Abstract
Antibodies to citrulline-modified proteins have a high diagnostic value in rheumatoid arthritis (RA). However, their biological role in disease development is still unclear. To obtain insight into this question, a panel of mouse monoclonal antibodies was generated against a major triple helical collagen type II (CII) epitope (position 359–369; ARGLTGRPGDA) with or without arginines modified by citrullination. These antibodies bind cartilage and synovial tissue, and mediate arthritis in mice. Detection of citrullinated CII from RA patients' synovial fluid demonstrates that cartilage-derived CII is indeed citrullinated in vivo. The structure determination of a Fab fragment of one of these antibodies in complex with a citrullinated peptide showed a surprising β-turn conformation of the peptide and provided information on citrulline recognition. Based on these findings, we propose that autoimmunity to CII, leading to the production of antibodies specific for both native and citrullinated CII, is an important pathogenic factor in the development of RA.
Rheumatoid arthritis (RA) is believed to be an autoimmune disease mainly caused by the longstanding observation of the presence of increased levels of autoantibodies. The classical autoantibodies are rheumatoid factors (RFs), i.e., antibodies reactive with IgG. Although these are likely to be relevant, as their occurrence predates the development of clinical arthritis (1), it is still unclear what their pathophysiologic role is. However, one of the most exciting recent discoveries is the finding that antibodies recognizing citrullinated proteins show a higher specificity for RA as compared with RFs (2, 3). Citrullination is a posttranslational modification of proteins in which a peptidyl arginine is converted into the nonstandard amino acid peptidyl citrulline. The reaction is catalyzed by calcium-dependent peptidyl arginine deiminase (PAD), an evolutionarily conserved protein with several isoforms in both mice and humans (PAD1–4 and PAD6) (4). The most prominent difference between the distinct PAD isotypes is the distribution of expression among specific tissues. PAD4 can be found in monocytes and macrophages, whereas both PAD2 and PAD4 have been observed in synovial fluid (5–7). Citrullination has been detected in many tissues and has been shown to occur in both mouse and human inflamed joints (5, 8, 9). Not only the expression but also the activation of PAD is required for citrullination. This activation requires a local calcium concentration of ∼10−5 mol/liter, which is much higher than normal cytosolic calcium concentration (∼10−7 mol/liter) (7). However, the calcium concentration is increased in the cytoplasm during apoptosis or necrosis (10), which allows PAD to be released. In inflamed tissues, the released PAD could therefore also citrullinate extracellular proteins like fibrinogen and collagen.
Antibodies against citrullinated proteins (ACPAs) have been identified in the synovium of a high number of RA patients (50–70%) (11, 12). In contrast, ACPAs are rarely found in healthy individuals or patients with other diseases (<2%). Interestingly, ACPAs share with RFs the fact that they can be detected in patient sera even before the onset of initial RA symptoms, and are therefore believed to play a pathogenic role (13). These findings have stimulated the search for the origin of ACPA production. The recognition of citrulline is dependent on the protein backbone, and it has therefore been of considerable interest to identify proteins that elicit and perpetuate the ACPA response. Clearly, ACPAs are produced in the joints (11, 14), and one possibility is that the recognized citrulline is an antigenic determinant that is preferentially associated with proteins deposited in joints like fibrin (15). In fact, immunization of mice with citrullinated fibrin has been reported to induce joint inflammation, which, however, differs considerably in its histopathologic features from those that are characteristic of RA or its well-established experimental models, such as collagen-induced arthritis (CIA) (16). In addition, fibrin deposition is not specific for RA joints (17). An alternative hypothesis is closely related to the discovery that citrullination of a vimentin-derived peptide increases its binding to the RA-associated MHC class II molecule DR4 (18). Similar to previous discoveries in celiac disease, the posttranslational modification of a potential T cell determinant could explain the breakdown of self-tolerance (19). Although tolerance remains restricted to the nonmodified self, it could easily allow for T cell activation in response to a presented citrullinated self-determinant, thereby giving rise to autoantigen-specific B cell help. However, so far there is no evidence for an enhanced T cell response to citrullinated vimentin neither in RA nor in animal models. An alternative perspective on the potential mechanism that may lead to the development of humoral immunity to citrullinated self-proteins in RA is offered by the assumption of a so-called linked recognition. According to this concept, the B cells with specificity for a citrullinated determinant get activated upon cooperation with T cells recognizing another (nonmodified cryptic) epitope on the same protein. In fact, the major cartilage protein, collagen type II (CII), provides such a possibility for recognition as a relevant joint-specific autoantigen. Importantly, if a linked recognition occurs, it could operate in two steps; i.e., B cells producing antibodies to other citrullinated proteins may capture citrullinated CII and subsequently activate pathogenic CII-reactive T and B cells. In this case, a response to CII should occur in RA joints.
Immunization of genetically susceptible animals with CII induces arthritis and is the basis for one of the most commonly used animal models for RA, CIA. The critical immune recognition structures have been identified in the mouse (20–22). T cells recognize a glycopeptide derived from CII position 260–270 on the MHC class II molecule Aq. Importantly, mice expressing the RA associated DR4 or DR1 class II molecules are also susceptible to arthritis because of recognition of an almost identical glycopeptide (CII261-273). In fact, T cell responses to this peptide, in particular to the galactosylated form, are raised in RA (20). Both mouse and human T cells predominantly recognize the posttranslationally modified lysine side chain at position 264. The B cell response in the CIA model is directed to a series of epitopes located at the triple helical form of the CII molecule, and these epitopes are also conserved and recognized in RA (23). Antibodies to certain epitopes correlate with arthritis severity in both CIA and RA, and mAbs directed to these epitopes induce arthritis in mice (the so-called collagen antibody–induced arthritis [CAIA]). Interestingly, these triple helical epitopes on the CII molecule contain a conserved motif that contains an arginine (G-X-R-G hydrophobic amino acid). In fact, some of the defined epitopes contain the conserved motifs in a tandem repeat like the dominating “C1” epitope at position 359–370 (24). Clearly, this opens up the possibility that these arginines could be citrullinated, exposing a second type of posttranslationally modified epitope in addition to the galactosylation of lysine 264 recognized by T cells. We have recently demonstrated that one of the major B cell epitopes on CII, the C1 epitope CII 359–370 (GARGLTGRPGDA), is also recognized in RA in its citrullinated form (25).
We have now made a panel of mAbs against the citrullinated C1 epitope and show that both CII- and citrulline-specific antibodies induce as well as promote arthritis in the mouse. Using these antibodies, citrullinated CII can be identified in mouse and human RA joints. The structural interactions between one of the antibodies recognizing the citrullinated epitopes were revealed, and it was found that the peptide forms a hairpin exposing the citrulline side chain into the combining site of the antibody. These data thus demonstrate that citrullination of CII occurs in RA, and the antibodies specific for at least one of the citrullinated collagen epitopes induces and promotes the development of arthritis.
RESULTS
Generation and specificity of mAbs to citrullinated CII ACC1–5
The mAb ACC1, specific for citrullinated CII, was isolated from a B10.RIII Cia5 congenic mouse immunized with bovine CII. The ACC2–4 antibodies were raised from DBA/1 or (B10.Q × DBA/1) F1 mice immunized with PAD4-treated B+T-Cit peptides emulsified in CFA. GB8 and ACC5 stem from B10.Q mice with chronic arthritis after CII/CFA immunization. The cloned hybridomas were characterized by VDJ sequencing, and the mAbs were purified and tested for specificity (Fig. 1 a and Table I).
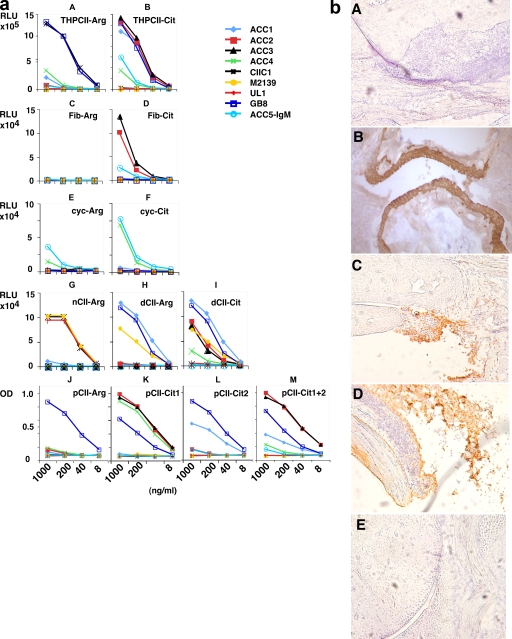
Figure 1.
ACC mAb antibody specificity in vitro and in vivo. (a) ACC mAb specificity in ELISA. Several arginine-containing peptides and their citrulline analogues have been tested to characterize the citrulline specificity of our generated antibodies. Some of the epitopes were accessible in the triple helical form of the citrulline-containing C1III epitope (THPCII-Cit) for some of the antibodies (ACC1–3 and ACC5; A and B). Nevertheless, this epitope seems not to be accessible for ACC4. Some of the antibodies cross react to selected citrullinated peptides even with a noncollageneous backbone. ACC2, ACC3, and ACC5 bind not only to CII but also to the citrullinated derivative of fibrinogen (C and D). Furthermore, ACC4 and ACC5 bind stronger to the citrullinated form of cyclic filaggrin (cyc-Cit) than to its arginine-containing form (cyc-Arg; E and F). Enzymatic deimination of CII with PAD4 generates neoepitopes, which are recognized by ACC2–4 (G–I). C1III peptides containing an additional biotinylated lysine have been synthesized, which in turn bind to NeutrAvidin-precoated ELISA plates (J–M). This setup allows free accessibility of the different antibodies specific for the citrulline-modified immunodominant CII epitopes. All of the assays were done in duplicate. (b) Staining of arthritic joints. Citrulline-specific antibodies bind to arthritic cartilage. Joint sections (10 mm) from arthritic BALB/c (A, C, and D), naive (BALB/c × B10.Q) F1 (B), or BALB/c (E) mice are shown. Results shown are representative histological pictures of arthritic (n = 4) and control (n = 3) mice used in the staining of joints with anticitrulline antibodies. Arthritis was induced in 4–6-mo-old naive male BALB/c mice (n = 36) by injecting 9 mg of an arthritogenic anti-CII mAb cocktail containing antibodies M2139 (binding to the J1 epitope) and CIIC1 (binding to the C1I epitope). The arthritis induction experiment was performed four times independently with 100% incidence and a mean maximum arthritis score of 32.9 ± 3. The four mice that were used for histology had arthritis scores of 59, 60, 47, and 50. Paw samples were taken on day 11 after antibody transfer (5 d after LPS injection). Sections were treated with no antibodies (A), ACC1 (B), or ACC4 (C–E) mAbs. Magnification, ×10.
Table I.
Summary of mAb specificities
| Clones | Sub- class | nCII- Arg | dCII- Arg | dCII- Cit | THPCII Arg | THPCII Cit | pCII- Arg | pCII Cit1 | pCII Cit2 | pCII Cit1+2 | cyc- Arg | cyc- Cit | FibArg | FibCit |
| ACC1 | γ2a | + | +++ | +++ | − | ++ | − | − | ++ | + | − | + | − | − |
| ACC2 | γ2b | − | − | ++ | − | ++ | − | +++ | − | +++ | − | − | − | +++ |
| ACC3 | γ2b | − | − | ++ | − | ++ | − | +++ | − | +++ | − | − | − | +++ |
| ACC4 | γ1 | − | − | + | − | + | − | +++ | − | − | + | +++ | − | − |
| ACC5 | μ | − | − | − | − | + | − | − | − | − | ++ | +++ | − | ++ |
| GB8 | γ2b | − | + | + | ++ | ++ | +++ | ++ | +++ | ++ | − | − | − | − |
| CIIC1 | γ2a | ++ | − | − | +++ | − | − | − | − | − | − | − | − | − |
| M2139 | γ2b | ++ | + | + | − | − | − | − | − | − | − | − | − | − |
| UL1 | γ2b | + | − | − | − | − | − | − | − | − | − | − | − | − |
Grading of values obtained with a 200-ng/ml antibody concentration. Scale for relative luminescence unit (RLU; CII assay) values: −, <20,000; +, >20,000; ++, >100,000; and +++, >200,000. Scale for RLU (THP assay): −, <100,000; +, >100,000; ++, >500,000; and +++, >1,000,000. Scale for RLU (FibC assay): −, <1,000; +, >1,000; ++, >5,000; and +++, >10,000. Scale for OD values: −, <0.05; +, >0.05; ++, >0.25; and +++, >0.5.
Sequencing revealed that ACC1, ACC4, GB8, and UL1 have unique cDNA V gene sequences, whereas the ACC2 and ACC3 clones shared both VH and VL (Figs. S1 and S2, available at http://www.jem.org/cgi/content/full/jem.20081862/DC1). The ACC2 and ACC3 clones were derived from the same mouse and are therefore likely to originate from the same clone in vivo. All of these ACC antibodies contained somatic mutations, indicating that they are produced by T cell–dependent follicular B cells (Table S1). All ACC antibodies bind to citrullinated forms of the C1 epitope. ACC1–3 and ACC5 bind to the triple helical citrullinated C1, and ACC4 binds to the citrullinated C1 epitope as an α-chain peptide. None of the ACC antibodies cross react to the respective noncitrullinated variant of the α chain of the C1 epitope. However, they cross react with variable degrees to citrullinated epitopes on other peptide backbones. ACC4 and ACC5 bind to cyclic citrullinated filaggrin peptide (i.e., CCP1), and ACC2 and ACC3 bind to citrullinated fibrinogen. Thus, these antibodies are mouse monoclonal ACPAs. Although they are all directed to the citrullinated C1 epitope, their specificities are not identical. The ACC1 antibody cross reacted to denatured CII as well as to the denatured triple helical C1 epitope. There are two arginines in the C1 epitope, which can be posttranslationally converted to citrulline. ACC2–4 recognize the first citrulline at position 360, and ACC1 recognizes the second citrulline at position 365.
To determine whether the citrullinated epitope is exposed in vivo, we stained joint sections from adult arthritic mice using the ACC1 and ACC4 antibodies. The ACC1 antibody stained cartilage and the ACC4 antibody stained the inflamed synovial tissue in the vicinity of cartilage (Fig. 1 b). Although the staining with ACC4 clearly demonstrates the occurrence of citrullinated denatured CII in the cartilage, the interpretation of the ACC1 staining is less clear because it might reflect either antibody recognition of citrullinated CII or cross-reactive binding to the denatured CII.
Induction of arthritis
To investigate the arthritogenic potential of the ACC mAbs, we purified the ACC1 (an IgG2c specific for triple helical C1 epitope), ACC4 (an IgG1 specific for the citrullinated C1 epitope on the α chain), and ACC5 antibodies (an IgM specific for the triple helical C1 epitope), as well as the anti-CII IgG antibodies M2139 (recognizing the J1 epitope), CIIC1 (recognizing the C1I epitope), and UL1 (recognizing the U1 epitope), and injected them in vivo in both naive mice and mice suffering from chronic CIA (Fig. 2; and Tables II and III). The ACC1 antibodies could induce mild arthritis in naive mice, and a more severe form of the arthritis was developed after a booster injection of LPS, similar to pathogenic anti-CII antibodies (Fig. 2 a). The severity of arthritis was enhanced when the ACC1 antibody treatment was combined with the anti-CII antibodies M2139 or CIIC1 (unpublished data). As the induction of arthritis with ACC1 could possibly be caused by its cross-reactivity with denatured CII, we also used the ACC4 antibody that showed no cross-reactivity with nonmodified CII and recognized the citrulline epitope on a single α-chain peptide. Injection of ACC4 antibody enhanced the arthritis when given concomitantly with another CII-specific antibody (M2139 ACC4) but could not induce arthritis by itself (Fig. 2 a). This lack of direct arthritogenic potential could be caused by the noncomplement-binding subclass of the ACC4 antibody (IgG1). Another citrulline-specific antibody, the IgM ACC5 mAb preferentially recognizing the noncollagenous citrullinated antigens filaggrin and fibrinogen, induces arthritis but with very low severity (Fig. 2 b). The monoclonal IgG2b antibody GB8 that recognized denatured CII was also unable to enhance arthritis in combination with anti-CII antibodies (Fig. 2 a). We conclude that antibodies to citrullinated CII can induce arthritis in naive mice by themselves, and the arthritis severity is enhanced by combining them with antibodies to noncitrullinated CII.
Figure 2.
Citrulline-specific antibodies mediate arthritis. (a) The frequency of arthritis on different days is shown. Groups (ACC1, n = 14; ACC4, n = 10; M2139 + PBS, n = 33; M2139 + ACC3, n = 10; M2139 + ACC4, n = 28; M2139 + ACC5, n = 10; M2139 + CIIC1, n = 20; and M2139 + GB8, n = 10) of 4-mo-old naive male B10.RIII mice were injected i.v. with 9 mg of a single mAb or an equal combination of two mAbs. M2139 with CIIC1 and 4.5 mg M2139 with PBS constituted positive and negative controls, respectively. 25 µg LPS per mouse was injected i.p. on day 5 to enhance the incidence and severity of arthritis. Results shown are pooled values from three similar experiments with balanced groups. M2139 + ACC4 versus M2139 + PBS, P ≤ 0.0113 (days 3–4) and P ≤ 0.001 (days 5–27); ACC1 versus ACC4, P ≤ 0.0034 (days 10–14 and days 21–27) and P ≤ 0.0013 (days 17–19). *, P < 0.05; **, P < 0.005; and ***, P < 0.001. (b) 2-mo-old QB = (BALB/c x B10.Q) F1 mice were injected with CII + CFA on day 0 and boosted with CII + IFA on day 35. These mice developed chronic arthritis that persisted for a minimum of 210 d. Mice that had no arthritis after 210 d were injected with 9 mg of a single mAb or an equal combination of two mAbs constituted different groups (ACC4, n = 9; PBS, n = 20; M2139 + PBS, n = 7; UL1 + PBS, n = 8; M2139 + ACC4, n = 10; and UL1 + ACC4, n = 9). M2139 with CIIC1 or UL1 and 4.5 mg M2139 or UL1 with PBS constituted positive and negative controls, respectively. LPS was not injected in these mice. The PBS-injected group denotes spontaneous relapse. All of the mice were used for calculations. Results shown are pooled values from two similar experiments with balanced groups. M2139 + ACC4 versus M2139 + PBS, P ≤ 0.026 (day 13) and P ≤ 0.026 (day 15); UL1 + ACC4 versus UL1 + PBS, P ≤ 0.0311 (day 5). *, P < 0.05 indicates a significant increase in arthritis frequency induced by ACC4 antibodies.
Table II.
Arthritogenicity of citrulline-specific mAbs in naive mice
| mAbs | Incidence | Mean max arthritis score (mean ± SEM) |
| ACC1 | 9/14** | 17 ± 5** |
| ACC4 | 0/10 | 0 |
| M2139 + PBS | 9/33 | 12 ± 2 |
| M2139 + ACC4 | 20/28*** | 27 ± 3**** |
| M2139 + ACC5 | 3/10 | 5 ± 3 |
| M2139 + CIIC1 | 18/20 | 26 ± 4 |
| M2139 + GB8 | 4/10 | 15 ± 5 |
Groups of 4-mo-old naive male B10.RIII mice were injected i.v. with 9 mg of a single mAb or an equal combination of two mAbs. M2139 with CIIC1 and 4.5 mg M2139 with PBS constituted positive and negative controls, respectively. 25 µg LPS per mouse was injected i.p. on day 5 to enhance the incidence and severity of arthritis. Cumulative incidence: M2139 + ACC4 versus M2139 + PBS, P ≤ 0.0006; and ACC1 versus ACC4, P < 0.001. Maximum arthritis score: M2139 + ACC4 versus M2139 + PBS, P < 0.0001; and ACC1 versus ACC4, P = 0.0024. Results shown are pooled values from three similar experiments. **, P < 0.005; ***, P < 0.001; and ****, P < 0.0001.
Table III.
Citrulline-specific antibodies mediate arthritis relapse
| mAbs | Incidence | Mean max arthritis score (mean ± SEM) |
| ACC4 | 4/9* | 20 ± 3* |
| PBS | 1/20 | 5 |
| M2139 + PBS | 2/7 | 3 ± 2 |
| UL1 + PBS | 4/8 | 18 ± 3 |
| M2139 + ACC4 | 7/10 | 14 ± 2* |
| M2139 + CIIC1 | 18/20 | 26 ± 4 |
| M2139 + UL1 | 9/9 | 25 ± 2 |
| UL1 + ACC4 | 4/9 | 15 ± 6 |
2-mo-old QB = (BALB/c x B10.Q) F1 mice were injected with CII + CFA on day 0 and boosted with CII + IFA on day 35. These mice developed chronic arthritis that persisted for a minimum of 210 d. Mice that had no arthritis after 210 d were injected with 9 mg of a single mAb or an equal combination of two mAbs. M2139 with CIIC1 or UL1 and 4.5 mg M2139 or UL1 with PBS constituted positive and negative controls, respectively. The PBS-injected group denotes spontaneous relapse. LPS was not injected in these mice. Results shown are pooled values from two similar experiments. Cumulative incidence: ACC4 versus PBS, P ≤ 0.0093. Maximum arthritis score: M2139 + ACC4 versus M2139 + PBS, P ≤ 0.0266; and ACC4 versus PBS, P ≤ 0.0073. *, P < 0.05.
To test whether the ACC antibodies could trigger a relapse of arthritis in an already ongoing chronic arthritis, we used a model in which chronic arthritis was induced in (BALB/c × B10.Q) F1 mice. These mice develop a chronic relapsing disease with a duration of >200 d, and we selected mice in remission from a large cohort of mice. Anti-CII antibodies induce arthritis relapses 1–2 d after injection in this cohort. Similarly, both ACC1 and ACC4 could both induce arthritis and enhance severity in this situation (Fig. 2 b). The induction of an arthritic relapse was, however, less pronounced but more prolonged as compared with anti-CII antibodies. We conclude that not only anti-CII antibodies but also antibodies specific for citrullinated CII induce relapses during the chronic phase of arthritis.
Immunoprecipitation of citrullinated CII from human RA and osteoarthritis (OA) joints
To determine whether citrullination of CII also occurs in human RA, we immunoprecipitated CII from synovial fluid (Fig. 3 a). For screening of citrullinated CII in 72 synovial fluid specimens from patients with OA (n = 47), RA (n = 10), and reactive arthritis (Reac A; n = 15), a capture ELISA procedure was designed. The titer plates were coated with a mixture of three earlier characterized mAbs (CIIE10, M2139, and D3) (24) recognizing distinct epitopes on native CII to capture the triple helical CII from the synovial fluid for subsequent detection with the biotinylated mAb ACC2 in combination with avidin-peroxidase. Citrullinated CII was detectable in synovial fluid from 7 out of 10 RA- and 20 out of 47 OA-derived samples (OD values > mean + 2SD of BSA control), whereas the results remained negative for all of the 15 specimens collected from Reac A patients, usually representing a nonerosive form of joint inflammation that does not lead to CII degradation (Fig. 3 b). For specificity control of the ELISA procedure, immunoprecipitation of selected synovial fluid samples was performed using the described cocktail of anti-CII mAbs followed by SDS-PAGE and immunostaining. Thus, precipitated CII was blotted onto a nitrocellulose membrane for subsequent detection of the CII-citrulline residues after a specific chemical modification procedure with an antimodified citrulline monospecific antibody (Fig. 3 a). In addition, a gel staining of citrullinated CII was performed using the specific mAb ACC2 as a detecting reagent (Fig. 3 b).
Figure 3.
Detection of citrullinated CII in human synovial fluid. (a) Immunoprecipitation of citrullinated CII from synovial fluid. Western blot staining with the antimodified citrulline antibody (AMC) upon appropriate pretreatment of the nitrocellulose membrane and immunostaining with the anticitrullinated CII mAb ACC2 are shown (+, ELISA-positive synovial fluid; −, ELISA-negative synovial fluid [compare with b]). Shown is a representative result of three independently performed experiments. (b) Capture ELISA for detection of citrullinated CII in synovial fluid specimens obtained from patients with OA, Reac A, and RA. Synovial fluid specimens were collected from patients with OA (n = 47), RA (n = 10), and Reac A (n = 15).
Molecular structure of ACC4 complexed with the citrullinated C1 epitope
To investigate the molecular interactions between the mAbs and citrullinated CII, we selected the ACC4 antibody that recognized the citrullinated CII single-chain peptide. The structure of the ACC4 (IgG1/κ) Fab fragment and its complex with a collagen C1 peptide citrullinated at position 360 (peptide pCII-Cit1) were determined, and data and refinement statistics are shown in Table IV. The Fab–peptide complex crystal belongs to space group P21, with two independent molecules per asymmetrical unit. The crystal of the ACC4 Fab alone has space group P212121, with one molecule per asymmetrical unit. The overall structure of ACC4 (Fig. 4 a) is similar to other Fab fragments for which the structure is known. The Fab fragment contains four Ig fold domains: two for the heavy chain (VH and CH) and two for the light chain (VL and CL). Each domain consists of a stable arrangement of hydrogen-bonded, antiparallel β strands, further stabilized by disulfide bond bridges between L23-L93, L139-L199, H22-H96, and H145-H200. The variable domains contain nine β strands and the constant domains contain seven β strands. The binding pocket is formed by residues from complementary determining region (CDR) loops H1, H2, H3, L1, L2, and L3 of the Fab fragment. CDR loops L1, L2, L3, H1, and H2 fall into canonical classes 4, 1, 1, 1, and 2, respectively (Table V). The elbow angle of the ACC4 peptide complex was determined by RBOW (26) as being 184.5° for the first molecule in the asymmetrical unit and 188.6° for the second, whereas the elbow angle of the unliganded Fab is 181.6°. These values are within the range (127–227°) for known Fab models.
Table IV.
Data processing and refinement statistics of ACC4 structures
| Dataset I (complex) | Dataset II (noncomplex) | |
| Data collection | ||
| Space group | P21 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 57.7, 128.4, 72.6 | 44.8, 70, 136.6 |
| α, β, γ (°) | 90, 106.1, 90 | 90, 90, 90 |
| Resolution (Å) | 2.21 | 1.45 |
| Rsym or Rmerge | 8.2 (27.2)* | 6.6 (27.8)* |
| I/σI | 14.6 (4.4)* | 20.8 (6.3)* |
| Completeness (%) | 96.6 (97.8)* | 97.3 (97.7)* |
| Redundancy | 3.8 | 3.6 |
| Refinement | ||
| Resolution (Å) | 24.23–2.21 | 68.2–1.45 |
| No. reflections | 45,714 | 64,156 |
| Rwork/Rfree | 23.4/28.5 | 18.8/22.7 |
| No. atoms | 6,894 | 3,888 |
| Water | 219 | 498 |
| RMS deviations | ||
| Bond lengths (Å) | 0.014 | 0.017 |
| Bond angles (°) | 1.84 | 1.783 |
| Ramachandran plot statistics (%) | ||
| Most favored regions | 88.4 | 92.3 |
| Additional allowed regions | 11 | 7.2 |
| Generously allowed regions | 0.4 | 0.3 |
| Disallowed region | 0.3 | 0.3 |
Asterisks indicate the statistics for the highest resolution shell. RMS, root mean square.
Figure 4.
Molecular structural analysis of the ACC4 interacting with citrullinated collagen. (a) The structure of ACC4 Fab complexed with pCII-Cit1 peptide. The light chain of the Fab is shown in cyan, the heavy chain is shown in green, and the bound pCII-Cit1 peptide is shown in red. The overall shape of ACC4 Fab is same as the other Fab structures in the Protein Data Bank. The peptide has bound to the interface between CDR loops on top of the variable domain, as anticipated. The images were created with PyMOL (reference 60). (b) The structure of the peptide pCII-Cit1. The difference (2Fo-Fc) electron density map is shown in blue. The density is contoured at 1 σ. The water molecule is shown as a red ball. The electron density is strong enough to build nine residues of peptides, shown as sticks. The PCII-Cit1 peptide adopts a β turn conformation. Cit6P represents citrulline residue 6 and Hyp12P represents hydroxyproline residue 12 of the peptide. (c) Surface representations of ACC4 Fab–peptide complex from the top (left) and the side (right). The pCII-Cit1 peptide is shown as sticks. The water molecule on the peptide is shown as a red ball. The peptide fills a cavity on top of the variable domain. Although the C-terminal side of the peptide is buried inside the cavity, the N-terminal side is solvent exposed. (d) Fab–peptide interactions. (A) The residues that contact with citrulline (Cit6P). (B) The residues that contact with arginine (Arg11P). The black dashed lines represent hydrogen bonds. The green residues are from the heavy chain and the cyan residues are from the light chain.
Table V.
Amino acids in CDR fragments of ACC4 in comparison with germline residues involved in interactions
| CDR | Sequence | Canonical form | ΔBSA |
| L1 | |||
| IMTG | 27QSLLDSDGKTY37 | Class 4 | 44.61 Å2 (8.1%) |
| Germline | |||
| Contact | D D Y | ||
| L2 | |||
| IMTG | 55LVS56 | Class 1 | 0 Å2 (0%) |
| Germline | |||
| Contact | |||
| L3 | |||
| IMTG | 94WQGTHFPLT102 | Class 1 | 122.56 Å2 (22.3%) |
| Germline | |||
| Contact | GTHF L | ||
| H1 | |||
| IMTG | 26GYTFTDYS33 | Class 1 | 94.26 Å2 (17.2%) |
| Germline | |||
| Contact | TDYS | ||
| H2 | |||
| IMTG | 51INTETGEP58 | Class 2 | 42.24 Å2 (7.5%) |
| Germline | |||
| Contact | NTE | ||
| H3 | |||
| IMTG | 97ARATTATELAY107 | 135.1 Å2 (24.6%) | |
| Germline | |||
| Contact | ATTATE | ||
| FR2 | |||
| Contact | H35 W47 W50 | 105.5 Å2 (19.1%) | |
| FR3 | |||
| Contact | T59 | 4.86 Å2 (1%) |
The CDR regions are determined according to IMGT rules. Residues that contact arginine7P are bolded. The residues that contact citrulline2P are underlined. FR, framework region.
Conformation of the bound citrullinated collagen peptide
The Fo-Fc maps revealed a clear density for bound peptide and allowed us to build 9 out of 18 residues unambiguously (A5-Cit6-G7-L8-T9-G10-R11-HyP12-G13; Fig. 4 b). The ordered part of the peptide corresponds to the C1 (359–367) epitope on CII. The second Fab molecule is relatively more disordered, and therefore only seven residues of bound peptide (Cit6-G7-L8-T9-G10-R11-HyP12-) are ordered; however, the conformation of the peptide is virtually the same as the for the one in the other molecule. The overall conformation of both pCII-Cit1 peptides is similar to a β hairpin with a type II β turn on the tip formed by Leu8P and Thr9P. The conformation of the peptide is stabilized by a network of intra- and interchain hydrogen bonds together with polar, apolar, and hydrophobic interactions. This conformation provides an overall cyclic shape to the peptides.
There are two NH…O=C hydrogen bonds between N(Gly10P)…O(Gly7P) and N(Gly7P)…O(Arg11P). An acceptor water molecule connects donor amide nitrogen of Thr9P, Arg11P, and the side chain of Thr9P by hydrogen bonds, which increases the stability of the conformation (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081862/DC1). Although the second peptide complex in the asymmetrical unit is more disordered, electron density of this water is present at lower σ levels. The plane of the hairpin is perpendicular to plane of the paratope cavity; therefore, only the lower edge and the tip of the hairpin plane are interacting with the Fab, whereas the upper edge is mostly solvent exposed. Arg11P, HyP12P, and Gly13P at the C terminus are twisted away from the β structure because a rotation of the Cα-C′ bond of Gly10P causes the carbonyl oxygen of Gly10P to be directed to the exterior rather than to the interior part (Fig. 4 c).
Fab–peptide interactions
The pCII-Cit1 peptide fills a groove in the antibody-combining site, which is surrounded by the CDR loops. The C-terminal half of the peptide has more extensive contacts with the Fab because this half is buried into the Fab, whereas the N-terminal half is partially exposed to the solvent and has fewer contacts with the Fab. The buried accessible surface areas (ΔBSAs) on the Fab and the peptide are 549.1 and 720.4 Å2, respectively. The ΔBSA on the Fab is formed mainly by the heavy chain (69.3%) involving CDRH1 (17.2%), CDRH2 (7.5%), FRH2 (19.1%), FRH3 (1%), and CDRH3 (24.6%), and the light chain (30.7%) involving CDRL1 (8.1%) and CDRL3 (22.3%; calculated using PISA software at EMBL-EBI) (27). The CDRL2 loop did not have any contact with the peptide. Although Ala5P has clear electron density, this residue does not form any contacts with Fab. The remaining eight visible residues of the peptide have contacts with CDR loops and framework residues (Table V).
Citrulline (Cit6P) is the second residue in the visible part of the pCII-Cit1 peptide. Although the N-terminal side of the peptide hairpin has only a limited number of interactions with the Fab, the citrulline side chain is involved in the most of these interactions (Fig. 4 d). Citrulline interacts with CDRH1, CDRH2, and FRH2. Thr30H, Asp31H, Tyr32H, and Ser33H from CDRH1 contact with citrulline with their main-chain atoms. These interactions are mainly Van der Waals interactions. The backbone carbonyl oxygen of Thr30H also forms a hydrogen bond with citrulline (N8). Citrulline is further stabilized by hydrogen bonds with the side chain of the residue Thr53H (N and OG1; Fig. 4 d). The hydrophobic moiety of the citrulline side chain interacts with Trp50H, which is a framework (FRH2) residue.
The tip of the peptide (Leu8P and Thr9P) has hydrophobic contacts with CDRL3 (Gly96L, Phe99L, and Leu101L) and with the heavy-chain second framework residues Trp47H and Trp50H. Another framework residue, Thr59H (FRH3), contacts with Leu8P via a bridging water molecule in the interface. Thr9P forms a hydrogen bond with Glu104H. The arginine (Arg11P) is located in the middle of the C-terminal half of the peptide and provides the most extensive interactions by making contacts with 11 different residues of the Fab (Fig. 4 d). The negatively charged residues from the light chain, Asp31L and Asp33L, are in close proximity to Arg11P, but the distance is too large to form a salt bridge. Although there is not a proper salt bridge, Arg11P contacts with Glu104H (CDRH3) and has cation–π interactions with Tyr37L (CDRL1). Arg11P forms a single hydrogen bond with the carbonyl oxygen of Gly96L. The CDRH3 loop contacts Arg11P with all of its residues. Hydroxyproline (Hyp12P) is involved in Van der Waals interactions with Ala99H, Thr100H, and Thr101 (CDRH3). Gly13P has contacts with Tyr32H and Asp31H.
The comparison of the peptide bound and unliganded Fab structures reveals that no significant conformational changes are required for binding. The overall root mean square deviation for the variable domains of the two structures is only 0.47 Å. There are only minor differences in the side-chain orientations of residues, which are close to the contact interface. Therefore, the binding of peptide follows the key-lock mechanism that requires a structurally stable antibody-combining site.
DISCUSSION
Antibody formation against CII is not only a common autoimmune phenomenon in RA but may also have pathogenic consequences. We now show that CII is citrullinated in the joints and that an antibody response to such epitopes could be a pathogenic link to CII autoimmunity. The present study provides the first molecular insights into how antibodies recognize a citrullinated epitope and the functional consequence of such an interaction. Mouse B cell hybridomas with specificities for citrullinated variants of the immunodominant CII epitope were established, and the respective mAbs were used to demonstrate their arthritogenicity in transfer experiments to naive mice as well as for the detection of citrullinated CII in human joints. Our systematic analysis of the pathogenicity of these mAbs is also the first study in the area of APCA research that provides a detailed characterization of the antibody fine specificities, including their relevant cross-reactivities. Because the availability of the latter information is critical for interpretation of the results of antibody transfer experiments, our study not only provides experimental evidence for the arthritogenicity of autoantibodies to citrullinated CII but also evidence for such a role of ACPAs in general. Controlled experiments have so far not been able to show a specific response to citrullinated proteins in several investigated arthritis models, including spontaneous arthritis in lupus mice, streptococcal cell wall–induced arthritis, pristane-induced arthritis in rats, and in some reports of CIA in DBA/1 mice (5, 28). There are some notable exceptions of mouse experimental arthritides: one study that uses bovine CII in CFA for induction of CIA (29), and another study of genetically engineered DBA/1 mice expressing human MHC class II molecules in the joints (30). Recently, another study showed spreading of antibody responses to citrullinated proteins in CIA in the DBA/1 mice (31).
It also has been previously shown that the injection of monoclonal IgM and IgG antibodies reactive with citrullinated fibrinogen enhance the development of CAIA (29). These antibodies were not cross-reactive with native CII, but it was not investigated if cross-reactivity to CII α chains or to citrullinated CII could account for their arthritogenic effect.
In the present study, the crystal structure analysis elucidates important new information on the specificity of the interaction between a mAb-derived Fab fragment and a citrullinated arginine in the conserved motif of a major CII epitope. Although the pCII-Cit1 peptide contains, besides the citrulline residue, flanking sites that form the entire citrullinated B cell epitope (GACitGLTGRPGDA), the interpretable electron density only covers a shorter stretch (ACitGLTGRP) that extends in length to the previously described noncitrullinated CII epitope C1 (24). The analysis of the crystal further uncovers that the citrulline is buried within the antibody-combining sites and is recognized via interactions with the CDR1 and CDR2 regions of the VH chain, whereas the collagen strand forms a distinct stable β hairpin with a type II β turn conformation. Thus, the bound CII peptide considerably differs from the deposited characteristic structures of triple helical collagen peptides in the database.
Adoption of a β hairpin is energetically more stable, and is therefore expected to be the preferred conformation of peptides in solution. This energetically favored peptide conformation is also likely to contribute to the predominance of β turns compared with other possible structures in peptide–antibody complexes (32, 33). Accordingly, the adoption of a β turn by the pCII-Cit1 peptide in complex with the ACC4 Fab might follow a more general principle. However, the elucidation of the pCII-Cit1 conformation in the ACC4 complex might provide a clue to a better understanding underlying the pathophysiologically relevant functional aspect of cross-reactive autoantibody recognition. Thus, we previously described human IgG antibodies in RA sera that exhibit cross-reactivities between the citrullinated C1 epitope and citrullinated cyclized peptides (25). Accordingly, we also detected a weak but significant cross-reactivity of the mouse ACC4 mAb with the cyclic citrullinated filaggrin peptide CCP1. It is therefore conceivable that the β turn adopted by the pCII-Cit1 peptide provides a cyclic shape that is similar to that recognized by the ACC4 mAb in the filaggrin peptide. Moreover, such β turn motifs are frequently encountered within the filaggrin sequences (34). However, filaggrin is not expressed in the joints, so autoantibody cross-reactivity to filaggrin is unlikely to be relevant for joint pathology. On the other hand, type II β turn motifs are quite different from the native structure of CII, a putative candidate cartilage–specific autoantigen that has a characteristic structure of an extended helical coil wrapped in a triple helical conformation (35–39).
This structural difference implies that those ACC mAbs capable of recognizing citrulline in the context of a triple helical conformation may differ from ACC4 with regard to citrulline side chain recognition. Alternatively, citrullination of the collagen triple helix could provoke a weakening of its rigid structure that eventually allows the citrulline residue to protrude into the CDR regions of the antibody. Thus, the partial unfolding or denaturation by citrullination of particular arginine residues could represent a mechanism causing conformational changes of distinct domains of the triple helix into β turns. In accordance, it has already been reported for noncollagenous proteins that citrullination can destabilize their tertiary structure and enhance their vulnerability to proteolytic digestion upon unfolding (34). Thus, an analogous partial unfolding of the triple helix upon PAD-induced deimination of certain arginine residues remains an intriguing possibility to trigger arthritogenic CII autoimmunity by the introduction of primary as well as tertiary structural changes into the tolerated self-protein. In turn, activation of adaptive and innate immune responses in the joints causes inflammation and proteolytic cartilage destruction with the subsequent liberation of native as well as denatured CII fragments into the synovium and synovial fluid. Subsequently, these peptides may get enzymatically deiminated by PADs, particularly PAD4. In agreement with such a scenario, we could not only demonstrate the occurrence of citrullinated CII in mouse cartilage and inflamed synovial tissue by immunohistology but also in synovial fluid specimens from RA patients. However, detection of these modified CII fragments also occurs in OA, thereby emphasizing that it is the immune response to the citrullinated peptide that is unique for RA rather than the deimination of joint proteins, which is in support of previous findings (40).
Citrulline modification within the structural frame of a relatively stable conformation such as a β turn or a triple helix may help to activate B cells. Importantly, activation of B cells that finally lead to the generation of highly specific IgG antibodies depends on appropriate T cell help. This requirement also applies to the induction of arthritogenic responses by CII-specific B cells that receive activating signals from CII-specific T cells recognizing an immunodominant determinant on CII that differs from the respective B cell epitopes (22, 41, 42). In both mice with the Aq MHC class II molecules or in humans with RA-associated shared-epitope MHC class II variants, this critical CII determinant has been localized to the domain 261–271 that harbors a galactosylated lysine side chain that is critical for T cell receptor recognition (20, 43). It is highly likely that the same type of T cell specificity can also trigger B cell responses to the presently studied citrullinated CII epitopes because they are found on the same protein backbone as the immunodominant C1 epitope on CII.
Humoral immune response to citrullinated proteins precedes the development of RA and is genetically associated with MHC class II alleles that confer increased risk for disease onset and severity (44, 45). In this context, our results suggest that the autoantibody responses to citrullinated proteins are likely to play a critical pathogenic role beyond their unequivocal value as early diagnostic markers. Their involvement in cross-reactive hapten-carrier mechanisms may lead to the activation of pathogenic B and T cells reactive with native and posttranslationally modified CII, thereby initiating an amplifying vicious circle of inflammatory responses in the joints. Furthermore, it is of interest to note that anticitrulline antibodies can also play an important role in inducing antibody-mediated arthritic relapses that could be of potential importance for future therapeutic intervention during the chronic phase of arthritis in both animals and humans.
To summarize, we have demonstrated the molecular structure of the specific interactions between an antibody and a citrullinated antigen and have shown that this interaction will have pathogenic consequences in vivo. We believe this will be useful for understanding the functional role of the antibody response to citrullinated proteins in RA and points toward a specific link to CII as one of the relevant self-antigens involved.
MATERIALS AND METHODS
Animals.
All mice used in this study were bred in the animal facilities of the Section for Medical Inflammation Research at Lund University, but the original founders for the B10.Q/rhd and B10.RIII/rhd mice were from University of Tübingen, and the original founders for the BALB/cJ mice were from the Jackson Laboratory. All animals were treated according to the Swedish guidelines for humane treatment of laboratory animals, and the experiments were approved by the Lund-Malmö laboratory animal ethical committee.
Peptides and proteins.
The following linear and triple helical peptides were synthesized using methods previously described in detail (23, 46). The linear peptide pCII-Arg contains the C1 epitope of mouse CII (GP-HyP-GARGLTGR-HyP-GDAGP-HyP), the peptide pCII-Cit1 has a citrulline at position 360 (GP-HyP-GACitGLTGR-HyP-GDAGP-HyP), peptide pCII-Cit2 has a citrulline at position 365 (GP-HyP-GARGLTGCit-HyP-GDAGP-HyP), and peptide pCII-Cit1+2 has citrulline residues at both position 360 and 365 (epitopes are denoted in bold). The triple helical peptide THPCII-Arg contains the C1 epitope of mouse CII in its triple helical form (GP-HYP-GP-HYP-GP-HYP-GP-HYP-GP-HYP-GARGLTGR-HyP-GDAGP-HyP-GP-HYP-G-ϵACA) (3), and the citrullinated version of the peptide, THPCII-Cit, has citrullines at both position 360 and 365. A citrullinated cyclic (cyc) filaggrin peptide (47), cyc-Cit (HQCHQEST(cit)GRSRGRCGRSGS; cit, citrulline; C, cysteines forming the disulfide bond upon oxidative cyclization), and a respective noncitrullinated peptide analogue, cyc-Arg (HQCHQESTRGRSRGRCGRSGS) (47), were obtained as commercially synthesized reagents (mass spectrometrically confirmed peptide identity and fast protein liquid chromatography–controlled purity >98%; Herman GbR Synthetische Biomoleküle). Fibrinogen and citrullinated fibrinogen were isolated as previously described in detail (48).
Generation of mAbs and induction of CAIA.
The CII-specific hybridomas have been previously generated and characterized (25, 46). The ACC1 hybridoma was established by immunizing and boosting a Cia5 congenic B10.RIII mouse (47) at the base of the tail with 100 or 50 µg of bovine CII in CFA or IFA (Difco), respectively. The other ACC hybridomas were produced using PAD4-treated triple helical CII peptides containing T and B cell epitopes for immunization and boosting of DBA/1 or (B10.Q × DBA/1) F1 mice. The GB8 and ACC5 clones originated from B10.Q mice, which had chronic arthritis after CII/CFA immunization. Specificity selection was done using native or denatured CII, CII peptides, and single and triple helical citrullinated or native C1 epitopes. mAbs were produced and purified as previously described (48). CAIA was induced in naive mice using a standard procedure (48), and antibody-induced relapses were induced as previously described (49).
ELISA.
For the characterization of the mAbs, either antigen-coated maxisorp immuno plates (Thermo Fisher Scientific) or biotinylated antigens linked to the plates via 2 µg/ml NeutrAvidin (PerkinElmer) were used. The direct coating of 10 µg/ml of antigens in PBS was used for the following polypeptides: heat-denatured (60°C for 15 min) CII (dCII-Arg), PAD4-treated CII (dCII-Cit), and native triple helical CII (nCII-Arg). The antigens cyc-Cit (citrullinated cyclic fibritin peptide), cyc-Arg (cyclic fibritin peptide), THPCII-Cit, and THPCII-Arg at 4 µg/ml, and Fib-Arg and Fib-Cit at 0.5 µg/ml were used for coating in PBS. All plates were blocked using 2% BSA (Sigma-Aldrich). After washing in Tris-based ELISA buffer, serial dilutions of the mAbs starting at 1,000 ng/ml were added and incubated for 2 h at room temperature. The κ light chain–specific mAb 187.1-biotin or goat anti–mouse IgM–biotin and SA-Eu+3 (PerkinElmer) were used for detection. The indirect coating via NeutrAvidin was used for the following antigens: biotinylated pCII-Arg, biotinylated pCII-Cit1, biotinylated pCII-Cit2, and biotinylated pCII-Cit1+2 at a concentration of 4 µg/ml in PBS. Plates were blocked and washed before serial dilutions of antibodies were added. Horseradish peroxidase–conjugated goat anti–mouse–IgG (Jackson ImmunoResearch Laboratories) or goat anti–mouse–IgM (SouthernBiotech) antibodies and ABTS tablets (Roche) were used for detection.
Synovial fluid specimens and patients.
The synovial fluid specimens were obtained from patients in the outpatient clinic of the Department of Internal Medicine III and the Department of Orthopedic Surgery of the Friedrich-Alexander-University of Erlangen-Nuremberg on the occasion of diagnostic and/or therapeutic knee joint punctures. The diagnosis of RA, OA, or Reac A was made by an experienced board-certified rheumatologist, thereby also ensuring that the respective existing classification criteria of the American College of Rheumatology (for RA and OA of the knee) were met. Written informed consent was obtained from all patients included in the study that had earlier been approved by the Ethical Committee of the Medical Faculty of the Friedrich-Alexander-University of Erlangen-Nuremberg.
Immunoprecipitation of citrullinated CII from synovial fluid.
Immunoprecipitation was performed using earlier characterized mouse IgG mAbs specific for distinct triple helical CII epitopes (CIIE10, M2139, and CIID3) (24) and a protein G immunoprecipitation kit (Seize X; Thermo Fisher Scientific). 500 µg IgG (166 µg of each mouse mAb) was covalently cross-linked to 400 µl of protein G slurry according to the manufacturer's instructions. 40 µl anti-CII–IgG cross-linked beads were added to 4 ml of RA synovial fluid (diluted 1:1 in binding buffer) and incubated overnight at 4°C. Upon centrifugation, the immunoprecipitated CII was eluted from the protein G slurry and 20 µl of each fraction was applied to an SDS gel for Western blot analysis.
Citrullinated CII was detected using the modified citrulline Western blot detection kit (Millipore). Alternatively, in-gel Western blot detection of immunoprecipitated citrullinated CII was performed using the citrulline-specific biotinylated ACC2 antibody and avidin-peroxidase (Sigma). For visualization of the results, an ECL plus detection kit (Millipore) was applied.
Capture ELISA for citrullinated CII in synovial fluid.
ELISA plates were coated with the CII-specific antibodies CIIE10, M2139, and CIID3 in PBS (2 µg/ml each). Subsequently, 100-µl specimens of hyaluronidase-pretreated synovial fluid were incubated for 90 min at room temperature. Incubation with BSA served as a negative control. Upon thorough washings, 0.3 µg/ml of the biotinylated ACC2 was added for 90 min at room temperature. Development was performed using avidin-peroxidase at a dilution of 1:750 in 1% BSA/PBS and ABTS as substrate. All assays were run in triplicates.
Immunohistochemistry and histopathology.
Paws from naive 4-mo-old male BALB/c or (BALB/c × B10.Q) F1 mice injected with 9 mg of arthritogenic anti-CII mAbs (M2139 and CIIC1) on day 11 were decalcified for 4 wk in an EDTA solution and frozen in optimum cutting temperature compound using isopentane on dry ice. The samples were stored at −70°C until cryosectioned at 10 µm at −30°C. Joint sections were stained with a panel of biotinylated anti-CII citrulline-specific mAbs (ACC1–4). Streptavidin peroxidase was used for detection. Diaminobenzidine staining was performed according to established procedures.
Statistics.
Statistical difference in the frequency (incidence) of disease between groups of mice was determined using a χ2 analysis on all scoring days. To compare the mean arthritis score between two experimental groups, the nonparametric Mann-Whitney U test was used. All of the statistical analyses were performed using the StatView program (version 1; SAS Institute), and P < 0.05 was considered significant.
Preparation of ACC4 Fab.
ACC4 Fab fragments were prepared by using an ImmunoPure Fab Preparation Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. In brief, 20 mg of ACC4 antibody was concentrated and diluted with 20 mM sodium phosphate and 10 mM EDTA buffer (pH 7) several times by using centrifugal filter devices (Amicon Ultra; Millipore). 20 mg of concentrated IgG was mixed with 0.5 ml of immobilized papain. The mixture was incubated by shaking overnight at 37°C. Crude digest was separated from immobilized papain and applied to a protein A column (AffinityPak; Thermo Fisher Scientific). Fab fragments were recovered in the flow through. Fc fragments and undigested IgG bound to the column were eluted with elution buffer. Collected Fab fragments were concentrated and further gel filtered on a Superdex 200 (10/30) column (GE Healthcare). The gel filtration column was previously equilibrated with 30 mM of Tris buffer (pH 8).
Antibody V gene sequence analysis.
The cDNA sequences of the heavy and light chains of anticollagen- and citrulline-specific antibodies were sequenced (see Supplemental materials and methods, available at http://www.jem.org/cgi/content/full/jem.20081862/DC1) and analyzed by IMGT/V-QUEST (50) and IMGT/Junction Analysis (51) from the IMGT database (52), and IgBLAST from the National Center for Biotechnology Information (available from GenBank/EMBL/DDBJ under accession nos. EU159566, EU159567, EU159568, EU159569, EU159570, EU159571, EU159572, EU159573, EU159574, EU159575, EU159576, and EU159577; Fig. S1).
Crystallization.
10 mg/ml ACC4 Fab in 30 mM of Tris buffer (pH 8) with and without the pCII-Cit1 peptide was crystallized using the hanging drop method using a Peg/Ion screen (Hampton Research). For Fab–peptide complex crystallization, the collagen peptide and Fab were mixed at a 5:1 molar ratio and incubated at 4°C overnight. Crystallization drops containing 2 µl of mother liquor and 2 µl of protein solution were incubated at 25°C. Crystals of the Fab–peptide complex appeared only in two conditions (#31 and #36), whereas crystals of the Fab alone appeared in several conditions. Promising conditions were optimized by decreasing the salt concentration and by adding glycerol. A crystal of the Fab–peptide complex grown in 0.15 M ammonium sulfate, 20% PEG 3350, and 10% glycerol was used for data collection, whereas for determining Fab structure, a crystal grown in 0.15 M ammonium nitrate and 25% PEG 3350 was used for data collection.
Data collection.
Datasets were collected at the I911-2 and I911-5 beam lines at MAX-lab. Before data collection, the crystals were soaked in a cryoprotecting solution of 20% glycerol (vol/vol) and mother liquor before exposure to synchrotron radiation. The crystals were flash cooled and data were collected at 100K. The noncomplex crystals diffracted to 1.5 Å and crystals of the complex diffracted to 2.1 Å. Both datasets were processed by XDS (53), and further data manipulation was performed using programs from the CCP4 suite (54). Crystal parameters and statistics from the data collection are shown in Table IV.
Structure determination and refinement.
The structures of the complex and noncomplex forms of the antibody were solved by molecular replacement using PHASER (55). The structure of anticollagen CIIC1 Fab (56) was used as a searching model. The model was split into variable and constant domains, and searching and fixing of these domains separately gave a single solution with an Rfree of 46%. For solving the ACC4 Fab–peptide complex structure, the ACC4 Fab structure was used intact as a search model. A single solution with an Rfree of 45% was obtained. Mismatching residues from the search model were changed to ACC4 residues, and missing loops were built by using the program COOT (57). Further refinement was performed by REFMAC (58) in the CCP4 suite (54). Restrained and TLS (59) refinement helped to decrease the R factor and Rfree to respectable values. For the refinement of the Fab–peptide complex structure, noncrystallographic symmetry restraints were applied. Two sulfate ions were identified in the model for the complex and one nitrate ion was added to the noncomplex Fab model. For building, the ligand Fo-Fc maps were calculated and the peptide was built into the positive density. In the final stage, water molecules were added to the models. Data and refinement statistics are shown in Table IV. The coordinates of the structures are available from the Protein Data Bank under accession nos. 2w60 and 2w65.
Online supplemental material.
Fig. S1 shows the amino acid sequences of antibody CDR regions, corresponding germline genes, and mouse strains that antibodies originated. Fig. S2 shows an amino acid sequence comparison of the CDR regions of the sequenced antibodies. Fig. S3 contains a stereoscopic view of the pCII-Cit1 peptide. Table S1 shows the somatic mutation statistics in light and heavy chains of antibodies. Supplemental materials and methods describes the DNA sequencing procedures. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081862/DC1.
Acknowledgments
We are grateful to C. Palestro for technical help.
This work was supported by the Swedish Research Council, the Strategic Science Foundation, the Research School in Pharmaceutical Sciences at Lund University, the Crafoord Foundation, the Kock Foundation, the Österlunds Foundation, the Alex and Eva Wallström Foundation, the European Union Grants Autocure (grant LSHB-2006-018661), MUGEN (grant LSHG-CT-2005–005203), and the Doktor Robert Pfleger Foundation (grant to H. Burkhardt).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ACPA, antibodies against citrullinated protein; CAIA, collagen antibody–induced arthritis; CDR, complementary determining region; CIA, collagen-induced arthritis; CII, collagen type II; ΔBSA, buried accessible surface area; OA, osteoarthritis; PAD, peptidyl arginine deiminase; RA, rheumatoid arthritis; Reac A, reactive arthritis; RF, rheumatoid factor; RLU, relative luminescence unit.
References
- Aho K., Palosuo T., Raunio V., Puska P., Aromaa A., Salonen J.T. 1995. When does rheumatoid disease start? Arthritis Rheum. 28:485–489 [DOI] [PubMed] [Google Scholar]
- Schellekens G.A., de Jong B.A., van den Hoogen F.H., van de Putte L.B., van Venrooij W.J. 1998. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies.J. Clin. Invest. 101:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbal-Neuhauser E., Durieux J.J., Arnaud M., Dalbon P., Sebbag M., Vincent G., Simon M., Senshu T., Masson-Bessière C., Jolivet-Reynaud C., et al. 1999. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues.J. Immunol. 162:585–594 [PubMed] [Google Scholar]
- Vossenaar E.R., Zendman A.J., van Venrooij W.J., Pruijn G.J. 2003. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease.Bioessays. 25:1106–1118 [DOI] [PubMed] [Google Scholar]
- Vossenaar E.R., Nijenhuis S., Helsen M.M., van der Heijden A., Senshu T., van den Berg W.B., van Venrooij W.J., Joosten L.A. 2003. Citrullination of synovial proteins in murine models of rheumatoid arthritis.Arthritis Rheum. 48:2489–2500 [DOI] [PubMed] [Google Scholar]
- Chang X., Yamada R., Suzuki A., Kochi Y., Sawada T., Yamamoto K. 2005. Citrullination of fibronectin in rheumatoid arthritis synovial tissue.Rheumatology (Oxford). 44:1374–1382 [DOI] [PubMed] [Google Scholar]
- Vossenaar E.R., Radstake T.R., van der Heijden A., van Mansum M.A., Dieteren C., de Rooij D.J., Barrera P., Zendman A.J., van Venrooij W.J. 2004. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages.Ann. Rheum. Dis. 63:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten D., Peene I., Union A., Meheus L., Sebbag M., Serre G., Veys E.M., De Keyser F. 2001. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies.Arthritis Rheum. 44:2255–2262 [DOI] [PubMed] [Google Scholar]
- Foulquier C., Sebbag M., Clavel C., Chapuy-Regaud S., Al Badine R., Méchin M.C., Vincent C., Nachat R., Yamada M., Takahara H., et al. 2007. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation.Arthritis Rheum. 56:3541–3553 [DOI] [PubMed] [Google Scholar]
- Schwab B.L., Guerini D., Didszun C., Bano D., Ferrando-May E., Fava E., Tam J., Xu D., Xanthoudakis S., Nicholson D.W., et al. 2002. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis.Cell Death Differ. 9:818–831 [DOI] [PubMed] [Google Scholar]
- Masson-Bessière C., Sebbag M., Durieux J.J., Nogueira L., Vincent C., Girbal-Neuhauser E., Durroux R., Cantagrel A., Serre G. 2000. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum.Clin. Exp. Immunol. 119:544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi D., Anouk M., Golan I., Paran D., Kaufman I., Wigler I., Levartovsky D., Litinsky I., Elkayam O. 2006. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis.Arthritis Rheum. 55:53–56 [DOI] [PubMed] [Google Scholar]
- Rantapää-Dahlqvist S., de Jong B.A., Berglin E., Hallmans G., Wadell G., Stenlund H., Sundin U., van Venrooij W.J. 2003. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis.Arthritis Rheum. 48:2741–2749 [DOI] [PubMed] [Google Scholar]
- Reparon-Schuijt C.C., van Esch W.J., van Kooten C., Schellekens G.A., de Jong B.A., van Venrooij W.J., Breedveld F.C., Verweij C.L. 2001. Secretion of anti-citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis.Arthritis Rheum. 44:41–47 [DOI] [PubMed] [Google Scholar]
- Masson-Bessière C., Sebbag M., Girbal-Neuhauser E., Nogueira L., Vincent C., Senshu T., Serre G. 2001. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin.J. Immunol. 166:4177–4184 [DOI] [PubMed] [Google Scholar]
- Hill J.A., Bell D.A., Brintnell W., Yue D., Wehrli B., Jevnikar A.M., Lee D.M., Hueber W., Robinson W.H., Cairns E. 2008. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice.J. Exp. Med. 205:967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy-Regaud S., Sebbag M., Baeten D., Clavel C., Foulquier C., De Keyser F., Serre G. 2005. Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides.J. Immunol. 174:5057–5064 [DOI] [PubMed] [Google Scholar]
- Hill J.A., Southwood S., Sette A., Jevnikar A.M., Bell D.A., Cairns E. 2003. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule.J. Immunol. 171:538–541 [DOI] [PubMed] [Google Scholar]
- Molberg O., McAdam S.N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P., et al. 1998. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease.Nat. Med. 4:713–717 [DOI] [PubMed] [Google Scholar]
- Bäcklund J., Carlsen S., Höger T., Holm B., Fugger L., Kihlberg J., Burkhardt H., Holmdahl R. 2002. Predominant selection of T cells specific for glycosylated collagen type II peptide (263-270) in humanized transgenic mice and in rheumatoid arthritis.Proc. Natl. Acad. Sci. USA. 99:9960–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsberg U., Gustafsson K., Jansson L., Michaëlsson E., Ahrlund-Richter L., Pettersson S., Mattsson R., Holmdahl R. 1994. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis.Eur. J. Immunol. 24:1698–1702 [DOI] [PubMed] [Google Scholar]
- Michaëlsson E., Andersson M., Engström A., Holmdahl R. 1992. Identification of an immunodominant type-II collagen peptide recognized by T cells in H-2q mice: self tolerance at the level of determinant selection.Eur. J. Immunol. 22:1819–1825 [DOI] [PubMed] [Google Scholar]
- Burkhardt H., Koller T., Engström A., Nandakumar K.S., Turnay J., Kraetsch H.G., Kalden J.R., Holmdahl R. 2002. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse.Arthritis Rheum. 46:2339–2348 [DOI] [PubMed] [Google Scholar]
- Schulte S., Unger C., Mo J.A., Wendler O., Bauer E., Frischholz S., von der Mark K., Kalden J.R., Holmdahl R., Burkhardt H. 1998. Arthritis-related B cell epitopes in collagen II are conformation-dependent and sterically privileged in accessible sites of cartilage collagen fibrils.J. Biol. Chem. 273:1551–1561 [DOI] [PubMed] [Google Scholar]
- Burkhardt H., Sehnert B., Bockermann R., Engström A., Kalden J.R., Holmdahl R. 2005. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis.Eur. J. Immunol. 35:1643–1652 [DOI] [PubMed] [Google Scholar]
- Stanfield R.L., Zemla A., Wilson I.A., Rupp B. 2006. Antibody elbow angles are influenced by their light chain class.J. Mol. Biol. 357:1566–1574 [DOI] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. 2005. Detection of protein assemblies in crystals. Computational Life Sciences. First International Symposium, CompLife 2005, Konstanz, Germany, September 25-27, 2005, Proceedings. Berthold M.R., Glen R., Diederichs K., Kohlbacher O., Fischer I., Springer, Berlin/Heidelberg, Germany: 163–174 [Google Scholar]
- Hoffmann M.H., Tuncel J., Skriner K., Tohidast-Akrad M., Türk B., Pinol-Roma S., Serre G., Schett G., Smolen J.S., Holmdahl R., Steiner G. 2007. The rheumatoid arthritis-associated autoantigen hnRNP-A2 (RA33) is a major stimulator of autoimmunity in rats with pristane-induced arthritis.J. Immunol. 179:7568–7576 [DOI] [PubMed] [Google Scholar]
- Kuhn K.A., Kulik L., Tomooka B., Braschler K.J., Arend W.P., Robinson W.H., Holers V.M. 2006. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis.J. Clin. Invest. 116:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S., Ota S., Sekine C., Tada T., Otsuka T., Okamoto T., Sønderstrup G., Peterlin B.M. 2006. Aberrant MHC class II expression in mouse joints leads to arthritis with extraarticular manifestations similar to rheumatoid arthritis.Proc. Natl. Acad. Sci. USA. 103:14465–14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd B.A., Ho P.P., Sharpe O., Zhao X., Tomooka B.H., Kanter J.L., Steinman L., Robinson W.H. 2008. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination.Arthritis Res. Ther. 10:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson H.J., Wright P.E. 1995. Antigenic peptides.FASEB J. 9:37–42 [DOI] [PubMed] [Google Scholar]
- Nair D.T., Singh K., Siddiqui Z., Nayak B.P., Rao K.V., Salunke D.M. 2002. Epitope recognition by diverse antibodies suggests conformational convergence in an antibody response.J. Immunol. 168:2371–2382 [DOI] [PubMed] [Google Scholar]
- Tarcsa E., Marekov L.N., Mei G., Melino G., Lee S.C., Steinert P.M. 1996. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin.J. Biol. Chem. 271:30709–30716 [DOI] [PubMed] [Google Scholar]
- Bella J., Eaton M., Brodsky B., Berman H.M. 1994. Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution.Science. 266:75–81 [DOI] [PubMed] [Google Scholar]
- Emsley J., Knight C.G., Farndale R.W., Barnes M.J., Liddington R.C. 2000. Structural basis of collagen recognition by integrin alpha2beta1.Cell. 101:47–56 [DOI] [PubMed] [Google Scholar]
- Emsley J., Knight C.G., Farndale R.W., Barnes M.J. 2004. Structure of the integrin alpha2beta1-binding collagen peptide.J. Mol. Biol. 335:1019–1028 [DOI] [PubMed] [Google Scholar]
- Stetefeld J., Frank S., Jenny M., Schulthess T., Kammerer R.A., Boudko S., Landwehr R., Okuyama K., Engel J. 2003. Collagen stabilization at atomic level: crystal structure of designed (GlyProPro)10foldon.Structure. 11:339–346 [DOI] [PubMed] [Google Scholar]
- Zong Y., Xu Y., Liang X., Keene D.R., Höök A., Gurusiddappa S., Höök M., Narayana S.V. 2005. A ‘Collagen Hug’ model for Staphylococcus aureus CNA binding to collagen.EMBO J. 24:4224–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar E.R., Smeets T.J., Kraan M.C., Raats J.M., van Venrooij W.J., Tak P.P. 2004. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue.Arthritis Rheum. 50:3485–3494 [DOI] [PubMed] [Google Scholar]
- Rosloniec E.F., Brand D.D., Myers L.K., Whittington K.B., Gumanovskaya M., Zaller D.M., Woods A., Altmann D.M., Stuart J.M., Kang A.H. 1997. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen.J. Exp. Med. 185:1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson E.C., Hansen B.E., Jacobsen H., Madsen L.S., Andersen C.B., Engberg J., Rothbard J.B., McDevitt G.S., Malmström V., Holmdahl R., et al. 1998. Definition of MHC and T cell receptor contacts in the HLA-DR4 restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice.Proc. Natl. Acad. Sci. USA. 95:7574–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Delwig A., Altmann D.M., Isaacs J.D., Harding C.V., Holmdahl R., McKie N., Robinson J.H. 2006. The impact of glycosylation on HLA-DR1-restricted T cell recognition of type II collagen in a mouse model.Arthritis Rheum. 54:482–491 [DOI] [PubMed] [Google Scholar]
- van der Helm-van Mil A.H., Verpoort K.N., le Cessie S., Huizinga T.W., de Vries R.R., Toes R.E. 2006. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis.Arthritis Rheum. 54:1117–1121 [DOI] [PubMed] [Google Scholar]
- Auger I., Sebbag M., Vincent C., Balandraud N., Guis S., Nogueira L., Svensson B., Cantagrel A., Serre G., Roudier J. 2005. Influence of HLA-DR genes on the production of rheumatoid arthritis-specific autoantibodies to citrullinated fibrinogen.Arthritis Rheum. 52:3424–3432 [DOI] [PubMed] [Google Scholar]
- Grab B., Miles A.J., Furcht L.T., Fields G.B. 1996. Promotion of fibroblast adhesion by triple-helical peptide models of type I collagen-derived sequences.J. Biol. Chem. 271:12234–12240 [DOI] [PubMed] [Google Scholar]
- Schellekens G.A., Visser H., de Jong B.A., van den Hoogen F.H., Hazes J.M., Breedveld F.C., van Venrooij W.J. 2000. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide.Arthritis Rheum. 43:155–163 [DOI] [PubMed] [Google Scholar]
- Vander Cruyssen B., Cantaert T., Nogueira L., Clavel C., De Rycke L., Dendoven A., Sebag M., Deforce D., Vincent C., Elewaut D., et al. 2006. Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays.Arthritis Res. Ther. 8:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar K.S., Johansson B., Björck L., Holmdahl R. 2007. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo.Arthritis Rheum. 56:3253–3260 [DOI] [PubMed] [Google Scholar]
- Giudicelli V., Chaume D., Lefranc M.P. 2004. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis.Nucleic Acids Res. 32:W435–W440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi Monod M., Giudicelli V., Chaume D., Lefranc M.P. 2004. IMGT/JunctionAnalysis: the first tool for the analysis of the immunoglobulin and T cell receptor complex V-J and V-D-J JUNCTIONs.Bioinformatics. 20:i379–i385 [DOI] [PubMed] [Google Scholar]
- Lefranc M.P., Giudicelli V., Ginestoux C., Bosc N., Folch G., Guiraudou D., Jabado-Michaloud J., Magris S., Scaviner D., Thouvenin V., et al. 2004. IMGT-ONTOLOGY for immunogenetics and immunoinformatics.In Silico Biol. 4:17–29 [PubMed] [Google Scholar]
- Kabsch W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants.J. Appl. Cryst. 26:795–800 [Google Scholar]
- Collaborative Computational Project, Number 4 1994. The CCP4 suite: programs for protein crystallography.Acta Crystallogr. D Biol. Crystallogr. 50:760–763 [DOI] [PubMed] [Google Scholar]
- Read R.J. 2001. Pushing the boundaries of molecular replacement with maximum likelihood.Acta Crystallogr. D Biol. Crystallogr. 57:1373–1382 [DOI] [PubMed] [Google Scholar]
- Uysal H., Sehnert B., Nandakumar K.S., Böiers U., Burkhardt H., Holmdahl R., Thunnissen M.M.G.M. 2008. The crystal structure of the pathogenic collagen type II specific mouse monoclonal antibody CIIC1 Fab: structure to function analysis.Mol. Immunol. 45:2196–2204 [DOI] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. 2004. Coot: model-building tools for molecular graphics.Acta Crystallogr. D Biol. Crystallogr. 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Vagin A.A., Dodson E.J. 1997. Refinement of macromolecular structures by the maximum-likelihood method.Acta Crystallogr. D Biol. Crystallogr. 53:240–255 [DOI] [PubMed] [Google Scholar]
- Winn M.D., Isupov M.N., Murshudov G.N. 2001. Use of TLS parameters to model anisotropic displacements in macromolecular refinement.Acta Crystallogr. D Biol. Crystallogr. 57:122–133 [DOI] [PubMed] [Google Scholar]
- DeLano W.L. 2002. Unraveling hot spots in binding interfaces: progress and challenges.Curr. Opin. Struct. Biol. 12:14–20 [DOI] [PubMed] [Google Scholar]