Abstract
Exercise training is known to induce several adaptations in the cardiovascular system, one of which is increased skeletal muscle blood flow at maximal exercise. Improved muscle blood flow, in turn, could in part be accounted for by augmented endothelium-dependent, nitric oxide (NO)-mediated vasodilation. Studies have indeed demonstrated that endothelium-dependent, NO-mediated dilation of conductance-type vessels is augmented after endurance exercise training; recently, this adaptation has been extended into resistance-type vessels within rodent skeletal muscle. With the latter, however, it appears that only resistance vessels supplying muscle active during training sessions exhibit this adaptation. These findings in rats are in contrast to those from human studies, in which increased endothelium-dependent dilation has been observed in vasculatures not associated with elevated blood flow during exercise. Increased expression of endothelial NO synthase (eNOS) appears to underlie enhanced endothelium-dependent, NO-mediated dilation of both conductance and resistance vessels. Greater eNOS expression may also underlie the preventive and (or) rehabilitative effect(s) of exercise training on atherosclerosis, given that NO inhibits several steps of the atherosclerotic disease process. Thus, exercise training may induce adaptations that benefit both vasodilation and vascular health.
Keywords: endothelium, conductance vessels, resistance vessels, nitric oxide synthase, exercise training, vasodilation, atherosclerosis
Introduction
The cardiovascular system exhibits numerous adaptations to chronic imposition of stress. One such chronic stress is endurance exercise training; that is, physical activity involving large muscles or muscle groups, which is performed at moderate intensity for prolonged sessions over a period of weeks to years. We use a rat model in our laboratory, with rats running on a motor-driven treadmill at 30 m/min up a 10% grade (*70% of exercise capacity) during 1 h daily training sessions, 5 d/week, over periods of 2–3 months. This exercise training program induces several well established adaptations, including increased skeletal muscle mitochondrial content and left ventricular hypertrophy (Delp et al. 1993, McAllister et al. 2005).
Increased maximal cardiac output is another adaptation to endurance exercise training. A study involving dogs demonstrated that this type of exercise training led to a 30% increase in cardiac output at maximal exercise levels (Musch et al. 1987). These investigators also determined regional blood flow distribution in their dogs using radiolabelled microspheres. They reported that 80% of the additional cardiac output at maximal exercise post-training was directed to skeletal muscle (e.g., hindlimb muscles such as the gastrocnemius). Although left ventricular adaptations undoubtedly made important contributions to greater muscle blood flow post-training, a nearly 25% reduction in total peripheral resistance suggested that vascular adaptations also contributed. We have focused our research on the latter possibility, examining adaptations to endurance exercise training in both conductance and resistance vessels supplying skeletal muscle with blood flow. Much of this review will therefore summarize findings from that research. In addition, since it appears that endurance exercise training confers protection against several chronic diseases, we will briefly discuss the possibility that training may protect against the vascular disease of atherosclerosis.
Conductance vessels
Conductance vessels are relatively large-diameter blood vessels. Whereas their resistance to blood flow is low, they have nonetheless been considered a logical place to begin examining adaptations to exercise training. When we determined dilatory responses of aortae isolated from endurance exercise-trained rats and their sedentary counterparts in vitro, a number of important findings emerged (Delp et al. 1993). First, dose-dependent relaxation responses to the endothelium-dependent agent acetylcholine (ACh), following norepinephrine-induced contraction, were greater in aortae from trained animals. In addition, both sensitivity to ACh and the maximal response to ACh were augmented with training. Second, dose-dependent responses to the endothelium-independent agent sodium nitroprusside (SNP) were not different between groups. Since SNP acts as an exogenous source of nitric oxide (NO), thereby bypassing endothelial-generated NO, the finding that SNP responses did not change with training suggested that the endothelium (but not vascular smooth muscle) adapted to endurance exercise training. Other experiments in this study, using the inhibitor of endothelial NO formation NG-nitro-l-arginine methyl ester, demonstrated that the augmented relaxation response to ACh with training was primarily due to greater NO formation in the endothelium. This finding was subsequently supported by several studies (e.g., McAllister et al. 2005) that demonstrated greater expression of endothelial NO synthase (eNOS), the protein that catalyzes NO formation in aortic endothelium, in trained animals. Collectively, these findings in a conductance vessel suggest that greater endothelium-dependent dilation may contribute to enhanced skeletal muscle blood flow after a period of endurance exercise training. Nonetheless, the small contribution that conductance vessels make to total peripheral resistance dictates that studies also examine how endurance training impacts the resistance vasculature. Although increased endothelium-dependent dilation of conductance vessels likely contributes minimally to greater muscle blood flow, increased NO availability may have other benefits in these vessels (e.g., inhibition of atherosclerosis; see below).
Resistance vessels
Resistance vessels are relatively small-diameter blood vessels. Given that their resistance to blood flow is high and therefore a primary determinant of tissue blood flow, they are considered important sites in the vasculature to examine adaptations to training. We recently conducted a study of the effects of endurance exercise training on skeletal muscle resistance vessels (McAllister et al. 2005). This study involved several experimental approaches, including in situ determination of vasodilatory responses, in vitro determination of vasodilatory responses, and determination of eNOS protein expression. These different experimental approaches yielded remarkably consistent findings concerning adaptations of resistance vessels to training.
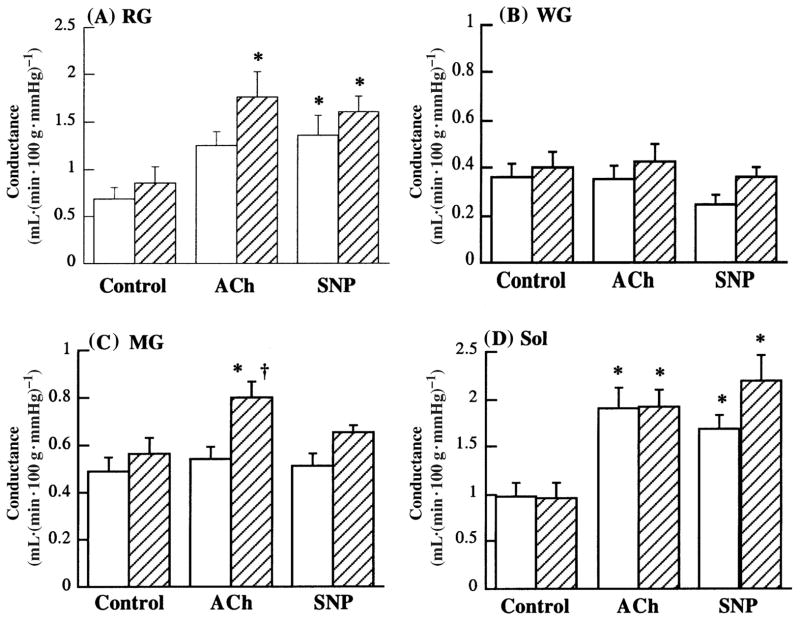
With the first approach, vasodilatory responses were determined in situ using the isolated perfused rat hindlimb preparation. Use of this preparation for determination of vascular function has been described in detail previously (McAllister 2003). Since *80% of the rat hindlimb mass is skeletal muscle, responses to infusion of agents such as ACh largely reflect those of resistance vessels located within muscles of the hindlimb. Importantly, the use of the radio-labelled microsphere technique, in conjunction with the hindlimb preparation, enables the examination of muscle-specific blood flows during infusion of vasoactive agents. When we examined muscle-specific responses, a number of findings emerged (Fig. 1). First, in the white gastrocnemius muscle section, which consists primarily of fast glycolytic muscle fibers, no vasodilation in response to ACh occurred in either the sedentary group or the endurance exercise-trained group (Fig. 1B). In the soleus muscle, which is composed almost exclusively of slow oxidative fibers, this response was similar between sedentary and trained rats, although robust vasodilation in response to ACh occurred (Fig. 1D). In the red section of the gastrocnemius muscle, consisting largely of fast oxidative glycolytic fibers, ACh-induced vasodilation was greater in trained than in sedentary rats (Fig. 1A; McAllister et al. 2005).
Fig. 1.
Conductance (quotient of blood flow and perfusion pressure) in the red section of gastrocnemius muscle (RG; A), white section of gastrocnemius muscle (WG; B), mixed section of gastrocnemius muscle (MG; C), and soleus muscle (Sol; D). Values are means ± SE; sedentary group, open bars; exercise-trained group, filled bars. ACh, acetylcholine; SNP, sodium nitroprusside. *, Significantly different from control within the same group (p < 0.05); {, significantly different between tissues from sedentary and exercise-trained rats (p < 0.05). (From McAllister et al. 2005, reproduced with permission from J. Appl. Physiol., Vol. 98, p. 756, # 2005 The American Physiological Society.)
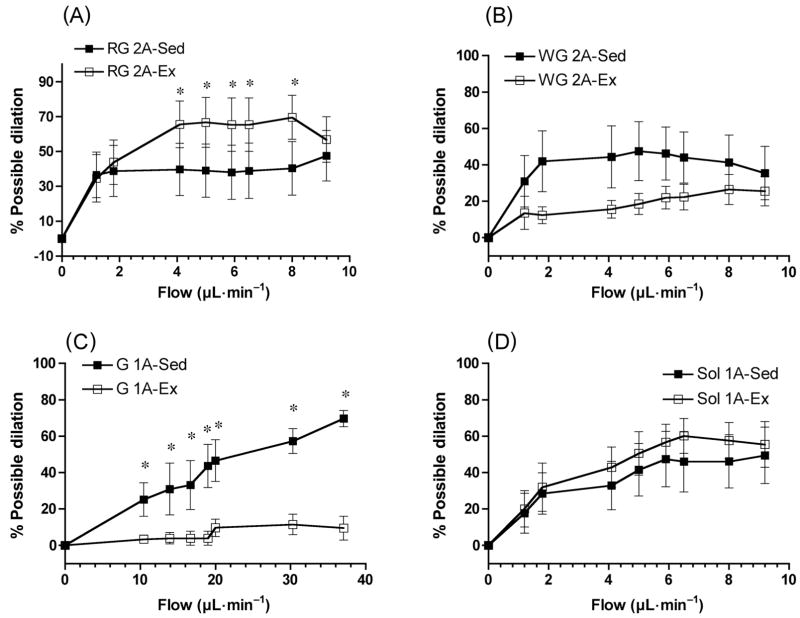
These findings, obtained in situ, led us to determine dilatory responses to increases in flow, a more physiological stimulus for endothelium-dependent dilation, through resistance vessels in vitro. We examined resistance vessels isolated from several hindlimb muscles and muscle sections, including the white gastrocnemius (2A arterioles), the soleus (1A arterioles), and the red gastrocnemius (2A arterioles). In each instance, we studied the first order of resistance vessel located intramuscularly. Vessels from all 3 muscles or muscle sections exhibited dilation in response to increases in flow (Fig. 2). Similar to responses determined in situ, dilatory responses to flow of 2A arterioles from red gastrocnemius of trained rats in vitro were greater than those of sedentary rats (Fig. 2A). Interestingly, dilation in response to SNP was also enhanced in these arterioles from trained animals, raising the possibility that enhancement of both endothelial NO formation and vascular smooth muscle sensitivity to NO contributed to augmentation of the dilatory response to flow (McAllister et al. 2005).
Fig. 2.
Dose-dependent dilatory responses to increases in the flow of red gastrocnemius (RG) 2A arterioles (A), white gastrocnemius (WG) 2A arterioles (B), gastrocnemius (G) 1A arterioles (C), and soleus (Sol) 1A arterioles (D). Values are means ± SE; percent possible dilation was calculated as a fraction of the difference in diameter between arteriole with basal tone and without tone (in Ca2+-free perfusate). *, Significantly different between tissues from sedentary (Sed) and exercise-trained (Ex) rats (p < 0.05). (From McAllister et al. 2005, reproduced with permission from J. Appl. Physiol., Vol. 98, p. 758. # 2005 The American Physiological Society.)
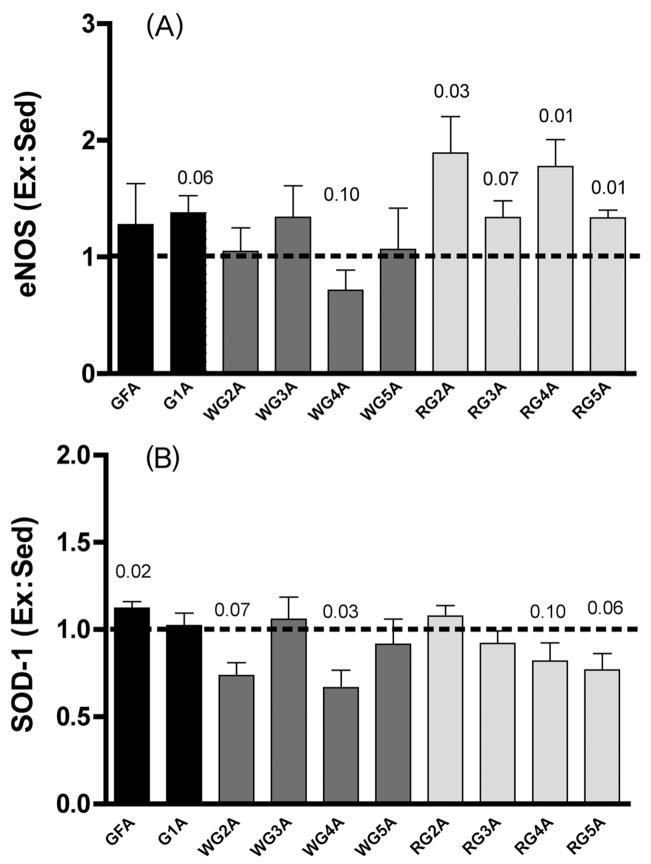
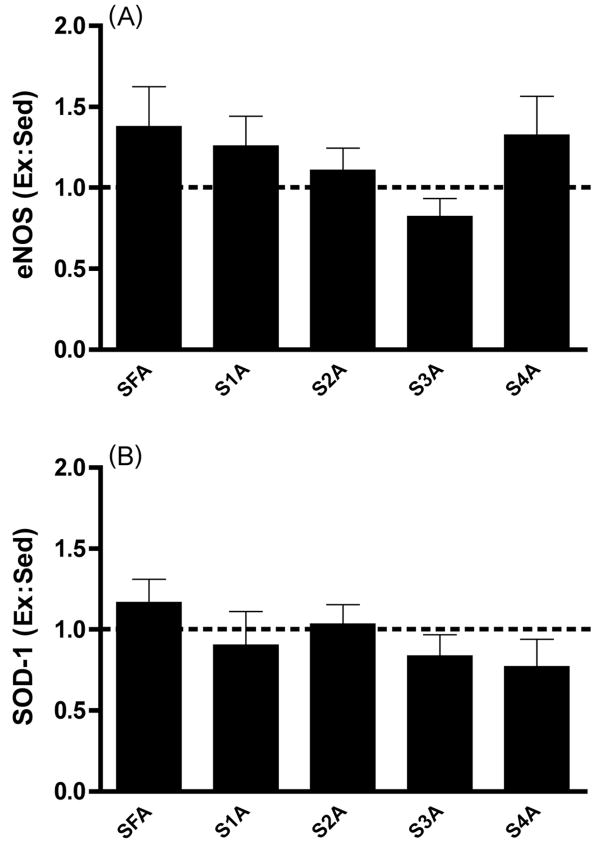
With our third approach, we determined eNOS protein expression in a variety of resistance vessel orders from the same muscles or muscle sections in which dilatory responses were examined. For these determinations, it was necessary to pool vessels from several rats to have sufficient protein for Western blotting. In agreement with the above-mentioned dilatory data, eNOS expression was augmented significantly by endurance exercise training only in vessels (2A, 4A, and 5A arterioles) from the red gastrocnemius muscle section (Fig. 3A). No resistance vessels from either the white gastrocnemius (Fig. 3A) or soleus muscle (Fig. 4A) exhibited greater eNOS expression in trained rats compared with their sedentary counterparts. Interestingly, expression of superoxide dismutase-1 (SOD-1), the Cu/Zn-dependent isoform of SOD-1, only increased in the gastrocnemius feed artery (Figs. 3B, 4B; McAllister et al. 2005). This suggests that increased NO production via eNOS, but not reduced NO scavenging by free radicals such as superoxide as a consequence of greater antioxidant action by SOD-1, is solely responsible for increased endothelium-dependent vasodilation.
Fig. 3.
(A) eNOS protein expression in gastrocnemius (G) resistance vessels. GFA, G feed artery. Values are means ± SE; heights of bars represent the ratios of expression in tissues from exercise-trained (Ex) rats to that in tissues from sedentary (Sed) rats. Ex is significantly greater than Sed (p < 0.05) for RG 2A, 4A, and 5A arterioles; borderline significance (0.05 < p < 0.10) is also indicated. WG, white gastrocnemius; RG, red gastrocnemius. (B) SOD-1 protein expression in G resistance vessels. Note that Ex is significantly greater than Sed in only GFA. (From McAllister et al. 2005, reproduced with permission from J. Appl. Physiol., Vol. 98, p. 760. #2005 The American Physiological Society.)
Fig. 4.
(A) eNOS protein expression in soleus (S) resistance vessels. SFA, soleus feed artery. Values are means ± SE; heights of bars represent the ratios of expression in tissues from exercise-trained (Ex) rats to that in tissues from sedentary (Sed) rats. Ex is not significantly greater than Sed (p < 0.05) in any arteriolar order. (B) SOD-1 protein expression in S resistance vessels. Note that Ex is not significantly greater than Sed in any arteriolar order. (From McAllister et al. 2005, reproduced with permission from J. Appl. Physiol., Vol. 98, p. 760, # 2005 The American Physiological Society.)
The consistency of our data, which we obtained using 3 different experimental approaches, suggests that endurance exercise training primarily induces endothelial adaptations in vessels that experience increased blood flow during training sessions. The fast oxidative glycolytic red gastrocnemius muscle section exhibits increased blood flow (*3-fold over resting level) during treadmill running at 30 m/min, whereas flows to the fast glycolytic white gastrocnemius and the slow oxidative soleus muscles increase only slightly (soleus) or not at all (white gastrocnemius; Laughlin and Armstrong 1982). These different blood flow responses to treadmill running at 30 m/min likely reflect differences in muscle fiber recruitment and metabolism (cf., Laughlin and Armstrong 1982). We have also investigated the effects of sprint exercise training (repeated short bursts of faster treadmill running at 60 m/min, separated by rest intervals; this type of training also increases recruitment of, as well as blood flow to, the fast glycolytic white gastrocnemius fibers (Laughlin and Armstrong 1982). Sprint training was found to enhance both ACh-induced dilatory responses in vitro and eNOS protein expression in certain resistance vessels of the white gastrocnemius muscle section (Laughlin et al. 2004). Collectively, these findings indicate that the magnitude of the increase in blood flow during exercise training sessions is a key determinant of vascular adaptations to exercise training in rodents. Interestingly, some studies in humans have demonstrated improved endothelium-dependent dilation in vessels that presumably did not experience increased blood flow (e.g., brachial artery with lower body exercise training; cf., Moyna and Thompson 2004). Finally, it is important to acknowledge that altered vascular structure likely also contributes to greater skeletal muscle blood flow with training. Increased arteriolar density, for example, would increase vessel numbers in parallel and therefore reduce resistance to blood flow. We recently reported that endurance training increased density of small-diameter arterioles in the gastrocnemius muscle (Laughlin et al. 2006). Thus, augmented endothelium-dependent, NO-mediated vasodilation is likely only one of several mechanisms accounting for greater skeletal muscle blood flow after a period of training.
Effects of endurance exercise training on atherosclerosis
The available human data (e.g., Myers et al. 2002) suggest that endurance exercise training prevents the development and (or) progression of atherosclerosis. Several steps of the atherosclerotic disease process, including leukocyte adhesion to the endothelium, vascular smooth muscle cell migration into the subendothelial space, and platelet aggregation in the vascular lumen (cf., Berliner et al. 1995), are inhibited by NO (cf., Gewaltig and Kojda 2002). Because exercise training augments endothelial NO formation, as detailed above, this may be an adaptation underlying the protective effect of exercise against atherosclerosis.
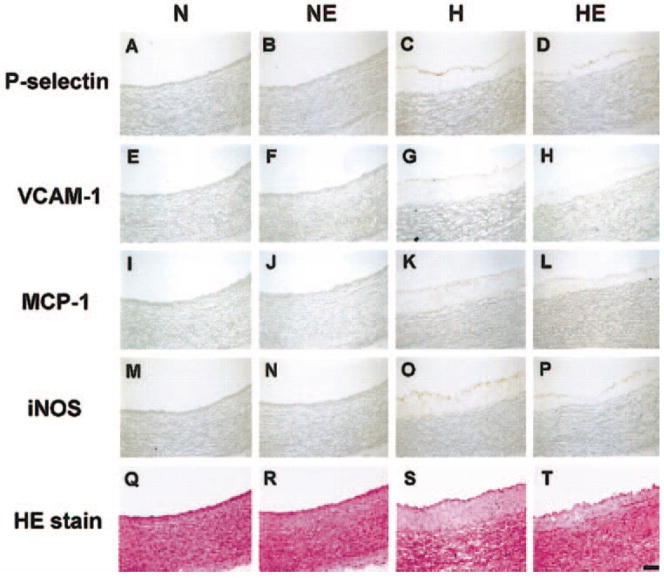
Concerning leukocyte adhesion, a recent study by Yang and colleagues (2003) examined the expression of P-selectin and vascular cell adhesion molecule-1 (VCAM-1), proteins that recruit leukocytes to the endothelium. These leukocytes may subsequently enter the subendothelial space, differentiate into macrophages, and become cholesterol-laden foam cells, a key constituent of an atherosclerotic lesion. Cholesterol-fed rabbits in this study expressed P-selection and VCAM-1 in aortic vascular endothelium, but exercise training on a treadmill blunted dietary cholesterol-induced expression of these proteins (Figs. 5D and 5H vs Figs. 5C and 5G). Importantly, intimal thickness (an index of extent of atherosclerosis) was reduced in parallel with the reduced expression of these proteins (Fig. 5T vs 5S).
Fig. 5.
Immunohistochemical staining of rabbit aorta for adhesion molecules (P-selectin and VCAM-1), inflammatory markers (MCP-1 and iNOS), and general morphology (hematoxylin–eosin stain). H, high cholesterol diet and sedentary; HE, high cholesterol diet and exercise-trained. Note the reduced expression of P-selectin and VCAM-1 in HE compared with H (P-selectin, D vs C; VCAM-1, H vs G), and less intimal thickness in HE compared with that in H (hematoxylin–eosin stain, T vs S). (From Yang et al. 2003, reproduced with permission from J. Appl. Physiol., Vol. 95, p. 1198. # The American Physiological Society.)
A study involving rats examined the anti-atherosclerotic effects of swimming training on vascular smooth muscle cell migration and platelet aggregation (Indolfi et al. 2002). In this study, carotid arteries were subjected to balloon angioplasty, a procedure known to induce smooth muscle migration and proliferation. The number of smooth muscle cells in the subendothelial space, an index of smooth muscle migration, was reduced in swim-trained rats compared with that in their sedentary counterparts. The extent of atherosclerosis was reduced in parallel. The co-administration of a NOS inhibitor abrogated both the training-induced increase in eNOS activity and the reduction in smooth muscle migration with swim training, suggesting that increased NO availability was linked to reduced migration of smooth muscle cells. Another beneficial adaptation to exercise training was a reduction in platelet aggregation in blood sampled from these rats.
These two studies require verification, but nonetheless suggest that increased NO formation in the vascular endothelium, a predicted outcome of greater eNOS expression with endurance exercise training, results in the inhibition of one or more steps of atherosclerosis. This adaptation may underlie the preventive effect of exercise training in healthy individuals, as well as the rehabilitative effect of training in patients with cardiovascular disease (Myers et al. 2002).
Acknowledgments
The authors acknowledge the important technical contributions to their studies cited in this review by Ivelisse Albarracin, Beth Degarmo, Molly Edmonds, Ann Melloh, Tara Smith, and Pam Thorne. Also acknowledged are our valued collaborators, Drs. Jeffrey Jasperse and Elmer Price. This work was supported by funds from the National Institutes of Health, the American Heart Association, and the American Physiological Society.
References
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- Gewaltig MT, Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res. 2002;55:250–260. doi: 10.1016/S0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, et al. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res. 2002;91:1190–1197. doi: 10.1161/01.RES.0000046233.94299.D6. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96:233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Cook JD, Tremble R, Ingram D, Colleran PN, Turk JR. Exercise training produces nonuniform increases in arteriolar density of rat soleus and gastrocnemius muscle. Microcirculation. 2006;13:175–186. doi: 10.1080/10739680600556829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RM. Endothelium-dependent vasodilation in different rat hindlimb skeletal muscles. J Appl Physiol. 2003;94:1777–1784. doi: 10.1152/japplphysiol.00901.2002. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol. 2005;98:753–761. doi: 10.1152/ japplphysiol.01263.2003. [DOI] [PubMed] [Google Scholar]
- Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113–123. doi: 10.1111/j.0001-6772.2003.01253.x. [DOI] [PubMed] [Google Scholar]
- Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol. 1987;62:1724–1732. doi: 10.1152/jappl.1987.62.4.1724. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Yang A-L, Jen CJ, Chen H-I. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol. 2003;95:1194–1200. doi: 10.1152/japplphysiol.00282.2003. [DOI] [PubMed] [Google Scholar]