Summary
The hairless (Hr) gene encodes a transcriptional co-repressor highly expressed in the mammalian skin. In the mouse, several null and hypomorphic Hr alleles have been identified resulting in hairlessness in homozygous animals, characterized by alopecia developing after a single cycle of relatively normal hair growth. Mutations in the human ortholog have also been associated with congenital alopecia. Although a variety of hairless strains have been developed, outbred SKH1 mice are the most widely used in dermatologic research. These unpigmented and immunocompetent mice allow for ready manipulation of the skin, application of topical agents, and exposure to UVR, as well as easy visualization of the cutaneous response. Wound healing, acute photobiologic responses, and skin carcinogenesis have been extensively studied in SKH1 mice and are well characterized. In addition, tumors induced in these mice resemble, both at the morphologic and molecular levels, UVR-induced skin malignancies in man. Two limitations of the SKH1 mouse in dermatologic research are the relatively uncharacterized genetic background and its outbred status, which precludes inter-individual transplantation studies.
Keywords: Hairless mice, Ultraviolet rays, Skin neoplasms, Wound healing, Skin aging
1. The hairless locus
The Hr gene and protein
The Hr gene and encoded protein are illustrated in Figure 1 [1-3]. Murine Hr lies at the 70 Mb position of mouse chromosome 14, a region syntenic with human chromosome 8 (http://www.ensembl.org). The gene spans almost 20 kb; it contains 19 exons and is transcribed into 3-kb and 6-kb mRNAs. Translation begins in exon 2 and ends 93 nucleotides upstream from the presumed polyadenylation site. Hr encodes a protein with a predicted length of 1182 amino acids and a molecular weight of 130 kDa. There is no hydrophobic leader or transmembrane domain, thus the protein is not exported from the cell [2]. A nuclear localization signal directs nuclear translocation [4]. The HR protein is a transcriptional co-repressor, binding to thyroid hormone, vitamin D, and retinoic acid receptor-related orphan receptors but not to retinoic acid or glucocorticoid receptors [5]. In the absence of hormone, the nuclear receptor and HR form a repressor complex, which associates with the nuclear matrix and interacts with histone deacetylases to repress transcription of target genes.
Fig. 1.
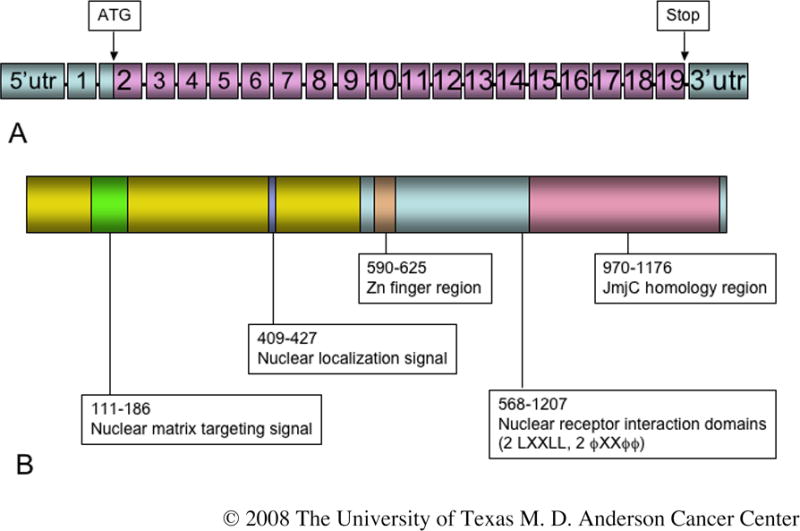
The hairless gene and encoded protein. (A) The hairless gene contains 19 exons; translation begins in exon 2 at the ATG indicated. (B) The encoded protein is 1182 amino acids in length in which a number of functional domains have been identified, as indicated. Information from which this figure was compiled was obtained from a variety of sources [1,4,5, 14,88-90].
Hr expression
In the mouse embryo, Hr is expressed in epithelia of mouth, tongue, nose, bladder, urethra, stomach, tooth bud, and submandibular salivary gland, in cartilage, and in nervous tissue [6]. Hr is not expressed in testis, liver, heart, or kidney. Hr mRNA appears in the periderm and basal layer of the fetal epidermis beginning at day 12.5. By the time of birth, Hr is strongly expressed in both the hair follicle and the interfollicular epidermis. Robust expression of Hr mRNA in the adult is seen only in brain and skin [1]. Hr mRNA is constitutively expressed in suprabasal layers of the interfollicular epidermis and hair follicle infundibulum, but expression of Hr mRNA in the proximal hair follicle is hair cycle-dependent [7]. During anagen, Hr expression appears sequentially in the hair follicle matrix, portions of the inner root sheath, and the innermost layer of the outer root sheath; with the onset of catagen, Hr expression progressively disappears from the hair follicle matrix and the inner root sheath and appears in the outer root sheath keratinocytes surrounding the lower end of the developing club hair; as the hair follicle enters telogen, only the keratinocytes associated with the dermal papilla remain Hr-positive. HR protein is not detected in keratinocytes in actively growing anagen follicles, but appears as the follicles enter catagen, in the nuclei of keratin 14-positive cells in the outer root sheath and keratin 14-negative hair bulb cells [8].
2. Varieties of hairless mice
Hr alleles
In 1924 a pair of wild hairless mice was identified in an aviary in London [9]. Dr. E.L. Green brought this mouse stock to the Jackson Laboratory (Bar Harbor, Maine), where a hairless male was mated to a female BALB/c; an inbred strain was derived by sibling mating and named HRS/J at generation F24 (http://jaxmice.jax.org/strain/000673.html). The SKH1 mouse (Crl:SKH1-hr) marketed by Charles River Laboratories (Wilmington, MA) was obtained from a commercial supplier in New York City via Temple University (http://www.criver.com/research_models_and_services/research_models/SKH1.html). The mutant allele carried by HRS/J and SKH1 mice is hairless (Hrhr). Like all Hr alleles, it is an autosomal recessive mutation. The hr allele contains a modified polytropic retrovirus stably integrated into intron 6 of the gene (Figure 2), which results in aberrant splicing of over 95% of Hr transcripts [1,6,10]. Eighteen murine Hr alleles are currently known (Table 1, http://www.informatics.jax.org/searches/allele_report.cgi?_Marker_key=9943). Mutations in orthologs of the murine Hr gene have been identified in other species, including man and rhesus macaque [11,12]. Two human autosomal recessive diseases (alopecia universalis congenita and atrichia with papular lesions) have been associated with HR mutations [13]. Hairless rats do not have mutations in the ortholog of mouse Hr [14].
Fig. 2.
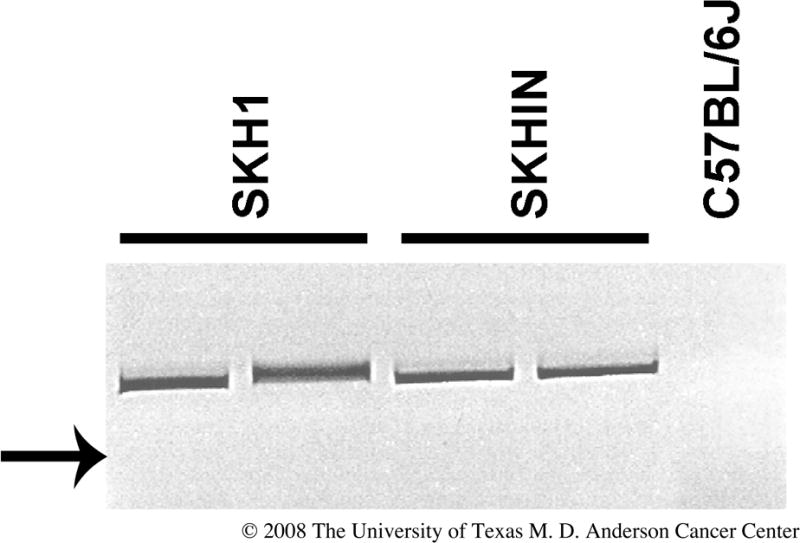
PCR identification of the hr allele. This shows the reverse image of an ethidium bromide-stained agarose gel of PCR products that identify the retroviral insertion in the Hr locus described for the hr allele. Primers employed were 5′-CAAGCCTTATTCGAACTAAC-3′ located within the retroviral insertion and 5′-AGATTTAACACAGGTGCTAG-3′ located in mouse genomic DNA. The presence of the 439 bp specific band in genomic DNA from SKH1 outbred and SKHIN inbred mice indicates that they carry the hr hypomorphic allele. The band was absent when C57BL/6 DNA was used in the reaction. We confirmed by direct sequencing that the PCR product contained retroviral sequences. The arrow indicates the position of the 400 basepair DNA size standard.
Table 1. Phenotypic alleles at Hr locusa.
| Allele symbol, name | Inheritance mode | Molecular alteration |
|---|---|---|
|
Hrba, bald |
Recessive | Undefined (extinct) |
|
Hrbldy, baldy |
Recessive | Missense mutation in exon 2 (ENU-induced) |
|
Hrhr, hairless |
Recessive | Stable retroviral insertion into intron 6 |
|
Hrm1Enu, m1Enu |
Recessive | Missense mutation at nucleotide 3572 (ENU-induced) |
|
Hrrh, rhino |
Recessive | Point mutation to termination codon in exon 6 |
|
Hrrh-J, rhino Jackson |
Recessive | Undefined |
|
Hrrh-2J, rhino 2 Jackson |
Recessive | Undefined |
|
Hrrh-7J, rhino 7 Jackson |
Recessive | Point mutation to termination codon in exon 3 |
|
Hrrh-8J, rhino 8 Jackson |
Recessive | Two bp substitution in exon 4 (extinct) |
|
Hrrh-9J, rhino 9 Jackson |
Recessive | Undefined (extinct) |
|
Hrrh-Chr, rhino Christiano |
Recessive | Missense mutation at codon 476 |
|
Hrrh-bmh, rhino-bald Mill Hill |
Recessive | Large deletion at 3′ end of gene |
|
Hrrh-Y, rhino Yurlovo |
Recessive | 13-bp insertion in exon 16 |
|
Hrrhsl, rhinocerotic and short lived |
Recessive | Nonsense mutation in exon 12 |
|
HrUSP, hairless USP |
Recessive | Undefined (ENU-induced) |
|
HrTg5053Mm, transgene insertion 5053, Miriam Meisler |
Recessive | Transgene insertion into locus |
|
Hr tm1Cct, targeted mutation 1, Catherine C Thompson |
Recessive | Genetically engineered knockout |
© 2008 The University of Texas M. D. Anderson Cancer Center
Strains of hairless mice
Commercially available strains of mice with the Hrhr allele are shown in Table 2. Perhaps the most widely used is the outbred albino SKH1. Inbred strains have been derived from SKH1 mice [15,16; Benavides, unpublished data]. Inbred SKH1 mice allow transplantation of immune cells and skin tumors. Other inbred strains of mice carrying the hr allele have been developed through successive generations of backcrosses to a recipient inbred strain (http://jaxmice.jax.org/; www.taconic.com). Outbred SKH1 mice have been used to introgress different transgenic or targeted loci through repetitive backcrosses [17,18]. Microinjecting a transgene construct into the pronuclei of fertilized oocytes from SKH1 hairless mice is a direct way to generate a transgenic line on an SKH1 genetic background [19].
Table 2. Strains and stocks carrying the hairless or rhino mutant alleles.
| Strain | Genetic background | Pigmentation | Source | Availability | Reference |
|---|---|---|---|---|---|
| HRS/J (hairless) | Inbred (HRS) | Albino | The Jackson Laboratory | Restricted | http://jaxmice.jax.org/info/index.html |
| RHJ/LeJ (rhino) | Inbred (RHJ) | Albino | The Jackson Laboratory | Restricted | http://jaxmice.jax.org/info/index.html |
| SKH2/J (hairless) | Inbred (SKH2) | Pigmented | The Jackson Laboratory | Cryopreserved | http://jaxmice.jax.org/info/index.html |
| WLHR/LeJ (hairless) | Inbred (WLHR) | Pigmented | The Jackson Laboratory | Cryopreserved | http://jaxmice.jax.org/info/index.html |
| SKH1 (hairless) | Outbred | Albino | Charles River Laboratory | Immediate | http://www.criver.com/research_models_and_services/research_models/mice_a_b.html |
| SKH2 (hairless) | Outbred | Pigmented | Charles River Laboratory | Restricted | http://www.criver.com/research_models_and_services/research_models/mice_a_b.html |
| B6.A-H2-T18a.HRS-Hrhr/J | Congenic (C57BL/6) | Pigmented | The Jackson Laboratory | Cryopreserved | http://jaxmice.jax.org/info/index.html |
| C3.Cg-Hrhr/Tac (HRLS model) | Congenic (C3H) | Pigmented | Taconic | Immediate | http://www.taconic.com/anmodels/HRLS.htm |
| D2. HRS-Hrhr/J | Congenic (DBA2) | Pigmented | The Jackson Laboratory | Cryopreserved | http://jaxmice.jax.org/info/index.html |
© 2008 The University of Texas M. D. Anderson Cancer Center
3. The skin of hairless mice
The role of HR
Repeated topical application of catalytic oligonucleotides that specifically target Hr mRNA for degradation recapitulates essentially all features of the hairless skin [20]. In addition, crossing Hr knockout mice with transgenic mice that constitutively express Hr in keratin 14-positive progenitor keratinocytes produces “transgenic rescue” mice that eventually develop normal fur [21]. Thus, expression of Hr in progenitor keratinocytes is both necessary and sufficient for normal hair growth. It has been postulated that Hr regulates keratinocyte progenitor cell differentiation by repressing expression of keratinocyte differentiation markers and promoting development of hair follicles [8]. As a transcriptional corepressor for hormone receptors, Hr likely acts by modulating hormone-dependent growth and development in the skin. Thyroid hormone deficiency and the hairless phenotype share characteristics of skin thickening and hair loss [5], and some mutations in the vitamin D receptor of man produce generalized atrichia virtually identical to the hairless phenotype [22].
Skin phenotype of hairless mice
The first hair coat of Hrhr/Hrhr mice (hereafter, hr/hr) develops normally (reviewed in [2,23]). Beginning 13-14 days after birth, however, there is rapid and complete hair loss beginning at the eyelids and proceeding caudally, with a sharp boundary between hairless and haired areas. By about 3 weeks of age, the animals are completely hairless except for a few vibrissae. A second wave of hair growth begins at 5 weeks of age, but the few primary tylotrich follicles that develop are abnormal. Vibrissae are shed repeatedly, becoming more abnormal and sparser with each regrowth. Hair and cilia are lost from the eyelids. Histologically, significant hair follicle abnormalities appear at the catagen stage of the first hair cycle, associated with dysregulation of the entire process of regression. Two characteristic structures appear, the utriculus and dermal cyst (Figure 3). The utriculus, an ampuliform structure lined by hyperkeratotic epithelium and connected to the skin surface, appears to arise from the infundibulum of the hair shaft. The dermal cyst is located in the deep dermis and is not connected to the overlying epidermis. The cyst is lined by keratinized epithelium and may contain sebocytes in the wall. It may derive from hair bulb progenitor cells undergoing unsuccessful sebaceous gland differentiation. There is also gradual enlargement of sebaceous glands and dermal granulomas develop. SKH1 skin is rugose and rugosity increases with age. Nails are long and twisted. The epidermis of the two sexes is similar in thickness, but the male dermis is thicker and the male hypodermis is thinner than the female (Oberyszyn, unpublished data).
Fig. 3.
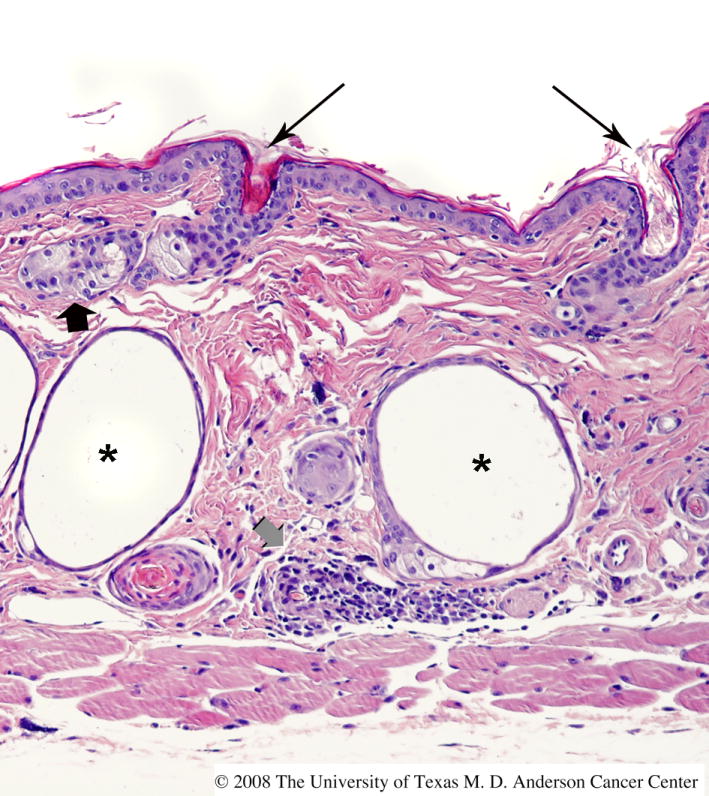
Histologic appearance of skin in mice homozygous for the hr allele. The skin of this FVB mouse homozygous for the hr allele exhibits characteristic features, including multiple dermal cysts (asterisks), utriculi connected to the skin surface (long black arrows), sebaceous gland hyperplasia (short black arrow), and dermal inflammation (short gray arrow).
Imaging implications
The lack of hair and skin pigmentation in hairless mice makes them ideal subjects for the application of new techniques of in vivo imaging, particularly light-based techniques. Luciferase-catalysed luciferin light emission, specific chemiluminescent probes and ultra-low-light imaging, and fiber optic confocal imaging in combination with fluorescent probes have been successfully employed [24-26].
Treatment implications
Skin offers several advantages for drug delivery, including avoidance of gastrointestinal upset, elimination of hepatic first pass effects, and more stable plasma drug levels (reviewed in [27]). The skin of hairless mice is widely used as a substitute for human skin to measure percutaneous drug penetration in vitro. In general, hairless mouse skin is more permeable than human skin [28]; it is less permeable to benzo[a]pyrene than the skin of haired mice but is equally permeable to testosterone [29]. Prolonged in vitro hydration of hairless mouse skin compromises barrier function and causes a dramatic increase in permeability, particularly to polar or ionized solutes [30]. Treatment of hairless skin with acetone does not alter its permeability to water [28]. Drug penetration of the stratum corneum may be enhanced. For example, drug-loaded liposomes enter the skin via hair follicles or diffuse through extracellular spaces in the stratum corneum to be internalized by keratinocytes [27,31].
Because of its large area and ready accessibility, the skin is an attractive target for gene delivery for immunization and gene therapy [32]. DNA has been applied to hairless skin in the form of naked plasmid DNA, liposomes, transferrin complexes, and plasmid-coated gold particles, with results ranging from no expression to rapid and efficient systemic expression of encoded genes [33-35]. Techniques such as arginine peptide complexing and electroporation may enhance gene delivery [36,37]. Hair follicles do not appear to be required for efficient vaccine delivery by microporation [38].
Skin diseases
Hairless SKH1 mice are susceptible to spontaneous skin abscesses caused by β-hemolytic Group G Streptococcus [39] and develop chloracne in response to dioxin [40].
4. Immunobiology of hairless mice
Major histocompatibility complex (MHC)
The haplotypes of both outbred SKH1 and newly developed inbred SKH1 mice are largely unknown. The H2 haplotype of one inbred SKH1 strain [15] was recently determined to be H2q by PCR (Charles River Laboratories, Wilmington, MA) (Benavides, unpublished data). These syngeneic mice are suitable for transplantation of skin tumors [41].
Lymphocyte number and function
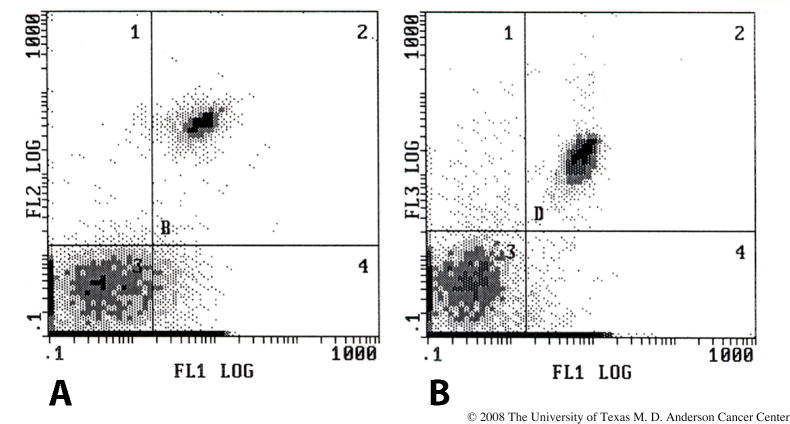
Commercially available antibodies allow the detection and quantification of SKH1 T lymphocytes by flow cytometry (Figure 4). Moreover, CD4 and CD8 T cells, as well as neutrophils, can be specifically deleted from SKH1 mice by treatment with appropriate monoclonal antibodies [42].
Fig. 4.
Flow cytometry of peripheral blood leukocytes of the SKH1 mouse. Blood was collected by retroorbital bleeding, red blood cells were lysed, and the remaining leukocytes were incubated with anti-CD4 (A) or CD8 (B). Staining and flow cytometry were performed as previously described [49].
In a study of 10 varieties of hairless mice with normal thymuses, including one HRS and several SKH1 stocks, spleen and lymph node mitogen assays, splenic antibody responses and T and B cell numbers in lymphoid organs were relatively normal in 8-13 week old mice [43]. Compared to haired mice, the primary antibody response to tetanus toxoid in HRS/J mice is delayed and reduced, but this effect is independent of hr genotype [44]. Spleens of HRS/J-hr/hr and HRS/J-+/hr mice contain comparable numbers of T and B cells, respond vigorously to mitogens, and generate normal cytotoxic responses to alloantigens [45]. However, spleen cells from HRS/J-hr/hr mice have a reduced proliferative response to alloantigens compared to those of HRS/J-+/hr mice. Immune abnormalities in the HRS/J strain are likely to be associated with a specific form of leukemia to which they are susceptible. T cell defects in HRS/J-hr/hr mice do not indicate a true immunodeficiency, for these mice can reject skin allografts and form antibodies normally.
5. Wound healing in hairless mice
Hairless mice are excellent for wound healing studies, as hair removal and associated inflammation are avoided and hair regrowth does not obscure the wound healing response. As in haired mice, stages of full-thickness wound healing in hairless mice include inflammation (clot formation and leukocyte influx), proliferation and tissue formation (reepithelialization, fibroplasia, and angiogenesis), and tissue remodeling (scar formation) [46]. Incisional and excisional wounding models employed in haired mice (reviewed in [47]) can readily be adapted to hairless mice, and studies of impaired healing due to ischemia or diabetes may be performed [48]. Full thickness incisional and excisional wounds in the dorsum that are allowed to heal by second intention exhibit marked contraction due to the activity of the panniculus carnosus. Such wound contraction can account for up to 90% of wound closure in mice, a situation very different from that in man [47]. This wound contraction occurs very early during wound healing and is quite distinct from the contracture due to fibroblast migration and myofibroblast activity occurring in the tissue remodeling phase of wound healing.
6. Photobiology of hairless mice
Effective wavelengths of light
The UVR spectrum is divided into UVC (100-280 nm), UVB (280-320 nm), and UVA (320-400 nm). Little UVC penetrates the ozone layer, thus studies using UVC wavelengths are not physiologically relevant. Sunlight reaching the earth's surface is a mixture of UVB and UVA. The wavelengths of UVR most effective in causing adverse effects on the skin of hairless mice, including sunburn, photoimmunosuppression, and skin carcinogenesis, are UVB [49-51]. Thus, most photobiology studies that employ hairless mice are carried out using UVR sources that emit UVB or mixed UVB and UVA wavelengths [52].
Sunburn
Hairless mice are valuable in the study of acute UVR effects, as background inflammation associated with hair removal by shaving, chemical depilation, or waxing is avoided. The minimal erythemal dose (MED) is the lowest dose of UVR causing perceptible cutaneous inflammation, as revealed by increased thickness of tented back skin 48 hours after exposure. In SKH1 mice, the MED for commonly employed UVR light sources (Kodacel-filtered Westinghouse FS or Phillips T12 sun lamps) is approximately 2240 J/m2 [53].
Hairless mice develop a typical sunburn reaction characterized by edema, erythema, and inflammation [54-56]. The peak inflammatory response in SKH1 mice occurs at 48 hours post-UVR. Increases in blood flow and vascular permeability and altered expression of vascular adhesion molecules promote the recruitment of inflammatory cells, particularly neutrophils and monocytes. Activated neutrophils produce myeloperoxidase, which catalyzes the production of reactive oxygen species (ROS). Monocytes and tissue macrophages phagocytize damaged tissue. Inflammatory cells secrete chemotactic and growth factors that promote further recruitment of inflammatory cells and tissue repair.
UVR directly induces cyclobutane pyrimidine dimers and (6-4) photoproducts in DNA [50,52]. Exposure to UVB also indirectly induces oxidative DNA damage, in part through the ROS generated by infiltrating inflammatory cells and activated keratinocytes. The most common type of DNA damage caused by ROS is the 8-oxo-deoxyguanosine adduct (reviewed in [57]), which serves a marker for oxidative stress. The hairless skin of the SKH1 mouse lends itself well to the study of topical compounds that alter the acute UVB induced inflammatory response [for example, 54,58].
Immunosuppression
UVR has profound immunosuppressive effects, including induction of suppressor T cell-mediated tolerance for immunogenic skin tumors and haptens such as oxazolone (reviewed in [51,59,60]). The ability of UVR to suppress Th1-mediated cellular immune responses like contact hypersensitivity and delayed type hypersensitivity has been explored extensively in a variety of mouse strains; hairless SKH1 mice appear to be more sensitive to the immunosuppressive effects of UVR than haired mice [61].
Photoaging
Aging changes in skins are due both to intrinsic and extrinsic causes, with sunlight being the most important extrinsic agent (reviewed in [62,63]). The SKH1 mouse is used extensively to study the mechanism, prevention, and treatment of age-related skin changes (reviewed in [64]). UVR-induced changes in the dermis of SKH1 mice include elastic fiber hyperplasia, collagen degradation, and increased glycosaminoglycans, associated with altered activity of matrix metalloproteases. Chronically UVR-exposed SKH1 mice develop wrinkles, which appear as prominent horizontal creases on the dorsum. These wrinkles differ from those in man by arising after only a few weeks of UVR exposure and by being associated with epidermal rather than dermal changes [65,66].
7. Skin carcinogenesis in hairless mice
Skin tumors in mice
Hairless mice are valuable for experimental carcinogenesis studies. Time-consuming depilation is not required, and the modifying effects of hair cycle on skin carcinogenesis are avoided. Carcinogens, promoters, chemopreventive agents, and chemotherapeutic compounds are readily applied to unperturbed hairless skin. Tumors are easily identified. Each mouse develops multiple independent skin tumors, which may exhibit significant differences in rate of development and aggressiveness. Tumors evolve through a series of reproducible stages that are associated with clearly defined morphologic and molecular hallmarks. Early during the course of tumor development, some tumors may undergo complete regression. Skin tumors are rarely fatal, thus they can often be followed for extended periods of time.
Skin tumors may be induced in mice by application of chemical carcinogens or chronic exposure to UVR. Skin tumors progress from foci of epithelial hyperplasia to papillomas and ultimately into squamous cell and spindle cell carcinomas. The process is conveniently divided into the stages of initiation, promotion, and progression (reviewed in [67]). Initiation is the irreversible process by which normal keratinocytes acquire (through somatic mutations) the irreversible capacity to form tumors. Promotion is a largely reversible process during which a clone of initiated keratinocytes expands to form a papilloma. During the process of tumor progression, a series of genetic and epigenetic events transforms the premalignant papilloma into a malignant squamous cell carcinoma (SCC). SCC of the skin induced by either chemical carcinogens or UVR may undergo conversion into spindle cell tumors composed of fibroblast-like cells that express vimentin [68]. Careful characterization of spindle cell tumors usually reveals keratin immunoreactivity and the presence of desmosomes in at least subpopulations of tumor cells, indicating that the tumors are of epithelial origin. Metastasis of skin tumors, both SCC and spindle cell tumors, although rare, does occur (Kusewitt, unpublished data).
Cup-shaped keratin-filled lesions resembling the keratoacanthoma of man have been reported in the skin of UVR-exposed SKH1 mice [69]. In man, such tumors frequently regress, while in mice, they regularly progress to SCC. In view of the differing clinical outcomes for keratoacanthomas in man and the mouse, application of the term to tumors of mice is probably inappropriate.
UVR carcinogenesis
The UVB waveband is responsible for most skin carcinogenesis; however, UVA has also been reported to be carcinogenic [50,70-73]. Several investigators have successfully induced tumors in mice using UVA, although these studies generally used extraordinarily high levels of UVA exposure. In some cases, however, UVA given before or at the same time as UVB has actually protected against UVB photocarcinogenesis in hairless mice [74-76].
SKH1 mice are highly susceptible to UVR induced skin cancer [77]; this susceptibility may be a function of the hairless gene itself, as C3H hairless mice are more susceptible to UVR carcinogenesis than haired mice of the same strain [78]. The SKH1 hairless mouse develops lesions resembling UVR-induced tumors in man and can be employed in studies that yield quantitative data suitable for both mechanistic studies and risk assessment purposes. For these reasons, it is widely considered to be the most suitable mouse model for studies of UVR carcinogenesis [79].
Our classification scheme for UVR-induced skin tumors in SKH1 mice accurately discriminates multiple stages in the carcinogenic cascade (Figure 5) [80]. Tumors are graded as papillomas (grades 1-3), microinvasive squamous cell carcinomas (MISCC; grades 1-3), or fully invasive SCCs, as follows: Papillomas are exophytic tumors that show no evidence of stromal invasion. A grade 1 papilloma is composed primarily of epithelium without a pronounced papillary pattern; a grade 2 papilloma is a well-differentiated papillary mass; a grade 3 papilloma is similar to a grade 2 papilloma, except that a few finger-like projections of atypical cells at the base of the mass are present. MISCC are characterized by penetration into the dermis with breaching of the basement membrane and, frequently, development of an inflammatory stromal response. Only tumors that invade into the panniculus carnosus are classified as fully invasive SCC. SCC with a primarily spindle cell morphology are termed spindle cell tumors and those that are composed of highly pleiomorphic tumors cells are termed anaplastic tumors. Papillomas are considered premalignant, while MISCC, SCC, spindle cell tumors, and anaplastic tumors are considered malignant.
Fig 5.
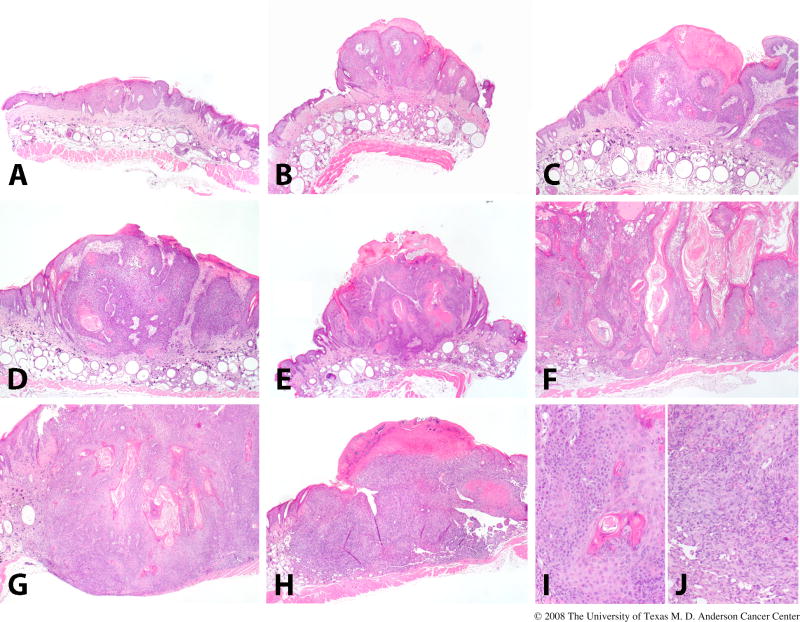
UVR-induced skin tumor development in the SKH1 mice. This figure illustrates the categories into which we classify skin lesions in SKH1 mice. Pre-malignant papillomas are shown in the upper panel, with grades 1, 2, 3, shown in (A), (B), and (C), respectively. Malignant micro-invasive SCC are shown in the middle panel, with grades 1, 2, 3, shown in (D), (E), and (F), respectively. A fully invasive SCC (G) and a spindle cell/anaplastic tumor (H) are shown in the lower panel. Closer views of the tumors in (G) and (H) are shown in (I) and (J). This detailed classification scheme has been useful in identifying small differences in skin tumor progression in several different protocols.
Chemical carcinogenesis in hairless mice
Hairless mice are not commonly employed for chemical carcinogenesis studies, although the outbred SKH1 hairless mouse is highly susceptible to both chemically induced and UVR-induced skin cancer [81,82]. However, a slightly lower incidence of skin tumors for HRS/J-hr/hr mice compared with +/hr mice after two-stage chemical carcinogenesis was observed [83].
Molecular alterations during skin carcinogenesis
In haired mice, the hallmark molecular change in chemically induced skin tumors is mutational activation of the H-ras oncogene (Hras1) [67]. However, such mutations are rare in UVR-induced tumors of hairless mice. Instead, inactivating mutations of the p53 (Trp53) tumor suppressor are characteristic, reportedly occurring in 50-75% of tumors [84]. P53 mutations have been reported in up to 100% of UVR-induced skin tumors in SKH1 mice, with many tumors demonstrating multiple p53 mutations [85]. p53 mutation is a very early event. Patches of keratinocytes immunohistochemically positive for P53 protein appear long before the emergence of tumors [86]. Mutations in P53-positive foci are essentially identical to those in UVR-induced tumors, thus the foci are true tumor precursors. Although fewer than 1 in 8300 of these foci progress to become tumors [87], the foci persist for long periods time, even in the absence of continued UVR exposure [85]. P53 mutations in UVR-induced skin tumors of hairless mice are primarily signature UVR mutations, C to T and CC to TT transitions at dipyrimidine sites, and are concentrated at particular hotspots in the p53 gene, especially in codon 270, a major hotspot for p53 mutation in nonmelanoma skin tumors of man [84-86]. In addition to the genetic changes that are hallmarks of skin carcinogenesis in hairless mice, there are also changes in global gene expression profiles. An interesting summary of such changes was provided by SAGE profiling of UVR-induced SCC in SKH1 hairless mice [87].
8. Conclusion
Hairless mice have been valuable experimental dermatology models for many years. Because the mice are hairless, depilation is not required before the initiation of wound healing or carcinogenesis studies or the application of toxic or therapeutic agents. The processes of wound healing, carcinogenesis, and inflammation are well characterized and readily observed in these mice. Hairless mice are particularly sensitive to the development of UVR-induced skin cancer, and develop epithelial tumors relevant to human skin cancer. However, the genetic background of many of the hairless mice used in dermatology studies is not well described. In addition, commonly used SKH1 hairless mice are outbred; these mice show considerable inter-individual variation and cannot be used for transplantation studies. Thus, hairless mice are often not suitable for the kinds of detailed genetic studies requiring inbred or genetically altered mice. Moreover, the immune responsiveness of hairless mice has been the subject of relatively little study, limiting their usefulness in studies of the skin immune system. A number of inbred hairless strains have been and are being developed, with an emphasis on developing mice carrying the hypomorphic hr allele. The widespread availability of inbred hairless mice on different genetic backgrounds will dramatically enhance the usefulness of hairless mice in many areas of experimental dermatology.
Acknowledgments
This work was supported by NIH grants #P30 CA16672 DHHS/NCI Cancer Center Support Grant to MD Anderson Cancer Center, #ES07784 NIEHS Center Grant, and NCI Training Grant #CA09480 (FB, DFK); #RO1 CA76598 and RO1 CA102340 (TMO); #R03 CA110054 (AMV); and #P30 CA16058 DHHS/NCI Cancer Center Support Grant to The Ohio State University (TMO, AMV, DFK). We thank Jennifer Thomas-Ahner for her valuable input into this manuscript.
Footnotes
Conflict of Interest Statement None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cachon-Gonzalez MB, Fenner S, Coffin JM, et al. Structure and expression of the hairless gene of mice. Proc Natl Acad Sci USA. 1994;91:7717–21. doi: 10.1073/pnas.91.16.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panteleyev AA, van der Veen C, Rosenbach T, et al. Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol. 1998;110:902–7. doi: 10.1046/j.1523-1747.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 3.Panteleyev AA, Botchkareva NV, Sundberg JP, et al. The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol. 1999;155:159–71. doi: 10.1016/S0002-9440(10)65110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djabali K, Aita VM, Christiano AM. Hairless is translocated to the nucleus via a novel bipartite nuclear localization signal and is associated with the nuclear matrix. J Cell Sci. 2001;114:367–76. doi: 10.1242/jcs.114.2.367. [DOI] [PubMed] [Google Scholar]
- 5.Potter GB, Beaudoin GM, 3rd, DeRenzo CL, et al. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev. 2001;15:2687–701. doi: 10.1101/gad.916701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cachón-González MB, San-José I, Cano A, et al. The hairless gene of the mouse: relationship of phenotypic effects with expression profile and genotype. Dev Dyn. 1999;216:113–26. doi: 10.1002/(SICI)1097-0177(199910)216:2<113::AID-DVDY3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Panteleyev AA, Paus R, Christiano AM. Patterns of hairless (hr) gene expression in mouse hair follicle morphogenesis and cycling. Am J Pathol. 2000;157:1071–9. doi: 10.1016/S0002-9440(10)64621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarach JM, Beaudoin GM, 3rd, Coulombe PA, et al. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development. 2004;131:4189–200. doi: 10.1242/dev.01303. [DOI] [PubMed] [Google Scholar]
- 9.Brooke HC. Hairless mice. J Hered. 1926;17:173–4. [Google Scholar]
- 10.Stoye JP, Fenner S, Greenoak GE, et al. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988;54:383–91. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad W, Panteleyev AA, Christiano AM. The molecular basis of congenital atrichia in humans and mice: mutations in the hairless gene. J Investig Dermatol Symp Proc. 1999;4:240–3. doi: 10.1038/sj.jidsp.5640220. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad W, Ratterree MS, Panteleyev AA, et al. Atrichia with papular lesions resulting from mutations in the rhesus macaque (Macaca mulatta) hairless gene. Lab Anim. 2002;36:61–7. doi: 10.1258/0023677021911777. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad W, Faiyaz U, Haque M, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279:720–4. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Panteleyev AA, Jahoda CA, et al. Genomic organization and analysis of the hairless gene in four hypotrichotic rat strains. Mamm Genome. 2004;15:975–81. doi: 10.1007/s00335-004-2383-3. [DOI] [PubMed] [Google Scholar]
- 15.Reeve VE, Greenoak GE, Boehm-Wilcox C, et al. Effect on topical 5-methoxypsoralen on tumorigenesis induced in albino and pigmented hairless mouse skin by UV irradiation. J Photochem Photobiol B. 1990;5:343–57. doi: 10.1016/1011-1344(90)85050-7. [DOI] [PubMed] [Google Scholar]
- 16.Sontag Y, Steerenberg P, Garssen J, et al. Time and dose dependence of acceptance of UV-induced syngeneic tumor implants in chronically UV-exposed hairless mice. Photochem Photobiol. 1997;65:342–6. doi: 10.1111/j.1751-1097.1997.tb08568.x. [DOI] [PubMed] [Google Scholar]
- 17.Cooper SJ, MacGowan J, Ranger-Moore J, et al. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;1:848–54. [PubMed] [Google Scholar]
- 18.Fischer SM, Pavone A, Mikulec C, et al. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinog. 2007;46:363–71. doi: 10.1002/mc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel A, Kopp-Schneider A, Zentgraf H, et al. E6/E7 expression of human papillomavirus type 20 (HPV-20) and HPV-27 influences proliferation and differentiation of the skin in UV-irradiated SKH-hr1 transgenic mice. J Virol. 2006;80:11153–64. doi: 10.1128/JVI.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cserhalmi-Friedman PB, Panteleyev AA, Christiano AM. Recapitulation of the hairless mouse phenotype using catalytic oligonucleotides: implications for permanent hair removal. Exp Dermatol. 2004;13:155–62. doi: 10.1111/j.0906-6705.2004.0143.x. [DOI] [PubMed] [Google Scholar]
- 21.Beaudoin GM, 3rd, Sisk JM, Coulombe PA, et al. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA. 2005;102:14653–8. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J, Djabali K, Chen T, et al. Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol. 2001;117:612–7. doi: 10.1046/j.0022-202x.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- 23.Sundberg JP. The Hairless (hr) and Rhino (hrrh) mutations, chromosome 14. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton: CRC Press; 1994. [Google Scholar]
- 24.Collaco AM, Geusz ME. Monitoring immediate-early gene expression through firefly luciferase imaging of HRS/J hairless mice. BMC Physiol. 2003;3:8. doi: 10.1186/1472-6793-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura H, Yasui H, Sakurai H. Generation and distribution of reactive oxygen species in the skin of hairless mice under UVA: studies on in vivo chemiluminescent detection and tape stripping methods. Exp Dermatol. 2006;15:891–9. doi: 10.1111/j.1600-0625.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 26.Bussau LJ, Vo LT, Delaney PM, et al. Fibre optic confocal imaging (FOCI) of keratinocytes, blood vessels and nerves in hairless mouse skin in vivo. J Anat. 1998;192:187–94. doi: 10.1046/j.1469-7580.1998.19220187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsayed MM, Abdallah OY, Naggar VF, et al. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007;332:1–16. doi: 10.1016/j.ijpharm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Hinz RS, Hodson CD, Lorence CR, et al. In vitro percutaneous penetration: evaluation of the utility of hairless mouse skin. J Invest Dermatol. 1989;93:87–91. doi: 10.1111/1523-1747.ep12277361. [DOI] [PubMed] [Google Scholar]
- 29.Kao J, Hall J, Helman G. In vitro percutaneous absorption in mouse skin: influence of skin appendages. Toxicol Appl Pharmacol. 1988;94:93–103. doi: 10.1016/0041-008x(88)90340-7. [DOI] [PubMed] [Google Scholar]
- 30.Bond JR, Barry BW. Limitations of hairless mouse skin as a model for in vitro permeation studies through human skin: hydration damage. J Invest Dermatol. 1988;90:486–9. doi: 10.1111/1523-1747.ep12460958. [DOI] [PubMed] [Google Scholar]
- 31.Yarosh DB. Liposomes in investigative dermatology. Photodermatol Photoimmunol Photomed. 2001;17:203–12. doi: 10.1034/j.1600-0781.2001.170501.x. [DOI] [PubMed] [Google Scholar]
- 32.Trainer AH, Alexander MY. Gene delivery to the epidermis. Hum Mol Genet. 1997;6:1761–7. doi: 10.1093/hmg/6.10.1761. [DOI] [PubMed] [Google Scholar]
- 33.Udvardi A, Kufferath I, Grutsch H, et al. Uptake of exogenous DNA via the skin. J Mol Med. 1999;77:744–50. doi: 10.1007/s001099900048. [DOI] [PubMed] [Google Scholar]
- 34.Yu WH, Kashani-Sabet M, Liggitt D, et al. Topical gene delivery to murine skin. J Invest Dermatol. 1999;112:370–5. doi: 10.1046/j.1523-1747.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 35.Kang MJ, Kim CK, Kim MY, et al. Skin permeation, biodistribution, and expression of topically applied plasmid DNA. J Gene Med. 2004;6:1238–46. doi: 10.1002/jgm.620. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Nolan E, Kreitschitz S, et al. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta. 2002;1572:1–9. doi: 10.1016/s0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 37.Kim HH, Choi HS, Yang JM, et al. Characterization of gene delivery in vitro and in vivo by the arginine peptide system. Int J Pharm. 2007;335:70–8. doi: 10.1016/j.ijpharm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Bramson J, Dayball K, Evelegh C, et al. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–60. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 39.Rojas IG, Padgett DA, Sheridan JF, et al. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- 40.Puhvel SM, Sakamoto M, Ertl DC, et al. Hairless mice as models for chloracne: a study of cutaneous changes induced by topical application of established chloracnegens. Toxicol Appl Pharmacol. 1982;64:492–503. doi: 10.1016/0041-008x(82)90247-2. [DOI] [PubMed] [Google Scholar]
- 41.Halliday GM, Reeve VE, Barnetson RS. Langerhans cell migration into ultraviolet light-induced squamous skin tumors is unrelated to anti-tumor immunity. J Invest Dermatol. 1991;97:830–4. doi: 10.1111/1523-1747.ep12491503. [DOI] [PubMed] [Google Scholar]
- 42.Hatton JL, Parent A, Tober KL, et al. Depletion of CD4+ cells exacerbates the cutaneous response to acute and chronic UVB exposure. J Invest Dermatol. 2007;127:1507–15. doi: 10.1038/sj.jid.5700746. [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Forbes PD, Linna TJ. Immune responses in nonhaired mice. Int Arch Allergy Appl Immunol. 1982;67:254–61. doi: 10.1159/000233027. [DOI] [PubMed] [Google Scholar]
- 44.Buerki H, Laissue J, Buchmann P, et al. Genetically determined disproportion between primary and secondary tetanus antitoxin responses and proliferative reactions in lymph nodes regional to the site of antigen injection: C57L/J compared with haired and hairless HRS/J mice. J Reticuloendothel Soc. 1981 Dec;30:531–8. [PubMed] [Google Scholar]
- 45.Morrissey PJ, Parkinson DR, Schwartz RS, et al. Immunologic abnormalities in HRS/J mice. I. Specific deficit in T lymphocyte helper function in a mutant mouse. J Immunol. 1980;125:1558–62. [PubMed] [Google Scholar]
- 46.Falabella AF, Falanga V. Wound healing. In: Freinkel RK, Woodley DT, editors. The biology of the skin. Pearl River: The Parthenon Publishing Group; 2001. [Google Scholar]
- 47.Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26:293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- 48.Uhl E, Rosken F, Sirsjo A, et al. Influence of platelet-derived growth factor on microcirculation during normal and impaired wound healing. Wound Repair Regen. 2003;11:361–7. doi: 10.1046/j.1524-475x.2003.11508.x. [DOI] [PubMed] [Google Scholar]
- 49.Forbes PD, Davies RE, Urbach F, et al. Simulated stratospheric ozone depletion and increased ultraviolet radiation: effects on photocarcinogenesis in hairless mice. Cancer Res. 1982;42:2796–803. [PubMed] [Google Scholar]
- 50.de Gruijl FR, Sterenborg HJ, Forbes PD, et al. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- 51.Reeve VE. Ultraviolet radiation and the contact hypersensitivity reaction in mice. Methods. 2002;28:20–4. doi: 10.1016/s1046-2023(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 52.Brown DB, Peritz AE, Mitchell DL, et al. Common fluorescent sunlamps are an inappropriate substitute for sunlight. Photochem Photobiol. 2000;72:340–4. [PubMed] [Google Scholar]
- 53.Wilgus TA, Ross MS, Parrett ML, et al. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat. 2000;62:367–84. doi: 10.1016/s0090-6980(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 54.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–68. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 55.Terui T, Okuyama R, Tagami H. Molecular events occurring behind ultraviolet-induced skin inflammation. Curr Opin Allergy Clin Immunol. 2001;1:461–7. doi: 10.1097/01.all.0000011061.54491.2e. [DOI] [PubMed] [Google Scholar]
- 56.Rivas JM, Ullrich SE. The role of IL-4, IL-10 and TNF- in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769–75. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 57.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Wilgus TA, Koki AT, Zweifel BS, et al. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 59.Kripke ML. Photoimmunology: the first decade. Curr Probl Dermatol. 1986;15:164–75. doi: 10.1159/000412100. [DOI] [PubMed] [Google Scholar]
- 60.de Gruijl FR. UV-induced immunosuppression in the balance. Photochem Photobiol. 2008;84:2–9. doi: 10.1111/j.1751-1097.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim TH, Ananthaswamy HN, Kripke ML, et al. Advantages of using hairless mice versus haired mice to test sunscreen efficacy against photoimmune suppressions. Photochem Photobiol. 2003;78:37–42. doi: 10.1562/0031-8655(2003)078<0037:aouhmv>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Farage MA, Miller KW, Elsner P, et al. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 63.Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 64.Kligman LH. The hairless mouse model for photoaging. Clin Dermatol. 1996;14:183–95. doi: 10.1016/0738-081x(95)00154-8. [DOI] [PubMed] [Google Scholar]
- 65.Kambayashi H, Yamashita M, Odake Y, et al. Epidermal changes caused by chronic low-dose UV irradiation induce wrinkle formation in hairless mouse. J Dermatol Sci. 2001;27:S19–25. doi: 10.1016/s0923-1811(01)00113-x. [DOI] [PubMed] [Google Scholar]
- 66.Moloney SJ, Edmonds SH, Giddens LD, et al. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem Photobiol. 1992;56:505–11. doi: 10.1111/j.1751-1097.1992.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 67.Slaga TJ, Budunova IV, Gimenez-Conti IB, et al. The mouse skin carcinogenesis model. J Investig Dermatol Symp Proc. 1996;1:151–6. [PubMed] [Google Scholar]
- 68.Klein-Szanto AJ, Larcher F, Bonfil RD, et al. Multistage chemical carcinogenesis protocols produce spindle cell carcinomas of the mouse skin. Carcinogenesis. 1989;10:2169–72. doi: 10.1093/carcin/10.11.2169. [DOI] [PubMed] [Google Scholar]
- 69.Canfield PJ, Greenoak GE, Reeve VE, et al. Characterization of UV induced keratoacanthoma-like lesions in HRA/Skh-1 mice and their comparison with keratoacanthomas in man. Pathology. 1985;17:613–6. doi: 10.3109/00313028509084762. [DOI] [PubMed] [Google Scholar]
- 70.De Gruijl FR, van der Leun JC. Action spectra for carcinogenesis. In: Urbach F, editor. Biological responses to ultraviolet A radiation. Overland Park: Valdenmar Publishing Company; 1992. [Google Scholar]
- 71.Van Weelden H, de Gruijl FR, van der Leun JC. Tumors induced by UV-A in mice. Photochem Photobiol. 1983;37:S79. [Google Scholar]
- 72.Kligman LH, Crosby MJ, Miller SA, et al. Carcinogenesis by long wavelength UVA and failure of reciprocity. In: Urbach F, editor. Biological responses to ultraviolet A radiation. Overland Park: Valdenmar Publishing Company; 1992. [Google Scholar]
- 73.Sterenborg HJCM, van der Leun JC. Tumorigenesis by a long wavelength UV-A source. Photochem Photobiol. 1990;51:325–30. doi: 10.1111/j.1751-1097.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 74.Van Weelden H, van der Leun JC. Photorecovery by UVA. In: Urbach F, Gange RW, editors. The biological effects of UVA radiation. New York: Praeger; 1986. [Google Scholar]
- 75.Forbes PD, Davies RE, Urbach F. Experimental ultraviolet carcinogenesis: wavelength interactions and time-dose relationships. Natl Cancer Inst Monograph. 1978;50:31–8. [PubMed] [Google Scholar]
- 76.Bech-Thomsen N, Wulf HCF, Poulsen T, et al. Pretreatment with long-wave ultraviolet light inhibits ultraviolet-induced skin tumor development in hairless mice. Arch Dermatol. 1988;124:1215–8. [PubMed] [Google Scholar]
- 77.Berton TR, Fischer SM, Conti CJ, et al. Comparison of ultraviolet light-induced skin carcinogenesis and ornithine decarboxylase activity in sencar and hairless SKH-1 mice fed a constant level of dietary lipid varying in corn and coconut oil. Nutr Cancer. 1996;26:353–63. doi: 10.1080/01635589609514491. [DOI] [PubMed] [Google Scholar]
- 78.Davies RE, Forbes PD. Effect of UV radiation on survival of non-haired mice. Photochem Photobiol. 1986;43:267–74. doi: 10.1111/j.1751-1097.1986.tb05604.x. [DOI] [PubMed] [Google Scholar]
- 79.de Gruijl FR, Forbes PD. UV-induced skin cancer in a hairless mouse model. Bioessays. 1995;17:651–60. doi: 10.1002/bies.950170711. [DOI] [PubMed] [Google Scholar]
- 80.Thomas-Ahner JM, Wulff BC, Tober KL, et al. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67:3468–74. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- 81.Steinel HH, Baker RS. Sensitivity of HRA/Skh hairless mice to initiation/promotion of skin tumors by chemical treatment. Cancer Lett. 1988;41:63–8. doi: 10.1016/0304-3835(88)90055-9. [DOI] [PubMed] [Google Scholar]
- 82.Widyarini S, Husband AJ, Reeve VE. Protective effect of the isoflavonoid equol against hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical cocarcinogen. Photochem Photobiol. 2005;81:32–7. doi: 10.1562/2004-06-02-RA-183. [DOI] [PubMed] [Google Scholar]
- 83.Sundberg JP, Sundberg BA, Beamer WG. Comparison of chemical carcinogen skin tumor induction efficacy in inbred, mutant, and hybrid strains of mice: morphologic variations of induced tumors and absence of a papillomavirus cocarcinogen. Mol Carcinog. 1997;20:19–32. doi: 10.1002/(sici)1098-2744(199709)20:1<19::aid-mc4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 84.van Kranen HJ, de Gruijl FR, de Vries A. Frequent p53 alterations but low incidence of ras mutations in UV-B-induced skin tumors of hairless mice. Carcinogenesis. 1995;16:1141–7. doi: 10.1093/carcin/16.5.1141. [DOI] [PubMed] [Google Scholar]
- 85.Melnikova VO, Pacifico A, Chimenti S, et al. Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: relationship to skin cancer development. Oncogene. 2005;24:7055–63. doi: 10.1038/sj.onc.1208863. [DOI] [PubMed] [Google Scholar]
- 86.Rebel H, Kram N, Westerman A, et al. Relationship between UV-induced mutant p53 patches and skin tumours, analysed by mutation spectra and by induction kinetics in various DNA-repair-deficient mice. Carcinogenesis. 2005;26:2123–30. doi: 10.1093/carcin/bgi198. [DOI] [PubMed] [Google Scholar]
- 87.Rundhaug JE, Hawkins KA, Pavone A, et al. SAGE profiling of UV-induced mouse skin squamous cell carcinomas, comparison with acute UV irradiation effects. Mol Carcinog. 2005;42:40–52. doi: 10.1002/mc.20064. [DOI] [PubMed] [Google Scholar]
- 88.Moraitis AN, Giguère V, Thompson CC. Novel mechanism of nuclear receptor corepressor interaction dictated by activation function 2 helix determinants. Mol Cell Biol. 2002;22:6831–41. doi: 10.1128/MCB.22.19.6831-6841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moraitis AN, Giguère V. The co-repressor hairless protects RORalpha orphan nuclear receptor from proteasome-mediated degradation. J Biol Chem. 2003;278:52511–8. doi: 10.1074/jbc.M308152200. [DOI] [PubMed] [Google Scholar]
- 90.Djabali K, Christiano AM. Hairless contains a novel nuclear matrix targeting signal and associates with histone deacetylase 3 in nuclear speckles. Differentiation. 2004;72:410–8. doi: 10.1111/j.1432-0436.2004.07208007.x. [DOI] [PubMed] [Google Scholar]