Abstract
Background
Mutations in genes encoding A-type lamins and emerin cause cardiomyopathy and muscular dystrophy. We previously showed activation of the extracellular signal-regulated kinase (ERK) branch of the mitogen-activated protein kinase (MAPK) cascade in hearts of mice with mutations in these genes. Here, we tested the hypothesis that reducing A-type lamins and emerin in cultured cells activate ERK signaling.
Methods
We used siRNA to knockdown A-type lamins and emeirn in HeLa and C2C12 cells. Activation of ERK was assessed by immunoblotting and immunofluorescence microscopy with antibodies against phosphorylated protein and by using real-time RT-PCR to measure RNAs encoded by genes for transcription factors stimulated by ERK.
Results
Knockdown of A-type lamins and emerin in HeLa and C2C12 stimulated phosphorylation and nuclear translocation of ERK as well as activation of genes encoding downstream transcription factors. A MAPK/ERK kinase (MEK) inhibitor reduced ERK phosphorylation in cells with reduced expression of A-type lamins and emerin.
Conclusions
These results provide proof for the hypothesis that altered expression of emerin and A-type lamins activates ERK signaling, which in turn can cause cardiomyopathy.
General Significance
ERK is a potential target for the pharmacological treatment of cardiomyopathy caused by mutations in the genes encoding emerin and A-type lamins.
Keywords: nuclear envelope, nuclear lamina, lamin, emerin, Emery-Dreifuss muscular dystrophy
1. Introduction
The nuclear envelope is composed of the nuclear membranes, the nuclear pore complexes and the nuclear lamina. The nuclear lamina is a meshwork of intermediate filament proteins call lamins and is primarily associated with the inner aspect of the inner nuclear membrane [1]. Clinical investigations over the past several years have shown that mutations in the genes encoding lamins and associated proteins of the inner nuclear membrane cause diverse diseases often referred to as laminopathies or nuclear envelopathies [2,3].
Emery-Dreifuss muscular dystrophy is characterized by skeletal muscle weakness and wasting in a humeroperoneal distribution, joint contractures and dilated cardiomyopathy with conduction defects [4,5]. The same clinical condition is inherited in an X-linked and an autosomal manner [4,5]. Mutations in EMD are the cause of X linked Emery-Dreifuss muscular dystrophy [6]. EMD encodes emerin, an integral protein of the inner nuclear membrane that is absent or has reduced expression in most cases of X-linked Emery-Dreifuss muscular dystrophy [6–8]. Mutations in LMNA cause autosomal dominant Emery-Dreifuss muscular dystrophy and more rare recessive cases [9,10]. LMNA encodes A-type nuclear lamins [11]. Most LMNA mutations causing Emery-Dreifuss muscular dystrophy generate single amino acid substitutions or deletions in A-type lamins but haploinsufficiency can also cause the disease [9,12–14]. While EMD and LMNA mutations were initially shown to cause the Emery-Dreifuss phenotype, the same mutations in these genes can cause cardiomyopathy with different, minimal or no apparent skeletal muscle involvement [12–18].
The molecular mechanism underlying how deficiency in emerin or A-type lamins causes striated muscle diseases is poorly understood. We previously identified abnormal activation of the extracellular signal-regulated kinase (ERK) and c-jun-N-terminal kinase (JNK) branches of the mitogen-activated protein kinase (MAPK) signaling pathway in hearts of Lmna H222P “knock in” mice, a model of autosomal Emery-Dreifuss muscular dystrophy [19]. We also observed activation of the ERK in hearts of emerin-deficient Emd−/y mice, a model of X-linked Emery-Dreifuss muscular dystrophy [20]. These studies suggested a connection between abnormalities in proteins of the nuclear envelope and a cell signaling pathway implicated in the pathogenesis of cardiomyopathy in tissues. However, data from model cellular systems have been limited to the demonstration that ERK and JNK are activated in transfected cultured cells overexpressing lamin A variants in striated muscle diseases [19]. Experiments showing that reducing expression of A-type lamins or emerin in cultured cells directly activates a MAPK are critical, as positive results would support the hypothesis that MAPK activation is a primary event rather than a secondary one occurring as a consequence of cardiomyopathy. We therefore tested the hypothesis that reducing A-type lamins and emerin in cultured cells activate ERK.
2. Materials and methods
HeLa cells and C2C12 cells were maintained in a 5% CO2 atmosphere at 37°C. Cells were cultured in Dulbeco’s modified Eagle’s medium supplemented with 10% calf bovine serum and 0.1% gentamicin. One day before transfection, HeLa and C2C12 cells were trypsinized, diluted with fresh medium without antibiotics and transferred to 24-well plates. Transient transfection of siRNAs (50 pM final concentration) was carried out using Oligofectamine (Invitrogen) as recommended by the manufacturer. Cells were assayed 72 hours after transfection for HeLa cells and 48 hours after transfection for C2C12 cells. Reduction of expression of targeted genes was confirmed in at least 3 independent experiments.
For measuring gene expression in cultured cells, media was removed from cultures reaching 80% and total RNA was extracted using the Rneasy isolation kit (Qiagen) according to the manufacturer’s instructions. Adequacy and integrity of extracted RNA were determined by gel electrophoresis. Concentrations were measured by ultraviolet absorbance spectroscopy. For real-time RT-PCR, RNA was reverse transcribed using SuperScript First-Strand Synthesis System according to the manufacturer’s instructions (Invitrogen). Real-time RT-PCR was performed as previously described [19,20]. Specificity of amplification was checked by melting-curve analysis. Relative levels of mRNA expression were calculated according to the Δ ΔCT method, normalized by comparison to Gapdh mRNA expression [21].
For immunoblotting, proteins were extracted from cells as previously described [19,20], separated by SDS-PAGE, transferred to nitrocellulose membranes and blotted with primary antibodies against ERK1/2 (Santa-Cruz), pERK1/2 (Cell Signaling), lamin A/C (Santa-Cruz), emerin (Novocatra), β-actin (Santa-Cruz) and Gapdh (Santa-Cruz). Secondary antibodies were horseradish peroxidase–conjugated (Amersham). Recognized proteins were visualized by enhanced chemiluminescence (ECL-Amersham) and visualized using Hyperfilm ECL (Amersham). Signal generated using antibody against β-actin was used as an internal control to normalize amounts of protein between immunoblots. Band densities were calculated using Scion Image software (Scion Corporation) and normalized to the appropriate total extract to control for protein loading. Data are reported as means ± standard deviations and compared with respective controls using a two-tailed t test.
For immunofluorescence microscopy, HeLa and C2C12 cells were grown on coverslips and washed with phosphate-buffered saline. Cells were fixed for 10 minutes in methanol at −20°C. HeLa and C2C12 cells were then incubated at room temperature with antibody against phosphorylated ERK (pERK) (Cell Signaling). Cells were then washed with phosphate-buffered saline and incubated with Texas Red conjugated goat anti-rabbit antibody in PBS (Molecular Probes). Cells were washed with phosphate-buffered saline and slides mounted in Mowiol (Santa-Cruz) with 0.1 μg/ml 4′,6-diamidino-2-phenylindole. Immunofluorescence microscopy was performed using an Axiophot microscope (Carl Zeiss). Micrographs were processed using Adobe Photoshop 6.0 (Adobe Systems).
For colorimetric analysis of ERK1/2 phosphorylation, cells were cultured for 24 hours in the presence of PD98059 (45 μM). ERK1/2 phosphorylation was measured using an enzyme linked immunosorbent assay (SuperArray CASE, ERK1/2 Kit) as per the manufacturer’s protocol. Signal intensities for pERK1/2 or total ERK1/2 were measured at an optical density (OD) of 450 nm and relative cell number was assayed in each well (OD of 595 nm). To determine ERK1/2 phosphorylation, we normalized the pERK1/2 signal ratio (OD450nm/OD595nm) to the total ERK1/2 signal ratio (OD450nm/OD595nm). Data are reported as means ± standard deviations and compared with respective controls using a two-tailed t test.
3. Results
To investigate if reduction of A-type lamins and emerin lead to activation of ERK signaling, we used a human epithelial cell line (HeLa cells) and a mouse myogenic cell line (C2C12 cells) and knocked down targeted genes using siRNA. Total RNAs and proteins were extracted from HeLa cells cultured without siRNA treatment (mock) or treated with Gapdh, Emd and Lmna siRNAs. When Gapdh, Emd and Lmna siRNAs were used, corresponding mRNAs (Fig. 1A) and proteins (Fig. 1B) were reduced by approximately 50%. In C2C12 cells, total RNA and proteins were extracted after mock treatment or treatment with Gapdh, Emd and Lmna siRNAs. When Gapdh, Emd and Lmna siRNAs were used, corresponding mRNAs (Fig. 1C) and proteins (Fig. 1D) were reduced by approximately 50%. Treatment with Lmna siRNA also led to modest but reproducible decreases in cellular emerin (Fig. 1C,D).
Fig. 1.
Knockdown of emerin and A-type lamins using siRNA. (A) Expression of mRNA encoded by Gapdh, Emd and Lmna in HeLa cells transfected with siRNA duplexes, using real-time quantitative RT-PCR. Bars indicate fold overexpression of the indicated mRNA. Values are means ± standard deviations for n=4 samples (*p<0.05). (B) Immunoblot showing expression of GAPDH, emerin and lamin A/C in HeLa cells transfected with siRNA duplexes. Antibody against actin was used as a loading control. (C) Expression of mRNA encoded by Gapdh, Emd and Lmna in C2C12 cells transfected with siRNA duplexes, using real-time quantitative RT-PCR. Bars indicate fold overexpression of the indicated mRNA. Values are means ± standard deviations for n=3 samples (*p<0.05). (D) Immunoblot showing expression of GAPDH, emerin and lamin A/C in C2C12 cells transfected with siRNA duplexes. Antibody against actin was used as a loading control.
To determine if siRNA treatment lead to ERK activation, we first evaluated phosphorylation of ERK isoforms 1 and 2 (ERK1/2). Immunoblotting with anti-pERK1/2 antibody demonstrated an increase in pERK1/2 in HeLa cells treated with Emd and Lmna siRNAs, whereas no significant increase was observed in mock treated cells or cells treated with Gapdh siRNA (Fig. 2A). pERK1/2 activates a series of downstream target genes, including those encoding c-Jun, Elk1 and Elk4. We analyzed expression of these transcripts using real-time quantitative RT-PCR. While these individual genes were not found to be significantly differentially expressed in mock treated cells or cells treated with Gapdh siRNA (Fig. 2B), treatment with Emd and Lmna siRNAs lead to 2-fold to 3-fold increased expression of c-Jun, Elk1 and Elk4 (Fig. 2B). Abnormal ERK1/2 activation was also observed in C2C12 cells treated with Emd or Lmna siRNAs (Fig. 2C). We also found 1.5-fold to 2-fold up-regulation of c-Jun when C2C12 cells were treated with Emd and Lmna siRNAs (Fig. 2D). In C2C12 cells, expression of Elk1 and Elk4 was increased approximately 1.5-fold only when treated with Lmna siRNA but not Emd siRNA (Fig. 2D).
Fig. 2.
Knockdown of emerin and A-type lamins leads to phosphorylation of ERK and increased expression of downstream genes. (A) Representative immunoblot showing expression of total ERK1/2 and pERK1/2 in HeLa cells transfected with siRNA duplexes. (B) Expression of downstream genes in ERK pathway in HeLa cells transfected with siRNA duplexes. Real-time RT-PCR results for c-Jun, Elk1 and Elk4 are shown. Bars indicate the fold overexpression of the indicated mRNA normalized to Gapdh. Values are means ± standard deviations for n=4 samples per group (*p < 0.05). (C) Representative immunoblot showing expression of total ERK1/2 and pERK1/2 in C2C12 cells transfected with siRNA duplexes. (D) Expression of downstream genes in ERK pathway in C2C12 cells transfected with siRNA duplexes. Real-time RT-PCR results for c-Jun, Elk1 and Elk4 are shown. Bars indicate the fold overexpression of the indicated mRNA normalized to Gapdh. Values are means ± standard deviations for n=4 samples per group (*p < 0.05).
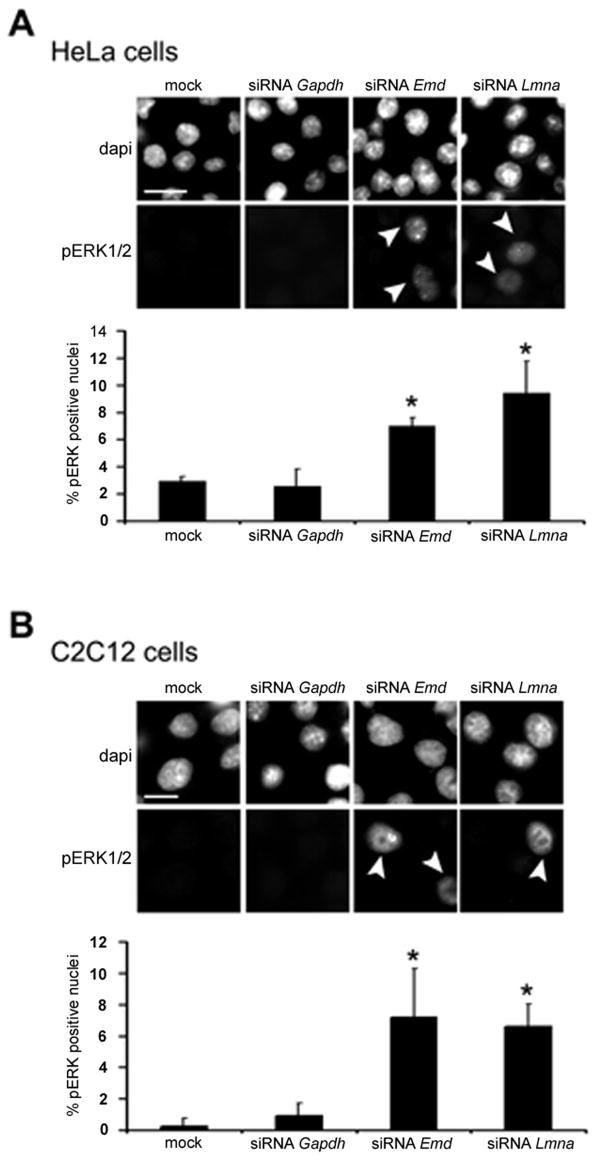
Translocation of pERK1/2 from cytoplasm to nucleus is necessary for activation of downstream genes. In mock treated HeLa cells and HeLa cells treated with Gapdh siRNA, pERK was weakly or not detectable and only approximately 2% of HeLa cells had detectable nuclear pERK (Fig. 3A). In contrast, treatment with Emd and Lmna siRNAs induced detectable levels of pERK in the nucleus in significantly more cells (Fig. 3A, arrowheads). Approximately 8% of HeLa cells treated with Emd siRNA and 10% of HeLa cells treated with Lmna siRNA had detectable nuclear pERK (Fig. 3A). In mock treated C2C12 cells and C2C12 cells treated with Gapdh siRNA, pERK was detectable in less than 1% of nuclei (Fig. 3B). When C2C12 cells were treated with Emd or Lmna siRNAs, there was a significant increase of cells (approximately 6%) with detectable nuclear pERK (Fig. 3B, arrowheads). Hence, knocking down expression of Emd and Lmna induces phosphorylation of ERK, nuclear translocation of pERK and activation of downstream targets.
Fig. 3.
Knockdown of emerin and A-type lamins leads to enhanced nuclear translocation of pERK. (A) Effect of siRNAs on nuclear translocation of pERK in transfected HeLa cells. Representative photomicrographs are shown for mock transfected cells, cells transfected with siRNA against Gapdh (siRNA Gapdh), Emd (siRNA Emd) and Lmna (siRNA Lmna). Top panels show labeling of nuclei with 4′,6-diamidino-2-phenylindole (dapi) and bottom panels labeling with antibodies against pERK1/2. Arrowheads show enhanced nuclear localization of pERK in cells transfected with Emd and Lmna siRNAs. Bar: 10 μm. Bar graph shows percentages of HeLa cells with pERK primarily in the nucleus (see arrowheads for example). Values are means ± standard deviations for n=200 cells per group (*p<0.05). (B) Effect of siRNAs on nuclear translocation of pERK in transfected C2C12 cells. Representative photomicrographs are shown for mock transfected cells, cells transfected with siRNA against Gapdh (siRNA Gapdh), Emd (siRNA Emd) and Lmna (siRNA Lmna). Top panels show labeling of nuclei with 4′,6-diamidino-2-phenylindole (dapi) and bottom panels labeling with antibodies against pERK1/2. Arrowheads show enhanced nuclear localization of pERK in cells transfected with Emd and Lmna siRNAs. Bar: 10 μm. Bar graph shows percentages of C2C12 cells with pERK primarily in the nucleus (see arrowheads for example). Values are means ± standard deviations for n=150 cells per group (*p<0.05).
MAPK/ERK kinase (MEK) is immediately upstream of ERK in this branch of the MAPK signaling cascade. MEK phosphorylates ERK to activate it. We analyzed the effect of the MEK inhibitor PD98059 on ERK1/2 activity in HeLa and C2C12 cells treated with siRNA against Emd and Lmna. Cells were cultured with or without PD98059 at a concentration of 45 μM for 24 hours. Immunoblotting with anti-pERK1/2 antibody demonstrated that the increase in pERK in HeLa and C2C12 cells treated with Emd and Lmna siRNAs was reduced with PD98059 added to the culture medium (Fig. 4A and 4B). Results from an enzyme-linked immunosorbent assay also confirmed that PD98059 inhibited activation of ERK1/2 by in the siRNA-treated cells (Fig. 4B).
Fig. 4.
MEK inhibitor PD98059 blocks ERK activation in cells with reduced emerin and A-type lamins. (A) Immunoblot showing effect of the MEK inhibitor PD98059 on the expression of total ERK1/2 and phosphorylated ERK1/2 in HeLa cells transfected with siRNAs against Gapdh, Lmna and Emd. (B) Upper part is an immunoblot showing effect of the MEK inhibitor PD98059 on the expression of total ERK1/2 and phosphorylated ERK1/2 in C2C12 cells transfected with siRNAs against Gapdh, Lmna and Emd. Lower part shows results of enzyme-linked immunosorbant assay showing effect of the MEK inhibitor PD98059 on the expression of total ERK1/2 and phosphorylated ERK1/2 in C2C12 cells transfected with siRNAs against Gapdh, Lmna and Emd. Bar graph shows the relative phosphorylation of ERK1/2. Values are means ± standard deviations for n=3 samples per group (*p<0.05 when compared to mock treatment, #p<0.05 when compared C2C12 cells with or without addition of PD98059).
4. Discussion
Our previous studies have shown that a missense mutation in LMNA encoding Atype lamins and loss emerin activate MAPKs in the hearts of genetically modified mice [19,20]. We have analyzed affected and unaffected tissues in LmnaH222P/H222P mice and found abnormal activation of genes downstream of ERK only in cardiac and to a more limited extent in skeletal muscle [19]. We have similarly demonstrated abnormal activation of ERK and downstream genes in hearts of emerin-deficient mice [20]. We have also demonstrated previously activation of MAPK signaling in transfected cultured cells overexpressing lamin A variants with amino acid substitutions found in subjects with Emery-Dreifuss muscular dystrophy [19]. Here we showed an aberrant increase of ERK activation and downstream transcription factors in siRNA-treated HeLa and C2C12 cells with decreased emerin and A-type lamin expression. It is unclear why in LmnaH222P/H222P mice ERK activation was observed only in cardiac and to a more limited extent in skeletal muscle while in the present study reduction of A-type lamins and emerin activated ERK in both myogenic and epithelial cell lines. This could potentially be explained by the fact the ERK activation is influenced by the composition of the extracellular matrix [22–24].
The present results show that decreasing the expression of A-type lamins and emerin activate MAPKs. We have also previously shown that expressing lamin A variants that cause cardiomyopathy in transfected cultured cells activate ERK [19]. These results, along with evidence of MAPK activation in hearts of LmnaH222P/H222P mice prior to the presence of clinically detectable cardiomyopathy [19], strongly suggest that abnormalities in nuclear envelope proteins are a cause of rather than a consequence of cardiomyopathy. Induced activation of ERK in hearts of transgenic mice induces a hypertrophic response and sustained hypertrophy is a leading predictor of heart failure [25]. Hence, the present results provide proof for the hypothesis that nuclear envelope abnormalities resulting from alterations in emerin and A-type lamin expression activate ERK that in turn contributes to the development of cardiomyopathy.
While most of the human LMNA mutations causing cardiomyopathy and skeletal muscle disease are missense, some patients carry nonsense mutations leading to haploinsufficiency [9,12–14]. Therefore, it was physiologically relevant to show that reductions in A-type lamins, as well as expression lamin variants [19], can activate ERK. While most mutations in EMD lead to a loss of emerin, some that cause Emery-Dreifuss muscular dystrophy lead to stable expression of emerin with amino acid substitutions [6–8,26,27]. It would be interesting to determine in future studies if expression of rare emerin variants with amino acid substitutions found in patients with Emery-Dreifuss muscular dystrophy similarly activate ERK.
The mechanism responsible for the activation of ERK in cells with abnormalities in A-type lamins and emerin remains to be determined experimentally. A-type lamins and emerin interact and depletion of A-type lamins leads to a redistribution from the inner nuclear membrane to the bulk endoplasmic reticulum [28–31]. Expression of some lamin A variants that cause cardiomyopathy and muscular dystrophy also leads to redistribution of some emerin from the nuclear envelope to the bulk endoplasmic reticulum [32,33]. Redistribution of emerin to the bulk endoplasmic reticulum is further associated with proteasome-mediated degradation of emerin [34] and cellular emerin levels were modestly reduced in the present study in cells treated with Lmna siRNA. Hence, loss of at least some emerin from the nuclear envelope along with decreased steady-state cellular levels may be common phenomena in striated muscle diseases caused by LMNA and EMD mutations.
Our results have potential practical implications because small molecule drugs can be used to inhibit ERK. The inhibitor we used, PD98059, is a selective inhibitor of MEK [35]. It mediates its inhibitory effects on ERK signaling by preventing phosphorylation of ERK. In this study, we showed that PD98059 reduces ERK1/2 activity in HeLa and C2C12 cells with reduced A-type lamins and emerin. We have recently shown that systemic administration of PD98059 prevents or delays the development of cardiomyopathy in LmnaH222P/H222P mice [36]. Hence, pharmacological inhibition of ERK signaling may also be a potential treatment of cardiomyopathy caused by mutations inducing A-type lamin haploinsufficiency or emerin loss in human subjects.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR048999) and from the Muscular Dystrophy Association (MDA4289) to H.J.W.
List of abbreviations
- ERK
extracellular signal-regulated kinase
- JNK
c-jun-N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- OD
optical density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Worman HJ, Courvalin JC. The inner nuclear membrane. J Membr Biol 2000. 2000;177:1–11. doi: 10.1007/s002320001096. [DOI] [PubMed] [Google Scholar]
- 2.Muchir A, Worman HJ. The nuclear envelope and human disease. Physiology. 2004;19:309–314. doi: 10.1152/physiol.00022.2004. [DOI] [PubMed] [Google Scholar]
- 3.Worman HJ, Bonne G. Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery AEH. Emery-Dreifuss muscular dystrophy - a 40 year retrospective. Neuromusc Disord. 2000;10:228–232. doi: 10.1016/s0960-8966(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 5.Muchir A, Worman HJ. Emery-Dreifuss muscular dystrophy. Curr Neurol Neurosci Rep. 2007;7:78–83. doi: 10.1007/s11910-007-0025-3. [DOI] [PubMed] [Google Scholar]
- 6.Bione S, Maestrini E, Rivella S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 7.Nagano A, Koga R, Ogawa M, et al. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat Genet. 1996;12:254–259. doi: 10.1038/ng0396-254. [DOI] [PubMed] [Google Scholar]
- 8.Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum Mol Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- 9.Bonne G, Di Barletta MR, Varnous S, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 10.Di Barletta MR, Ricci E, Galluzzi G, et al. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2000;66:1407–1412. doi: 10.1086/302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 12.Bonne G, Mercuri E, Muchir A, et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000;48:170–180. [PubMed] [Google Scholar]
- 13.Sébillon P, Bouchier C, Bidot LD, et al. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J Med Genet. 2003;40:560–567. doi: 10.1136/jmg.40.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetti S, Menditto I, Degano M, et al. Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology. 2007;69:1285–1292. doi: 10.1212/01.wnl.0000261254.87181.80. [DOI] [PubMed] [Google Scholar]
- 15.Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 16.Muchir A, Bonne G, van der Kooi AJ, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky GL, Muntoni F, Miocic S, Sinagra G, Sewry C, Mestroni L. Lamin A/C gene mutation associated with dilated cardiomyopathy with variable skeletal muscle involvement. Circulation. 2000;101:473–476. doi: 10.1161/01.cir.101.5.473. [DOI] [PubMed] [Google Scholar]
- 18.Astejada MN, Goto K, Nagano A, et al. Emerinopathy and laminopathy clinical, pathological and molecular features of muscular dystrophy with nuclear envelopathy in Japan. Acta Myol. 2007;26:159–164. [PMC free article] [PubMed] [Google Scholar]
- 19.Muchir A, Pavlidis P, Decostre V, et al. Activation of MAPK pathway links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchir A, Pavlidis P, Bonne G, Hayashi Y, Worman HJ. Activation of MAPK in hearts of Emd null mice: similarities between mouse models of X-linked and autosomal dominant Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 2007;16:1884–1895. doi: 10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- 21.Ponchel F, Toomes C, Bransfield K, et al. Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reusch HP, Chan G, Ives HE, Nemenoff RA. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun. 1997;237:239–244. doi: 10.1006/bbrc.1997.7121. [DOI] [PubMed] [Google Scholar]
- 23.Kudirka JC, Panupinthu N, Tesseyman MA, Dixon SJ, Bernier SM. P2Y nucleotide receptor signaling through MAPK/ERK is regulated by extracellular matrix: involvement of beta3 integrins. J Cell Physiol. 2007;213:54–64. doi: 10.1002/jcp.21087. [DOI] [PubMed] [Google Scholar]
- 24.Hong H, McCullough CM, Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28:3824–3833. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 26.Yates JR, Bagshaw J, Aksmanovic VM, et al. Genotype-phenotype analysis in X-linked Emery-Dreifuss muscular dystrophy and identification of a missense mutation associated with a milder phenotype. Neuromuscul Disord. 1999;9:159–165. [PubMed] [Google Scholar]
- 27.Ellis JA, Yates JR, Kendrick-Jones J, Brown CA. Changes at P183 of emerin weaken its protein-protein interactions resulting in X-linked Emery-Dreifuss muscular dystrophy. Hum Genet. 1999;104:262–268. doi: 10.1007/s004390050946. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan T, Escalante-Alcalde D, Bhatt H, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairley EA, Kendrick-Jones J, Ellis JA. The Emery-Dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane. J Cell Sci. 1999;112:2571–2582. doi: 10.1242/jcs.112.15.2571. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya Y, Hase A, Ogawa M, Yorifuji H, Arahata K. Distinct regions specify the nuclear membrane targeting of emerin, the responsible protein for Emery-Dreifuss muscular dystrophy. Eur J Biochem. 1999;259:859–865. doi: 10.1046/j.1432-1327.1999.00112.x. [DOI] [PubMed] [Google Scholar]
- 31.Clements L, Manilal S, Love DR, Morris GE. Direct interaction between emerin and lamin A. Biochem Biophys Res Commun. 2000;267:709–714. doi: 10.1006/bbrc.1999.2023. [DOI] [PubMed] [Google Scholar]
- 32.Östlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J Cell Sci. 2001;114:4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 33.Raharjo WH, Enarson P, Sullivan T, Stewart CL, Burke B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J Cell Sci. 2001;114:4447–4457. doi: 10.1242/jcs.114.24.4447. [DOI] [PubMed] [Google Scholar]
- 34.Muchir A, Massart C, van Engelen BG, Lammens M, Bonne G, Worman HJ. Proteasome-mediated degradation of integral inner nuclear membrane protein emerin in fibroblasts lacking A-type lamins. Biochem Biophys Res Commun. 2006;351:1011–1017. doi: 10.1016/j.bbrc.2006.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 36.Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn343. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]