida-specific systemic cell-mediated immune reactivities in human immunodeficiency virus-positive persons with mucosal candidiasis.J. Infect. Dis. 183:277–285 [DOI] [PubMed] [Google Scholar]
- Fidel P.L., Jr 2004. History and new insights into host defense against vaginal candidiasis.Trends Microbiol. 12:220–227 [DOI] [PubMed] [Google Scholar]
- Fidel P.L. 2003. Immune regulation and its role in the pathogenesis of candida vaginitis.Curr. Infect. Dis. Rep. 5:488–493 [DOI] [PubMed] [Google Scholar]
- Ryan K.R., Hong M., Arkwright P.D., Gennery A.R., Costigan C., Dominguez M., Denning D., McConnell V., Cant A.J., Abinun M., et al. 2008. Impaired dendritic cell maturation and cytokine production in patients with chronic mucocutaneous candidiasis with or without APECED.Clin. Exp. Immunol. 154:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]