Abstract
Allogeneic (allo) hematopoietic stem cell transplantation is an effective therapy for hematological malignancies but it is limited by acute graft-versus-host disease (GVHD). Dendritic cells (DC) play a major role in the allo T cell stimulation causing GVHD. Current immunosuppressive measures to control GVHD target T cells but compromise posttransplant immunity in the patient, particularly to cytomegalovirus (CMV) and residual malignant cells. We showed that treatment of allo mixed lymphocyte cultures with activated human DC-depleting CD83 antibody suppressed alloproliferation but preserved T cell numbers, including those specific for CMV. We also tested CD83 antibody in the human T cell–dependent peripheral blood mononuclear cell transplanted SCID (hu-SCID) mouse model of GVHD. We showed that this model requires human DC and that CD83 antibody treatment prevented GVHD but, unlike conventional immunosuppressants, did not prevent engraftment of human T cells, including cytotoxic T lymphocytes (CTL) responsive to viruses and malignant cells. Immunization of CD83 antibody-treated hu-SCID mice with irradiated human leukemic cell lines induced allo antileukemic CTL effectors in vivo that lysed 51Cr-labeled leukemic target cells in vitro without further stimulation. Antibodies that target activated DC are a promising new therapeutic approach to the control of GVHD.
Allogeneic (allo) hematopoietic stem cell transplantation (HSCT) is an effective therapy for many malignant and nonmalignant hematological and some nonhematological conditions. Conditioning the recipient with radiation and chemotherapy enables donor hematopoietic and immune systems to engraft and provide immune effectors, which confer protective immunity and, for leukemia patients, the desired therapeutic graft versus leukemia (GVL) effect. Donor T cell–mediated acute graft-versus-host disease (GVHD), which targets recipient skin, gut, liver, lung, and lymphoid tissue, is an inevitable consequence of alloHSCT and a major cause of morbidity and mortality (1). GVHD arises within the conditioning-induced inflammatory milieu as donor CD4+ and CD8+ T cells are stimulated to generate alloreactive antihost effector cells. Mouse alloHSCT models indicate that the donor antihost T cell response is stimulated by direct alloantigen presentation by host APC, particularly DC (2, 3). Donor APC also contribute, presumably via the indirect pathway by processing and presenting host antigens to donor T cells (4). That they may be an appropriate therapeutic target in their own right is further supported by recent studies showing that donor APC propagate GVHD initiated by host APC (5), that they can independently induce GVHD (6), and that they play a key role in HSCT rejection (7).
Prophylactic, and often additional therapeutic immunosuppression, is used to control GVHD but, being nonspecific, this neither spares preexisting donor memory cells nor discriminates between alloreactive and nonalloreactive donor T cells. Thus, although GVHD can be controlled, it is at the cost of increased incidence of graft failure, leukemia relapse (8), and compromised immunity to posttransplant infection, particularly to CMV (9). GVHD and/or immunosuppression-associated complications prevent the application of alloHSCT to older patients and limit its wider use for the treatment of nonhematopoietic tumors, common nonmalignant conditions (autoimmune disease, thalassemia, and immunodeficiencies), and for gene replacement therapy (10).
An alternative strategy that primarily targets DC might prevent GVHD without the complications associated with T cell immunosuppression. Depletion of APC (including DC) in mice with liposomal clodronate reduced development of liver GVHD (11), and UV radiation to deplete host skin DC prevented mouse skin GVHD (12). More practical methods aimed at DC are required for clinical therapy. Antibodies can be used to target specific cells and some are available for therapeutic T cell depletion and immunosuppression. However, there are no pan-DC–specific antibodies; therefore, it is not currently possible, and possibly not desirable, to specifically deplete all human DC to achieve immunosuppression. However, a proportion of human DC spontaneously up-regulate the DC surface activation markers CD83 and CMRF-44 after overnight culture (13). These activated DC are the prime stimulators of allo T cell proliferation in vitro and their depletion with antibody specific for CD83 (14) or CMRF-44 antigen (15, 16) significantly reduces the allo proliferative response, suggesting that such antibodies may have a role in the control of GVHD. We show in this paper that treatment of MLC with anti-human CD83 antibody markedly reduced allo T cell proliferation but preserved preexisting antiviral, particularly anti-CMV effector/memory CD8+ T cells. In contrast, the therapeutic immunosuppressive antibody alemtuzumab (Campath-1H) prevented allo T cell proliferation by depleting virtually all cells including virus-specific T cells.
To investigate the antihuman CD83 antibody in vivo, we used a chimeric human/mouse model of xenogeneic GVHD (17) in which SCID mice are engrafted with human PBMC. These hu-SCID mice develop a fatal human CD4+ T cell–dependent GVHD-like syndrome affecting multiple organs, which has histological features similar to those seen in allo human and mouse GVHD (18). We show in this paper that human DC were required to induce GVHD in this model. Treatment of the hu-SCID mice with CD83 antibody prevented GVHD yet allowed human leukocyte engraftment and preserved T cells, including CTL precursors specific for CMV, influenza, and the malignancy-associated antigen Mart1. Moreover, CD83 antibody treatment of hu-SCID mice did not impair in vivo induction of antileukemic cytolytic T cell effectors in response to immunization with human leukemic cell lines.
RESULTS
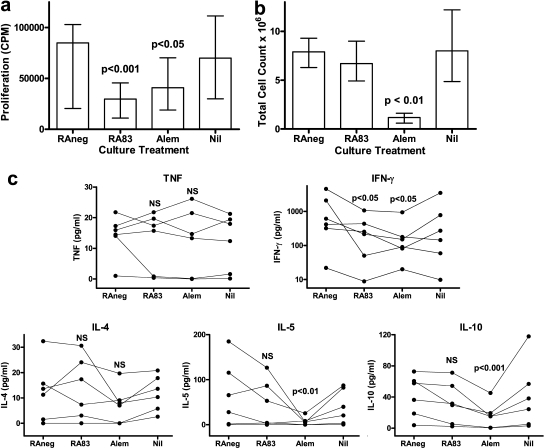
Anti-CD83 antibody in allo MLC reduces T cell proliferation and IFN-γ expression but maintains cell numbers
Polyclonal rabbit antihuman CD83 (RA83) induces antibody-dependent cellular cytotoxic (ADCC) lysis of activated DC, thereby reducing DC-stimulated allo T cell proliferation in MLC (Fig. 1 a) (14). The therapeutic antibody alemtuzumab, which depletes most human PBMC by ADCC and complement-dependent cytotoxicity, also reduced T cell proliferation (Fig. 1 a) but, unlike RA83, it substantially reduced the total number of viable leukocytes recovered at day 7 from the allo MLC (Fig. 1 b). Of the five cytokines assayed at day 7, RA83 treatment reduced only IFN-γ secretion into the culture medium (Fig. 1 c). Alemtuzumab reduced IFN-γ, IL-5, and IL-10. However, TNF and IL-4 were not affected by either antibody treatment despite the large reduction in cell numbers induced by alemtuzumab.
Figure 1.
RA83 reduces T cell proliferation and expression of IFN-γ in allo MLC without nonspecific ablation of leukocytes. (a) Cell proliferation ([3H]thymidine incorporation; CPM) was significantly reduced in MLCs treated with 5 µg/ml of RA83 or with 5 µg/ml of alemtuzumab (Alem), compared with 5 µg/ml of RAneg (nonimmune rabbit IgG-negative control antibody). Median and interquartile range (error bars) are shown for n = 9 stimulator/responder combinations. (b) The number of viable leukocytes recovered from 7-d MLCs was not affected by RA83 but was substantially reduced by alemtuzumab. Median viable cell count and interquartile range (error bars) are shown for n = 11 stimulator/responder combinations. (c) RA83 reduced 7-d MLC concentrations of IFN-γ (note log scale) by a median of 64%, but TNF, IL-4, IL-5, and IL-10 were not significantly affected. Alemtuzumab similarly reduced 7-d MLC concentrations of IFN-γ (median 75% reduction), IL-5, and IL-10. Graphs show, for each antibody treatment, individual cytokine concentrations for n = 6 stimulator/responder combinations, each linked by lines. Raw cytokine data contained zero values; therefore, 1.0 pg/ml was added to all cytokine data to enable log transformation for statistical analysis (P > 0.05). NS and p-values are for repeated measures ANOVA followed by Bonferroni-corrected multiple comparisons posttests for RA83 and alemtuzumab each compared with RAneg treatment. Data are also shown for untreated (Nil) MLCs, which were not statistically significantly different from RAneg-treated cultures.
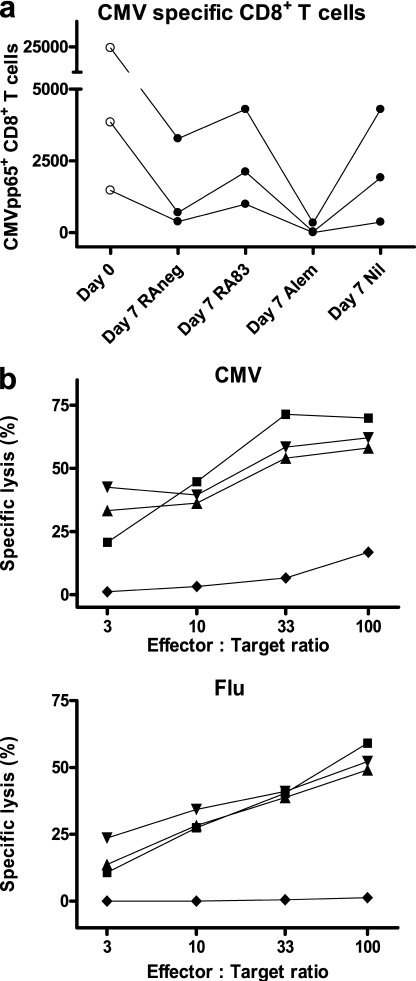
Anti-CD83 treatment of the MLC preserves specific T cell immunity
The RA83-mediated reduction in T cell alloproliferation (Fig. 1 a), without T cell loss (Fig. 1 b), suggested the hypothesis that this approach to immunosuppression would not compromise T cell memory. To investigate this, antibody-treated 7-d allo MLCs using CMV+ HLA-A*0201+ responder PBMC were tested for the presence of CMV and influenza-specific CD8+ T cells. For each of three donors, approximately similar absolute numbers of CMVpp65-specific CD8+ T cells were recovered from untreated MLCs and those treated with RA83 or negative control antibody (RAneg). Much lower numbers were recovered from alemtuzumab-treated MLCs (0, 1.3, and 7.7% of respective RA83 values for the three donors; Fig. 2 a). The same number of total live cells from each MLC were stimulated with CMVpp65 peptide or influenza matrix protein (FMP) peptide and irradiated autologous PBMC and then tested for specific cytotoxic activity. Cells expanded from RA83, RAneg, and untreated MLCs all lysed CMV peptide or FMP peptide-loaded 51Cr-labeled T2 target cells. In contrast, alemtuzumab-treated MLC-derived cells lysed significantly fewer target cells, indicating that most CMV- and FMP-specific precursors in the MLC had been eliminated by this antibody (Fig. 2 b). These data support our hypothesis that antibody that targets activated DC could control GVHD yet maintain protective T cell memory.
Figure 2.
RA83 treatment preserves virus-specific T cell immunity in allo MLC. (a) Number of CMVpp65 pentamer-positive CD8+ T cells surviving after 7 d in antibody-treated 10-ml MLCs. The day 0 column shows the starting number of cells (data shown is for three different HLA-A*0201+ CMV+ donors; lines link data from the same donor). (b) A substantial viral antigen-specific functional CTL response was generated from 7-d RA83- (▴), RAneg- (▾), and Nil (▪)-treated MLCs but not from alemtuzumab (♦)-treated MLCs. Graphs show the mean percentage of lysis of CMV and FMP peptide-loaded 51Cr-labeled target cells by CTL effectors generated from the treated MLCs. P < 0.0001 for CMV (n = 3 donors) and P < 0.02 for FMP (n = 4 donors) for repeated measures ANOVA. Subsequent Bonferroni-corrected multiple comparisons testing showed that alemtuzumab treatment was significantly different from each of the other treatments (P < 0.001 for CMV and P < 0.01 for FMP).
Human DC are required for GVHD in the chimeric human PBMC-transplanted SCID mouse model (hu-SCID)
To test this hypothesis in vivo, we used the well established chimeric hu-SCID mouse model, in which human donor CD4+ T cells mediate GVHD in SCID mice (17, 18). In our hands, conditioned SCID mice injected i.p. with 50 × 106 human PBMC reliably developed a fatal GVHD-like syndrome within 8–13 d. Histological examination showed periportal lymphocytic infiltration (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070723/DC1), which is typical of GVHD in this model (19) and in patients (20). Mice were killed when an overall GVHD score of 5 was attained, reflecting severe GVHD, at which time human donor cells were detected in spleen, bone marrow, and peritoneal cavity (Fig. S2).
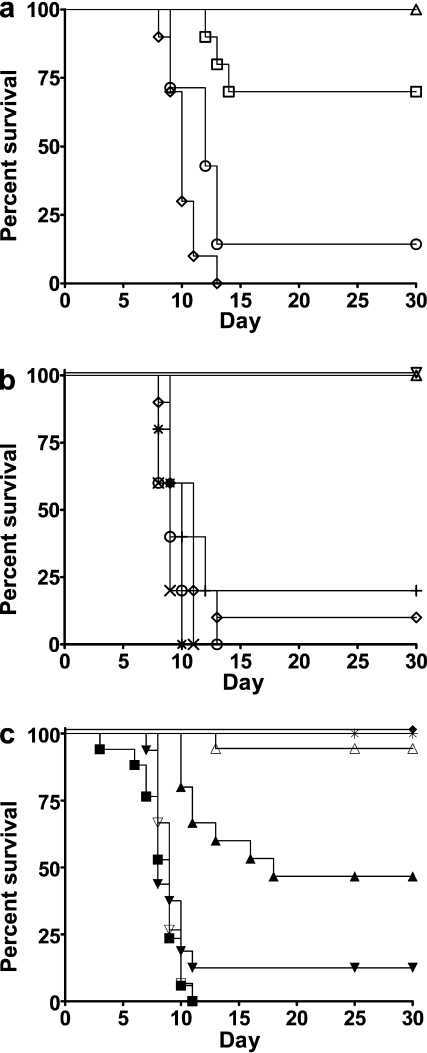
Mouse APC are weak stimulators of human T cells in vitro (21, 22); therefore, we considered it likely that APC in the human donor PBMC graft provided the primary stimulation of the human CD4+ T cell GVHD effectors in the hu-SCID model. Supporting this view, purified human T cell (97% CD3+) grafts alone induced GVHD in only 30% of SCID mice (Fig. 3 a). A high incidence of GVHD was fully restored by coadministration of 2.5% human autologous monocyte–derived DC with the purified T cells (Fig. 3 a). Thus, human APC are required to induce full GVHD in the hu-SCID model. To explore the role of other human APC besides DC and of other human leukocytes, we depleted PBMC, before transplant, of CD14+ cells (monocytes), CD19+ cells (B cells), CD16+/CD56+ cells (NK cells), and CD8+ cells (T cells). None of these depletions significantly affected induction of GVHD (≥80% of mice achieved a GVHD score of 5 within 13 d; Fig. 3 b). This confirmed that human DC are required to stimulate the human antimouse CD4+ T cell effectors to induce GVHD and validated the hu-SCID model for evaluating human DC targeted therapy.
Figure 3.
Hu-SCID model of GVHD. (a) Human DC enable full GVHD induction. Administration of purified T cells (97% CD3+) alone induced severe GVHD in only 3 out of 10 mice (□; not significantly different from untransplanted controls [▵]), but coadministration of 2.5% autologous monocyte-derived DC (MoDC) restored the incidence of severe GVHD (six out of seven mice; ○) to PBMC levels (10 out of 10 mice; ⋄, P > 0.05 for MoDC + T cells vs. PBMC). P = 0.025 for T cells only versus MoDC + T cells (combined data from two experiments using two different PBMC donors). (b) Monocytes and B cells are not required for GVHD induction. In vitro depletion of monocytes (X), B cells (+), CD8+ T cells (○), and NK cells (*) from human PBMC before administration to mice did not prevent or delay development of GVHD (each depletion was tested on n = 5 mice and 1 PBMC donor; P > 0.05 for each depletion vs. undepleted PBMC transplanted mice [⋄]). Administration of irradiated (3000cGy) PBMC (▿) or of vehicle alone (untransplanted [▵]) did not induce GVHD as assessed by GVHD score. (c) In vivo treatment with anti-CD83 antibody prevents GVHD. i.p. injection of conditioned SCID mice with RA83 (125 µg, ▵; 25 µg, ▴) or alemtuzumab (5 µg, ♦) 3 h before PBMC administration prevented GVHD (combined data from three experiments using three different PBMC donors; 8–18 mice for each treatment; *, no transplant; ▪, nil antibody; ▾, 25 µg RAneg; ▿,125 µg RAneg). P < 0.002 for RA83 versus RAneg for 125- and 25-µg doses.
In vivo anti-CD83 antibody treatment prevents GVHD and alters circulating human cytokine concentrations in the hu-SCID model
We administered RA83 to hu-SCID mice after determining by ELISA that the antibody had a circulating half life of ∼10 d (unpublished data). RA83 administration attenuated GVHD in the hu-SCID model, as assessed by blinded GVHD scoring, in a dose-dependent manner (Fig. 3 c). 94% of mice injected i.p. with 125 µg RA83 per mouse at the time of transplant survived for 30 d, which is in contrast to RAneg-treated control mice, all of which developed severe GVHD within 11 d. In the same experiment, a lower dose of 25 µg RA83 per mouse protected only 47% of the mice for the full 30-d experimental period.
Alemtuzumab also prevented GVHD in this model (Fig. 3 c). From day 3 after transplant, body weights increased and GVHD scores decreased for mice treated with RA83 (125 µg/mouse), alemtuzumab (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070723/DC1), or for control untransplanted mice. Higher circulating human IFN-γ (P = 0.01) and IL-5 (P < 0.05) concentrations were observed in RA83-treated mice at day 30 compared with alemtuzumab-treated mice, indicating greater T cell engraftment of the RA83-treated mice at this time (Fig. S4). Administration of RA83 i.p. was as effective as the i.v. route (unpublished data) so i.p. injections were used in subsequent experiments.
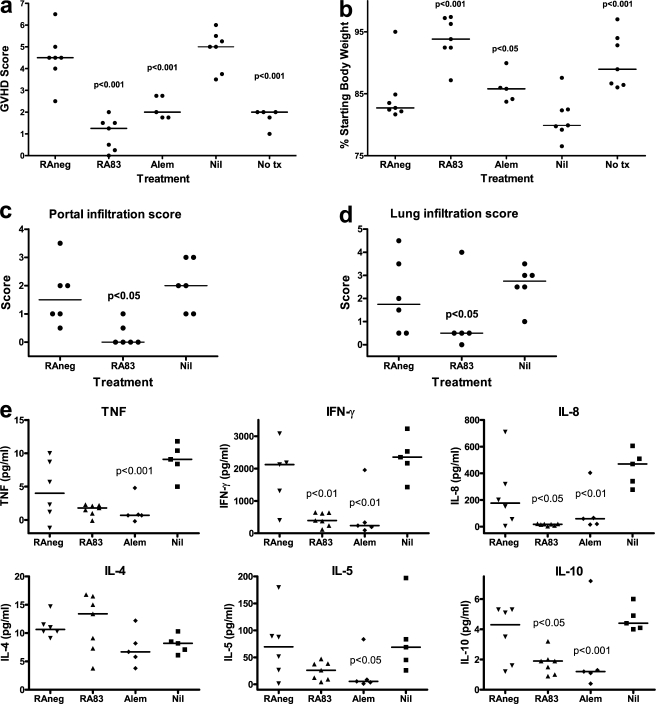
To make direct temporal comparisons between treatments, each time a mouse attained a GVHD score of ≥5, it was killed together with the highest scoring mouse from each other treatment group in the experiment. This occurred 8–13 d after transplant, depending on the donor. At the time that control hu-SCID mice were suffering severe GVHD, untransplanted mice and transplanted mice treated with either RA83 or alemtuzumab had significantly reduced GVHD scores (Fig. 4 a) and body weight loss (Fig. 4 b). Liver and lung from the RA83-treated hu-SCID mice had reduced GVHD-associated lymphocytic infiltration and cell damage (Fig. 4, c and d). RA83 and alemtuzumab treatments each substantially reduced the circulating concentrations of the human cytokines IFN-γ, IL-8, and IL-10 (P < 0.05) but maintained IL-4 (Fig. 4 e). TNF and IL-5 concentrations were also reduced by alemtuzumab treatment (P < 0.05), but any reductions caused by RA83 did not reach statistical significance (Fig. 4 e).
Figure 4.
RA83 treatment reduces signs of GVHD in the hu-SCID model. In vivo treatment of hu-SCID mice with anti-CD83 antibody significantly reduced GVHD score (a), weight loss (b), lymphocyte infiltration in liver (c) and in lung (d) and circulating human IFN-γ, IL-8, and IL-10 (e). RA83 (125 µg/mouse) was always compared with RAneg (125 µg); alemtuzumab (5 µg) and untransplanted (No tx) mice were always compared with Nil antibody-treated transplanted mice. P-values are shown only when <0.05. The RA83 outlier in d and the alemtuzumab outliers in e for IL-5 and IL-10 were omitted for statistical analysis. Combined data from two experiments are shown, both using the same PBMC donor. n = 5–7 mice per treatment, each killed 8–11 d after transplant when a Nil- or RAneg-treated control mouse developed severe GVHD (score ≥ 5). Horizontal lines are median values. Symbols are the same as in Fig. 2 b.
In vivo anti-CD83 antibody treatment prevents GVHD without ablative immunosuppression and loss of T cell immunity
To assess the effects of RA83 treatment on the recovery of CMV-specific human CD8+ T cells, we repeated the temporal comparison from the previous section using a CMV+ HLA-A*0201+ PBMC donor. For this donor, 25 µg RA83 per mouse prevented GVHD as effectively as 125 µg. Median GVHD scores were 0.5 (range 0.5–1.5) for 25 µg RA83, 1.0 (0.5–2.25) for 125 µg RA83, and 0.5 (0–0.5) for 5 µg alemtuzumab (P < 0.001 for each treatment when compared with 6.0 [5.0–6.5] for 25 µg RAneg and 5.5 [5.0–6.0] for 125 µg of RAneg-treated controls; n = 4–5 mice per treatment, 8–11 d after transplant). We also tested the therapeutic immunosuppressant anti-thymocyte globulin (ATG), a rabbit polyclonal antihuman thymocyte globulin which, like alemtuzumab, has broad specificity for human leukocytes. ATG was as effective as RA83; the mean GVHD score for hu-SCID mice treated with 125 µg ATG was 1.0 (0–2.25).
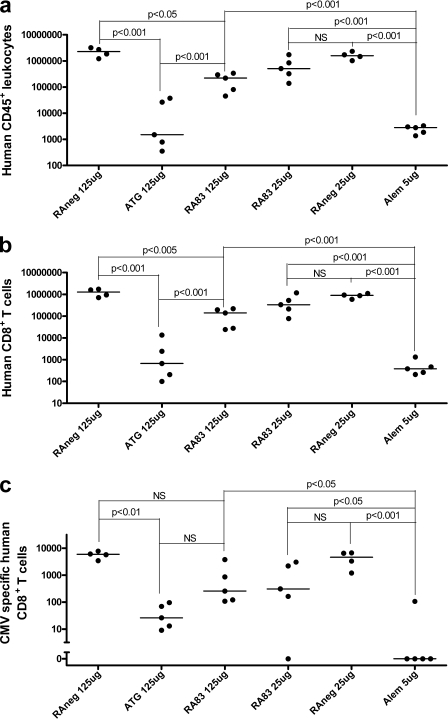
The numbers of live cells in spleen, femoral bone marrow, and peritoneal washings from each killed mouse for all treatments were counted at the time that the control hu-SCID mice developed severe GVHD. The three samples from each mouse were then pooled for flow cytometric analysis, enabling calculation of absolute numbers of human CD45+ leukocytes, total human CD8+ T cells, and CMVpp65 pentamer+ CD8+ T cells recovered from each mouse. Very low numbers of human CD45+ leukocytes were recovered from hu-SCID mice treated with ATG and alemtuzumab, compared with controls (Fig. 5 a). The median number of human CD45+ leukocytes recovered from RA83 (125 µg)-treated mice was 147 times greater than for ATG-treated mice (P < 0.001; Fig. 5 a), even though both antibodies protected mice from GVHD equally well. Recoveries of total CD8+ T cells and CMVpp65 pentamer+ CD8+ T cells followed similar trends (Fig. 5, b and c; and Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20070723/DC1). Notably, the median number of CMVpp65 pentamer+ CD8+ T cells recovered from each RA83 (125 µg)-treated mouse was 255, compared with zero for alemtuzumab treated mice (P < 0.05).
Figure 5.
RA83 treatment did not prevent engraftment of human leukocytes (a), total CD8+ T cells (b), or CMV-specific CD8+ T cells (c) in the hu-SCID mouse model of GVHD. Dots show, for each treated hu-SCID mouse, the total number of human cells recovered from bone marrow, spleen and peritoneal cavity, combined, 8–11 d after transplant. Heavy horizontal lines show median values. Raw CMV data contained zero values; therefore, 1.0 was added to all CMV data to enable log transformation for statistical analysis (p-values are shown for selected posttests). n = 5 hu-SCID mice per antibody treatment (one experiment using 1 CMV+ HLA-A*0201+ PBMC donor).
To further assess the effect of RA83 treatment on retention of immunity we harvested cells from RA83- and RAneg-treated hu-SCID mice transplanted with PBMC from a different HLA-A*0201+ donor. Human cells were isolated (see Materials and methods) and stimulated with irradiated autologous PBMC loaded with HLA-A*0201+ peptides from FMP and from the model tumor antigen Mart1. This induced expansion of FMP and Mart1 pentamer+ CTL (not depicted), which specifically lysed FMP and Mart1 peptide-loaded T2 cells, respectively, regardless of RA83 or RAneg treatment of the hu-SCID mice from which the precursor cells were recovered (Fig. 6, a and b).
Figure 6.
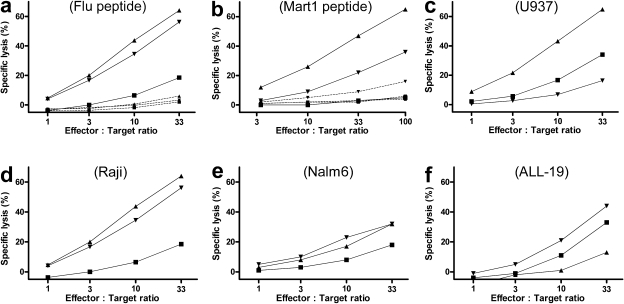
RA83 treatment of hu-SCID mice did not impair subsequent in vitro induction of antiviral and allo antileukemic cytotoxic T cell effectors from cells recovered from hu-SCID mice. 10-d posttransplant hu-SCID mice treated with 125 µg RA83 (n = 19 mice; GVHD score = 0.5 on day 9) or RAneg (n = 5 mice; GVHD score = 3.25 on day 9) were killed, cells from spleen, bone marrow, and peritoneal washings were combined, and human leukocytes recovered (see Materials and methods). These cells and, as a control, an equal number of freshly thawed PBMC from the same donor, were stimulated in vitro with irradiated autologous PBMC plus either peptide antigen or irradiated leukemic cell lines. After two rounds of stimulation, T cell–mediated lysis of FMP peptide-loaded T2 cells (a), U937 (c), Raji (d), Nalm6 (e), and ALL-19 (a human primary ALL passaged in NOD-SCID mice [reference 40]; f) leukemic cell lines was measured by 51Cr release assay. Specific killing of T2 cells loaded with peptide from the naive melanoma-associated antigen Mart1 was assayed after four rounds of stimulation (b). (▴, RA83; ▾, RAneg; ▪, freshly thawed donor PBMC). Dashed lines in a and b show minimal lysis of T2 cells loaded with irrelevant HIV peptide (RA83, P < 0.01 for FMP and 0.001 for Mart1 compared with HIV). Data are from one representative experiment of three using one HLA-A*0201+ PBMC donor.
We assessed the potential for GVL by also stimulating in vitro the cells recovered from the RA83- and RAneg-treated hu-SCID mice in the experiment described in the previous paragraph with irradiated autologous PBMC and irradiated allo human leukemic cell lines. For three of the four leukemic cell lines tested, cells from RA83 treated mice produced T cell–mediated lytic responses equal to or greater than the cells from RAneg-treated hu-SCID mice (Fig. 6, c–f).
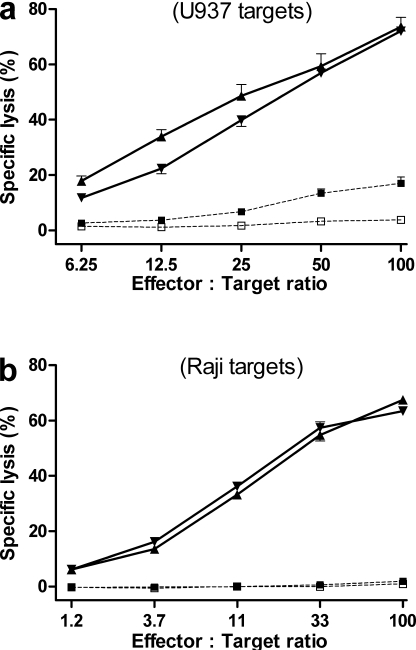
The effect of RA83 treatment on the induction of GVL in vivo was investigated in two separate experiments using different PBMC donors. RA83- and RAneg-treated hu-SCID mice were immunized on days 0 and 7 after transplant with irradiated U937 or Raji cells (107 cells i.p.). All hu-SCID mice were killed on day 9 or 10 (donor dependent), when the GVHD clinical score for RAneg-treated mice was significantly greater than for RA83 treated mice (P ≤ 0.001). Human cells recovered from the mice (see Materials and methods) were used as effectors, without further stimulation, in a chromium release assay with 51Cr-labeled U937 or Raji cells as targets. Human cells recovered from nonimmunized hu-SCID control mice were poor lytic effectors against U937 or Raji targets, whereas cells from immunized hu-SCID mice lysed the appropriate targets, regardless of the previous RA83 or RAneg antibody treatment of the mice (Fig. 7).
Figure 7.
RA83 treatment did not impair in vivo induction of human allo antileukemic cytotoxic T cell effectors as a result of immunization of hu-SCID mice with irradiated leukemic cell lines. RA83- or RAneg-treated hu-SCID mice were immunized by i.p. injection on days 0 and 7 with 107 irradiated (3000cGy) U937 cells (a), Raji cells (b), or vehicle alone. All mice were killed on days 10–11, and cells from peritoneal cavity, spleen, and bone marrow were combined within each cohort. After removal of dead cells, erythrocytes and mouse CD45+ cells, the remaining cells (>95% human CD45+), were tested for cytotoxic activity, without further stimulation, in a 51Cr release assay using U937 (a) or Raji (b) cells as targets. Effectors were from immunized mice (solid lines) treated with RA83 (▴) or with RAneg (▾) or from negative controls (dotted lines; □, freshly thawed donor PBMC; ▪, nonimmunized RAneg-treated hu-SCID mice). Data are from two experiments using two different PBMC donors. Error bars show technical reproducibility (1SEM) and are each calculated from five replicate wells.
DISCUSSION
We show in this paper that antibody specific for the DC activation marker CD83 is a potential new therapeutic option for the control of GVHD in alloHSCT. Our in vitro and in vivo evidence shows that CD83 antibody not only limits the uncontrolled T cell proliferative response that characterizes GVHD but preserves the donor T cell repertoire, in particular, potentially life saving antiviral memory T cells and antileukemic effectors. Current GVHD prophylaxis, be it ex vivo T cell depletion of the graft before transplant or nonspecific immunosuppression, does not spare these vital components of the donor graft (23).
Our in vitro comparison of the polyclonal anti-CD83 antibody (RA83) with the CD52 mAb alemtuzumab provided insight into their mechanisms of immunosuppression. Both antibodies reduced allo T cell proliferation and expression of the TH1 cytokine IFN-γ, but alemtuzumab also reduced the TH2 cytokine IL-5 and the immunosuppressive cytokine IL-10, presumably as a result of its pan-leukocyte–depleting capacity. RA83 had little direct effect on T cells, as it did not significantly reduce the number of viable mononuclear cells recovered from the allo MLC (Fig. 1 b), nor did it destroy preexisting CMV-specific nor flu-specific CD8+ T cells (Fig. 2).
Alloproliferating CD4+ T cell blasts, which were generated during MLC and found in the highest numbers after 96 h, express low levels of CD83 and are also susceptible targets of RA83-mediated ADCC lysis (14) but to a lesser extent than activated CD83+ DC (4.4-fold less lysis at 10:1 E/T ratio [calculated from data in reference 14]). Delayed addition of RA83 to the MLC reduced its inhibitory effect on alloproliferation, completely negating its effect, when administered after 96 h to target the alloresponding T cells (14). Thus, the functional cellular target of RA83 is present early rather than late during the course of MLC, which is consistent with the principal target being the DC.
To obtain in vivo evidence for immunosuppressive efficacy of CD83 antibody, we chose a chimeric human/mouse model of GVHD because antibodies specific for human CD83 and CD52 antigens, such as RA83 and alemtuzumab, can be tested. The SCID mouse was used in preference to other immunodeficient strains, despite the requirement for higher donor cell doses (24), because the hu-SCID model of GVHD has been used more extensively than others (25), it is complement replete (26), and functional human NK cells are present (27), allowing for antibody-mediated complement-dependent cytotoxicity and ADCC lysis of human target cells in vivo. The hu-SCID model of GVHD requires human CD4+ T cells (17), but the stimulatory cells had not previously been identified and mouse APC are known to be weak stimulators of human T cells (21, 22, 28, 29). We therefore considered it likely that human DC in the graft would play a major role, and this was supported by finding that purified human T cells required supplementation with human DC to be fully effective at inducing GVHD in conditioned SCID mice (Fig. 3 a).
Using the hu-SCID model, we found that RA83 prevented GVHD (Fig. 3 c). RA83-treated mice had significantly lower GVHD scores and less weight loss than RAneg-treated or untreated controls when the latter had severe GVHD (Fig. 4, a and b). Circulating levels of human IFN-γ and IL-8 were substantially reduced in RA83-treated mice but not IL-4 (Fig. 4 e), which is consistent with a GVHD-ameliorative TH2 cytokine milieu predicted by the in vitro studies (Fig. 1 c). RA83 treatment is expected to leave potentially tolerogenic nonactivated (CD83−) DC intact, and these may induce regulatory T cells (30) with potential allosuppressive benefits. RA83 may also selectively retain TH2 inducing plasmacytoid DC (31), as they express comparatively low levels of CD83 when activated (13).
Alemtuzumab treatment also prevented GVHD in the hu-SCID model but at the expense of T cell engraftment, particularly CMV-specific CD8+ T cells (Fig. 5 c). In marked contrast, RA83 treatment prevented GVHD without the loss of specific donor T cell immune memory. Treating transplant patients with CD83 antibody at the time of conditioning should limit the generation of a large pool of alloreactive GVHD inducing effector/memory T cells immediately after transplantation, which, at least in an allo mouse model, can induce GVHD at any time subsequently in the absence of host DC (32–34). Antibody that targets activated DC, such as that studied here, should also preserve donor T cell immunity to common infections such as CMV, which cause major posttransplant mortality and morbidity. Current immunosuppressants that target T cells, exemplified here by alemtuzumab and ATG, compromise posttransplant immunity, particularly to CMV (35) and other infectious agents.
T cell depletion also compromises the GVL effect and predisposes to recurrence of leukemia (8, 36). Theoretically, specific depletion of activated DC to control GVHD in clinical alloHSCT should preserve the antileukemia T cell repertoire. Supporting this, from cells recovered from RA83 treated hu-SCID mice, we obtained human effector T cell responses to the naive tumor-associated antigen Mart1 (Fig. 6 b) and to allo human leukemic cell lines, particularly to U937 (AML; Fig. 6 c) and Raji (B cell lymphoma; Fig. 6 d) cells.
A potential disadvantage of targeting CD83+ DC for the prevention of GVHD in alloHSCT patients is that these DC may be required for the induction of GVL effectors from antileukemic precursors, be they T or NK cells. Reddy et al (37) showed in a mouse “acute leukemia” model that host and, to a lesser extent, donor DC are required for effective GVL after alloHSCT, although the role of DC activation was not explored. Encouragingly, we found that RA83 did not prevent in vitro induction of allo cytotoxic antileukemic cell line activity by cocultured PBMC (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20070723/DC1). Furthermore, RA83 treatment of hu-SCID mice immunized with the human leukemic cell lines U937 and Raji did not impair in vivo induction of antileukemic cytotoxic T cell effectors (Fig.7). Nevertheless, if GVL proves to be compromised by peritransplant DC-targeted treatment, it could be managed by subsequent vaccination with leukemia antigen-loaded donor DC or by donor leukocyte infusions, perhaps boosted by donor vaccination before transplantation. Alternatively, to retain peritransplant antileukemia priming by host CD83+ DC, antibody treatment might await the appearance, after transplant, of activated DC in the circulation, an event which precedes clinical GVHD (38).
Any significant improvement in the control of GVHD as a result of targeting DC may allow wider utilization of alloHSCT for malignant conditions and for nonmalignant conditions, which do not require GVL. Our data provides compelling evidence that depletion of activated human DC is a promising alternative GVHD prevention strategy that warrants further investigation. A DC targeted therapy, which prevents alloreactive GVHD-inducing T cell generation, even allowing immature DC-mediated tolerance induction, which nonetheless, still preserves protective and therapeutic T cells, would also have wider applications in allotransplantation.
MATERIALS AND METHODS
Antibodies.
Rabbit polyclonal IgG anti–human CD83 (RA83) was prepared as described previously (14, 39) but with an added CD83 antigen affinity purification step (see Supplementary Materials and methods [available at http://www.jem.org/cgi/content/full/jem.20070723/DC1] for preparation and validation). Clinical grade ATG (Fresenius) and alemtuzumab (Schering) were obtained from the Mater Health Services Pharmacy.
Human PBMC and cell preparations.
PBMC were obtained with informed consent from normal healthy donors either as whole blood donations or by leukapheresis (approved by the Mater Human Research Ethics Committee). PBMC were purified by Ficoll-Hypaque centrifugation, cryopreserved, and stored at −180°C until required. Specific leukocyte populations were depleted from PBMC using AUTOMACS (Miltenyi Biotec) and either directly conjugated CD14 or CD16 + CD56 microbeads (Miltenyi Biotec) or indirectly conjugated with CD8 or CD19 antibody followed by anti–mouse IgG microbeads. T cells (97.0% CD3+) were prepared by staining PBMC with a mixture of unconjugated antibodies (CD11c clone MCA 2087 [AbD Serotec]; CD14 CMRF-31 [in house]; HLA-DR L243 [American Type Culture Collection]; CD16 3G8, CD19 J4.119, CD20 B9.E9, and CD56 n901 [Beckman Coulter]; and CD34 HPCA-1 [BD]), followed by anti–mouse Ig microbeads. MoDC were generated as previously described (13) from monocytes purified from PBMC by CD14 immunomagnetic selection. CMV-positive donors were identified by serological testing from a panel of HLA-A*0201+ normal donors and confirmed by CMVpp65 pentamer staining (see CTL induction).
MLC.
Irradiated human PBMC stimulators and nonirradiated human PBMC responders from another donor were cultured together, each at 106/ml for 7 d. Cultures were performed in 96-well microplates for proliferation assays (14) or in 10-ml vol T25 flasks for cytokine and antiviral immune memory assays.
Cytokine analysis.
Human cytokines in MLC supernatants and in hu-SCID mouse sera were analyzed using Human Cytokine Flex Sets (BD) and an LSR II flow cytometer (data analysis by FCAP software [BD]). Cross-reactivity with mouse cytokines was minimal, as determined by analyzing sera from mouse alloHSCT experiments and untransplanted controls.
CTL induction.
Cells recovered from MLCs or from hu-SCID mice, at 106/ml in media containing 10 ng/ml IL-7 were stimulated with peptide antigen (HCMV pp65 495–504 NLVPMVATV, Influenza A MP 58–66 GILGFVFTL, Mart1/MelanA 27–35 ELAGIGILTV, Malaria CS 334–342 YLNKIQNSL, or HIV Gag 77–85 SLYNTVATL) or with irradiated human leukemic cell lines (ALL-19 [reference 40], U937, Raji, or Nalm6) and irradiated (30Gy) autologous PBMC. 25 IU/ml IL-2 was added every 2–3 d. At 7-d intervals, cells were restimulated with irradiated autologous PBMC and peptide or irradiated leukemic cell line. T cell–mediated lysis of 51Cr-labeled leukemic cell lines or peptide-loaded T2 cells (103/well) was assayed (41). Excepting the experiment shown in Fig. S6, NK cell–mediated lysis was blocked with unlabeled K562 cells (≥5 × 104/well). Lysis of malaria or HIV peptide-negative controls was minimal. CD3-PE+, CD8-PerCPCy5.5+, and HLA-A*0201/NLVPMVATV pentamer–APC+ (ProImmune) cells were enumerated by flow cytometric staining.
Hu-SCID mouse model of GVHD.
Animal procedures were approved by the University of Queensland Animal Ethics Committee. Female SCID mice (C.B-17-Igh-1b-Prkdcscid) were purchased from the Animal Resource Centre (Perth, WA, Australia), housed in sterile microisolator cages, and given autoclaved food and water. On day −1, 5–7-wk-old mice were injected i.p. with 20 µl asialo-GM-1 (Wako Chemicals USA, Inc.) and irradiated (137Cs, 325cGy). 50 × 106 washed human PBMC in 200 µl were injected i.p. on day 0 (17). Mice were assessed daily using a GVHD scoring system that assesses weight loss, posture, activity, fur texture, and skin integrity (42) modified by addition of diarrhea. The overall score for each mouse was the sum of the six individual scores (0–2 for each). Mice with severe GVHD (overall score ≥5) were killed and tissues, blood, and peritoneal washings (in RPMI1640) taken for analysis. Some hu-SCID mice were immunized i.p. on days 0 and 7 with the U937 or Raji human leukemic cell lines (107 cells irradiated at 3000cGy).
Antibodies (RAneg, RA83, alemtuzumab, and ATG) were administered by i.p. injection 3 h before human PBMC injection on day 0. The RA83 circulating half-life was estimated by ELISA of blood samples drawn from SCID mice up to 14 d after a single i.p. injection.
Cell and tissue analysis.
Flow cytometric analyses were performed using FACSCalibur and LSR II flow cytometers (BD). Cells from peritoneal cavity, femoral bone marrow, and spleen were treated with red cell ACK lysis buffer and live cells counted by Trypan blue exclusion. PBMC and cells from mice were stained with fluorophore-conjugated antibodies (human CD3 clone SK7, CD8 SK1, CD45 2D1, and mouse CD45 30-F11 [BD]). Flow cytometry data were analyzed using FCS Express software. In some experiments, cells harvested from hu-SCID mice, as in the previous section, were combined and human leukocytes recovered by density gradient centrifugation (Ficoll Hypaque) and depletion with mouse CD45 immunomagnetic beads (Miltenyi Biotec), for subsequent in vitro CTL experiments.
Mouse tissues were fixed in 10% formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. The degree of lymphocytic infiltration was assessed by examination of one to two entire full face sections. Liver was scored between 0 (nil lymphocytes) and 2.5 (moderate infiltration), to which 1.0 was added for any focal apoptosis and hepatitis. Lung was scored between 0 and 3.5 for degree of perivascular lymphocytic infiltration, and 1.0 was added for any peribronchiolar infiltration.
Statistical analysis.
We used GraphPad Prism 4.0 (GraphPad Software, Inc.) and SYSTAT 10.2 (SYSTAT Software, Inc). Survival data were analyzed using the Kaplan-Meier log-rank test, with Bonferroni corrected posttest multiple comparisons. All other data (log transformed where clearly non-Gaussian and indicated in figure legends) were analyzed by ANOVA (repeated measures where indicated in legends) and, if statistically significant (P < 0.05), Bonferroni-corrected multiple comparisons posttests were done.
Online supplemental material.
Fig. S1 shows histology of liver from a hu-SCID mouse with GVHD and Fig. S2 shows human leukocyte engraftment of hu-SCID mouse spleen, bone marrow, and peritoneal cavity by flow cytometric staining. Fig. S3 shows weight change and GVHD scores for antibody-treated and control hu-SCID mice, whereas Fig. S4 shows human leukocyte engraftment 30 d after transplant in hu-SCID mice treated with RA83. Fig. S5 shows human CMV-specific CD8+ T cells from a RA83-treated hu-SCID mouse. Fig. S6 shows the effect of RA83 on in vitro induction of antileukemic cell line cytotoxicity. Supplementary Materials and methods, with Figs. S7–S10 embedded, describes the preparation and validation of the RA83 antibody. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070723/DC1.
Acknowledgments
We thank Ms Ann-Marie Burns for help and advice with SCID mice, Ms Ning Huang for preparation of CD83-Ig fusion protein used in rabbit immunizations and ELISA, Mater Pathology for histology preparation, and the Mater Research Support Centre for statistical advice.
This study was funded by the National Health and Medical Research Council of Australia (grant ID#281803) and The Cancer Council Queensland. Mater Medical Research Institute is affiliated with The University of Queensland, which supported J. Wilson with an Australian Postgraduate Award. A European Commission funded Marie Curie Outgoing Fellowship supported H. Cullup (Contract Number: MCOIF-CT-2004-509939).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ADCC, antibody-dependent cellular cytotoxic; allo, allogeneic; ATG, anti-thymocyte globulin; FMP, influenza matrix protein; GVHD, acute graft-versus-host disease; GVL, graft versus leukemia; HSCT, hematopoietic stem cell transplantation; MoDC, monocyte-derived DC.
References
- Welniak L.A., Blazar B.R., Murphy W.J. 2007. Immunobiology of allogeneic hematopoietic stem cell transplantation.Annu. Rev. Immunol. 25:139–170 [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D., Couzens M.S., Tang C.B., McNiff J., Robert M.E., Liu J., Shlomchik M.J., Emerson S.G. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells.Science. 285:412–415 [DOI] [PubMed] [Google Scholar]
- Duffner U.A., Maeda Y., Cooke K.R., Reddy P., Ordemann R., Liu C., Ferrara J.L., Teshima T. 2004. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease.J. Immunol. 172:7393–7398 [DOI] [PubMed] [Google Scholar]
- Matte C.C., Liu J., Cormier J., Anderson B.E., Athanasiadis I., Jain D., McNiff J., Shlomchik W.D. 2004. Donor APCs are required for maximal GVHD but not for GVL.Nat. Med. 10:987–992 [DOI] [PubMed] [Google Scholar]
- Tivol E., Komorowski R., Drobyski W.R. 2005. Emergent autoimmunity in graft-versus-host disease.Blood. 105:4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.E., McNiff J.M., Jain D., Blazar B.R., Shlomchik W.D., Shlomchik M.J. 2005. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ.Blood. 105:2227–2234 [DOI] [PubMed] [Google Scholar]
- Taylor P.A., Ehrhardt M.J., Lees C.J., Panoskaltsis-Mortari A., Krieg A.M., Sharpe A.H., Murphy W.J., Serody J.S., Hemmi H., Akira S., et al. 2008. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection.Blood. 112:3508–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.E., Thompson J.S., Carter S.L., Kernan N.A. 2005. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell depletion trial): a multi-centre, randomised phase II-III trial.Lancet. 366:733–741 [DOI] [PubMed] [Google Scholar]
- Chakraverty R., Robinson S., Peggs K., Kottaridis P.D., Watts M.J., Ings S.J., Hale G., Waldmann H., Linch D.C., Goldstone A.H., Mackinnon S. 2001. Excessive T cell depletion of peripheral blood stem cells has an adverse effect upon outcome following allogeneic stem cell transplantation.Bone Marrow Transplant. 28:827–834 [DOI] [PubMed] [Google Scholar]
- Copelan E.A. 2006. Hematopoietic stem-cell transplantation.N. Engl. J. Med. 354:1813–1826 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shlomchik W.D., Joe G., Louboutin J.P., Zhu J., Rivera A., Giannola D., Emerson S.G. 2002. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease.J. Immunol. 169:7111–7118 [DOI] [PubMed] [Google Scholar]
- Merad M., Hoffmann P., Ranheim E., Slaymaker S., Manz M.G., Lira S.A., Charo I., Cook D.N., Weissman I.L., Strober S., Engleman E.G. 2004. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease.Nat. Med. 10:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K.P.A., Munster D., Clark G.C., Dzionek A., Schmitz J., Hart D.N.J. 2002. Characterization of human blood dendritic cell subsets.Blood. 100:4512–4520 [DOI] [PubMed] [Google Scholar]
- Munster D.J., MacDonald K.P.A., Kato M., Hart D.N.J. 2004. Human T lymphoblasts and activated dendritic cells in the allogeneic mixed leukocyte reaction are susceptible to NK cell-mediated anti-CD83-dependent cytotoxicity.Int. Immunol. 16:33–42 [DOI] [PubMed] [Google Scholar]
- Koppi T., Munster D.J., Brown L., Munster D., MacDonald K.P.A., Hart D.N.J. 2003. CMRF-44 antibody-mediated depletion of activated human dendritic cells: a potential means for improving allograft survival.Transplantation. 75:1723–1730 [DOI] [PubMed] [Google Scholar]
- Collin M.P., Munster D., Clark G., Wang X.N., Dickinson A.M., Hart D.N. 2005. In vitro depletion of tissue-derived dendritic cells by CMRF-44 antibody and alemtuzumab: implications for the control of Graft-versus-host disease.Transplantation. 79:722–725 [DOI] [PubMed] [Google Scholar]
- Sandhu J.S., Gorczynski R., Shpitz B., Gallinger S., Nguyen H.P., Hozumi N. 1995. A human model of xenogeneic graft-versus-host disease in SCID mice engrafted with human peripheral blood lymphocytes.Transplantation. 60:179–184 [PubMed] [Google Scholar]
- Duchosal M.A., Mauray S., Ruegg M., Trouillet P., Vallet V., Aarden L., Tissot J.D., Schapira M. 2001. Human peripheral blood leukocyte engraftment into SCID mice: critical role of CD4(+) T cells.Cell. Immunol. 211:8–20 [DOI] [PubMed] [Google Scholar]
- Krams S.M., Dorshkind K., Gershwin M.E. 1989. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice.J. Exp. Med. 170:1919–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A., Morey A., Rainer S., Turner J., Munro V. 2004. Histopathology of hematopoietic stem cell transplantation. Clinical Bone Marrow and Blood Stem Cell Transplantation. Atkinson K., Champlin R., Ritz J., Fibbe W.E., Ljungman P., Brenner M., Cambridge University Press, Cambridge, UK: 1634–1658 [Google Scholar]
- Alter B.J., Bach F.H. 1990. Cellular basis of the proliferative response of human T cells to mouse xenoantigens.J. Exp. Med. 171:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P.J., Shearer G.M., Neudorf S., Gress R.E. 1990. The human antimurine xenogeneic cytotoxic response. I. Dependence on responder antigen-presenting cells.J. Immunol. 144:4548–4554 [PubMed] [Google Scholar]
- Goker H., Haznedaroglu I.C., Chao N.J. 2001. Acute graft-vs-host disease: pathobiology and management.Exp. Hematol. 29:259–277 [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Ishikawa F., Greiner D.L. 2007. Humanized mice in translational biomedical research.Nat. Rev. Immunol. 7:118–130 [DOI] [PubMed] [Google Scholar]
- van Rijn R.S., Simonetti E.R., Hagenbeek A., Hogenes M.C., de Weger R.A., Canninga-van Dijk M.R., Weijer K., Spits H., Storm G., van Bloois L., et al. 2003. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice.Blood. 102:2522–2531 [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B., McKenna S., Mobraaten L., Rajan T.V., Greiner D.L., et al. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice.J. Immunol. 154:180–191 [PubMed] [Google Scholar]
- Shpitz B., Chambers C.A., Singhal A.B., Hozumi N., Fernandes B.J., Roifman C.M., Weiner L.M., Roder J.C., Gallinger S. 1994. High level functional engraftment of severe combined immunodeficient mice with human peripheral blood lymphocytes following pretreatment with radiation and anti-asialo GM1.J. Immunol. Methods. 169:1–15 [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Bennett M., Anver M.R., Baseler M., Longo D.L. 1992. Human-mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions.Eur. J. Immunol. 22:1421–1427 [DOI] [PubMed] [Google Scholar]
- Lucas P.J., Bare C.V., Gress R.E. 1995. The human anti-murine xenogeneic cytotoxic response. II. Activated murine antigen-presenting cells directly stimulate human T helper cells.J. Immunol. 154:3761–3770 [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. 2000. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells.J. Exp. Med. 192:1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpinati M., Green C.L., Heimfeld S., Heuser J.E., Anasetti C. 2000. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells.Blood. 95:2484–2490 [PubMed] [Google Scholar]
- Zhang Y., Louboutin J.P., Zhu J., Rivera A.J., Emerson S.G. 2002. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell- mediated acute graft-versus-host disease.J. Clin. Invest. 109:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Joe G., Hexner E., Zhu J., Emerson S.G. 2005. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease.J. Immunol. 174:3051–3058 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Joe G., Hexner E., Zhu J., Emerson S.G. 2005. Host-reactive CD8+ memory stem cells in graft-versus-host disease.Nat. Med. 11:1299–1305 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Mackinnon S., Chopra R., Kottaridis P.D., Peggs K., O'Gorman P., Chakraverty R., Marshall T., Osman H., Mahendra P., et al. 2002. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution.Blood. 99:4357–4363 [DOI] [PubMed] [Google Scholar]
- Maraninchi D., Gluckman E., Blaise D., Guyotat D., Rio B., Pico J.L., Leblond V., Michallet M., Dreyfus F., Ifrah N., et al. 1987. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias.Lancet. 330:175–178 [DOI] [PubMed] [Google Scholar]
- Reddy P., Maeda Y., Liu C., Krijanovski O.I., Korngold R., Ferrara J.L. 2005. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses.Nat. Med. 11:1244–1249 [DOI] [PubMed] [Google Scholar]
- Lau J., Sartor M., Bradstock K.F., Vuckovic S., Munster D.J., Hart D.N. 2007. Activated circulating dendritic cells after hematopoietic stem cell transplantation predict acute graft-versus-host disease.Transplantation. 83:839–846 [DOI] [PubMed] [Google Scholar]
- Hock B.D., Kato M., McKenzie J.L., Hart D.N.J. 2001. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera.Int. Immunol. 13:959–967 [DOI] [PubMed] [Google Scholar]
- Lock R.B., Liem N., Farnsworth M.L., Milross C.G., Xue C., Tajbakhsh M., Haber M., Norris M.D., Marshall G.M., Rice A.M. 2002. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse.Blood. 99:4100–4108 [DOI] [PubMed] [Google Scholar]
- Radford K.J., Turtle C.J., Kassianos A.J., Hart D.N. 2006. CD11c+ blood dendritic cells induce antigen-specific cytotoxic T lymphocytes with similar efficiency compared to monocyte-derived dendritic cells despite higher levels of MHC class I expression.J. Immunother. 29:596–605 [DOI] [PubMed] [Google Scholar]
- Hill G.R., Crawford J.M., Cooke K.R., Brinson Y.S., Pan L., Ferrara J.L. 1997. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines.Blood. 90:3204–3213 [PubMed] [Google Scholar]