Abstract
Despite the fact that many nuclear receptors are ligand dependent, the existence of obligate regulatory ligands is debated for some receptors, including steroidogenic factor 1 (SF-1). Although fortuitously bound bacterial phospholipids were discovered in the structures of the SF-1 ligand-binding domain (LBD), these lipids might serve merely as structural ligands. Thus, we examined whether exogenously added phospholipids would exchange for these bacterial lipids and bind to SF-1. Here, we report the first crystal structure of the SF-1 LBD bound by the exchanged phosphatidylcholine. Although the bound phosphatidylcholine phospholipid mimics the conformation of bound bacterial phosphoplipids, two surface loops, L2-3 and L11-12, surrounding the entrance to the pocket vary significantly between different SF-1 LBD structures. Based on this observation, we hypothesized that a bound ligand might control the conformations of loops L2-3 and L11-12, and that conserved residues in these dynamic loops could influence ligand binding and the receptor function. Consistent with this hypothesis, impaired phospholipid exchange and diminished transcriptional activity were observed for loop L11-12 SF-1 mutants and for the loop L2-3 human mutant R255L. The endocrine disease associated with this L2-3 mutation coupled with our cellular and biochemical data suggest that critical residues at the mouth of the ligand-binding pocket have evolved for efficient binding of phospholipid ligands and for achieving optimal SF-1 activity.
The crystal structure of SF-1 bound by an exchanged-exogenous phospholipid ligand reveals two variable surface loops important for both ligand uptake and transcriptional activity.
Steroidogenic factor-1 (SF-1) is a member of the nuclear receptor (NR) subfamily NR5A. This transcription factor is critical for development of multiple endocrine organs and, as such, is required for reproduction, energy homeostasis, and for mounting a normal stress response. SF-1 also coordinately regulates a network of genes required for steroidogenesis and peptide hormone signaling and is important for male sexual differentiation (1). In contrast to nuclear receptors that activate gene transcription after hormone binds, SF-1 activating hormones have not yet been identified. As found for other nuclear receptors, posttranslational modifications, including phosphorylation, acetylation, and sumoylation either enhance or diminish SF-1 activity (2,3,4,5).
Crystallographic studies on the mouse and human SF-1 ligand-binding domain (LBD) revealed different bacterial phospholipids including phosphatidylglycerol (PG), phosphatidylethanol (PE), or phosphatidylethanolamine fortuitously bound in its hormone-binding pocket (6,7,8). Similar results were reported for the close relative of SF-1, liver receptor homolog 1 (LRH-1) (7,8,9,10), except for mouse LRH-1, which appears to be stable in an apo state (11). All SF-1 LBDs revealed a large ligand binding pocket of approximately 1300 Å (3) with the acyl chains of bound phospholipids fitting snugly into the hydrophobic cavity. The phosphate group in all ligands was found to be specifically coordinated at the entrance to the ligand-binding pocket, with different ligand head groups exposed to the solvent. Protein-lipid overlay and liposome-mediated ligand exchange experiments showed that the structurally similar phosphatidylinositols, which are present in the nucleus (12,13), are also able to bind to the SF-1 LBD (14). These results, coupled with the fact that phospholipid pools are dynamic and can be affected by extracellular signaling, suggested phosphatidylinositols as potential ligands for the NR5A receptors in vivo (14). Other studies suggest that phosphatidic acid might function as an agonist of SF-1 (14), whereas sphingolipids are proposed to antagonize the receptor activity (15,16). These combined data are provocative and raise the question as to whether one preferred phospholipid functions as the true ligand for SF-1 or whether preferential ligand uptake depends on relative levels of phospholipid species present in different subcellular pools or in specific cell types.
Aside from identifying the precise phospholipid ligand of SF-1 in different cells, the functional role of these bound phospholipids in regulating SF-1 activity remains unclear. For established ligand-dependent nuclear receptors, binding of ligand facilitates coregulator binding to either enhance or diminish transcription. Most known transcriptional coregulators bind to the AF-2 activation function site, a hydrophobic cleft on the surface of the receptor very near the buried bound hormone (17). The proximity of the AF-2 region to the hormone pocket explains why specific characteristics of a bound ligand, including its size, shape, and charge, are expected to influence the receptor/coregulator association and its stability. For receptors such as SF-1, the structural determinants for ligand binding and the influence of different ligands on coactivator binding have yet to be established.
To probe how different ligands bound to SF-1 LBD might affect receptor activity, we surveyed a variety of nonbacterial phospholipids including phosphatidylcholine (PC) and phosphatidylinositol mono-, di- and triphosphates (PIP, PIP2, and PIP3) in ligand exchange studies. Data provided here show that the endogenous bacterial PG bound to SF-1 is readily replaced by nonbacterial phospholipids. In this work, we present the crystal structure of mouse (m)SF-1 bound by an exchanged phospholipid ligand (PC) and coregulatory peptide derived from the peroxisomal proliferator-activated receptor-γ coactivator PGC-1α. Analysis of this structure reveals the loops L2-3 and L11-12 surrounding the entrance to the SF-1 hormone-binding pocket to be dynamic. This observation, combined with biochemical and cellular data for SF-1 mutants affecting conserved L2-3 and L11-12 residues, suggests that specific configurations of these loops at the entrance to the hormone pocket might be essential for efficient uptake of phospholipids and for maximal SF-1 activity.
Results and Discussion
Preparation of SF-1 with different bound ligands
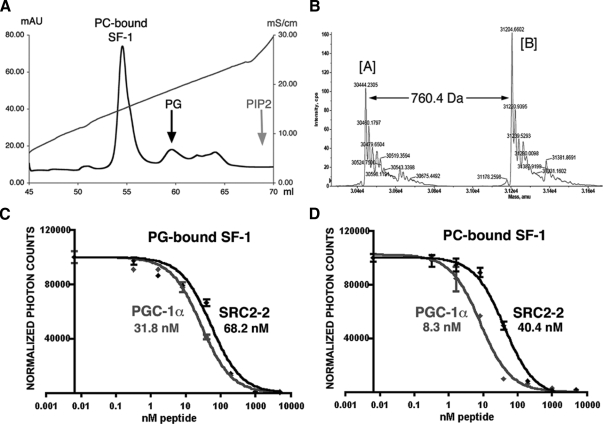
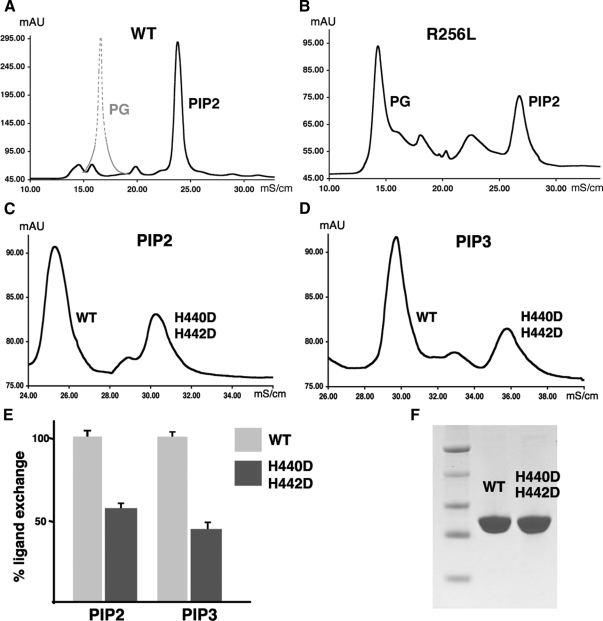
To determine whether exogenous nonbacterial phospholipids might exchange into the SF-1 hormone-binding pocket, liposome-mediated or solution-based ligand exchange experiments were used to exchange the bacterial PG for nonbacterial phospholipids, PC, PIP, PIP2, or PIP3, as described in Materials and Methods. After incubation of the PG-bound SF-1 LBD with liposomes, the protein was purified, and its ligand content confirmed by mass-spectrometry analysis. All tested lipids except for PIPs could substitute efficiently for the PG in SF-1 (Fig. 1, A and B, and data not shown). Furthermore, different phospholipid-bound species of SF-1 could be readily separated and purified due to the charge differences in the solvent-exposed ligand head groups (shown for PC-bound SF-1 in Fig. 1, A and B).
Figure 1.
Preparation of the PC-bound SF-1 protein. A, Eluates from a MonoQ column. The peak corresponding to the purified SF-1 with the exchanged PC ligand is indicated. Positions of the peaks corresponding to the SF-1 bound to original bacterial PG and with the exchanged PIP2 ligand are indicated by the black and gray arrows. B, Mass spectrometry analysis of the purified PC-bound SF-1 LBD. Spectra are shown with the apo-[A] and the PC-bound [B] species indicated. The molecular mass difference between the two peaks (760.4 Daltons) corresponds to the molecular mass of the exchanged PC. C and D, Binding affinities of PGC-1α and SRC-2 peptides estimated in AlphaScreen based competition assays for the PG-bound (C) and PC-bound (D) SF-1 proteins. The peptide binding curves are shown in gray for PGC-1α and black for SRC-1 peptides. The corresponding EC50 values are indicated.
To test whether the overall fold and conformational state of the LBD remained preserved, the relative affinities of SF-1 for two different coactivator peptides, steroid receptor coactivator 2 (SRC-2) and PGC-1α, were determined before and after the ligand exchange. These experiments showed that the peptide binding by SF-1 LBD was not affected by the ligand exchange procedure, as shown for PC-bound SF-1 (Fig. 1, C and D). We also found that the PGC-1α peptide associates with the PG- and PC-bound form of the SF-1 LBD more tightly than the SRC-2 coactivator peptide (Fig. 1, C and D), with the PC-bound SF-1 showing a significant difference in preference. These results differ for that observed with LRH-1, which showed a clear preference for SRC peptides (10). Based on our peptide-binding data with SF-1, we chose to use the PGC-1α peptide to enhance stability of the ligand-exchanged SF-1 LBD in our crystallization experiments. To date, of all ligand-exchanged SF-1 species tested, only the PC-bound LBD complexed with PGC-1α peptide produced crystals suitable for high-resolution crystallographic analysis.
Structure of the PC-bound SF-1 LBD
The structure of the PC-bound receptor-peptide complex was determined using the molecular replacement method and refined to 2.2 Å with Rfree/R values of 25.0/22.1. Details on crystallization and structure determination are provided in Materials and Methods and summarized in Table 1.
Table 1.
Data collection and refinement statistics
| Crystallization | |
|---|---|
| Unit cell dimensions | |
| a (Å) | 46.9 |
| b (Å) | 67.2 |
| c (Å) | 82.7 |
| Space group | P212121 |
| Molecules per asymmetric unit | 1 PC-SF-1/PGC-1 complex |
| Resolution (Å) | 2.2 |
| Number of unique reflections | 13267 |
| Completeness (%)a | 95.9 (91.2) |
| Data redundancya | 3.7 (2.9) |
| <I/ς(I)a* | 15.1 (3.0) |
| Rsymm (%)a,b | 7.0 (24.0) |
| Refinement (25.0–2.2 Å) | |
| ς-cutoff | None |
| Ra | 22.1 (28.3) |
| Rfreea,c | 25.0 (30.0) |
| Rms deviation from ideality | |
| Bond length (A)* | 0.007 |
| Bond angle (degrees) | 1.81 |
| Average B factor (Å2) | 36.8 |
Numbers in parentheses show the last resolution shell (2.3–2.2 Å). rms, Root mean square.
Rsymm = ΣhIh – I /Σh I, where (I) is the mean intensity of reflection h.
Rfree is for 5% of total reflections.
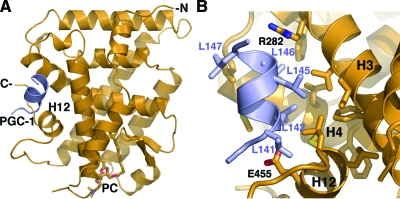
The structure of the PC-bound SF-1 (Fig. 2A) adopts the general NR LBD fold consisting of 12 α-helices distributed in three layers and two short antiparallel β-strands forming a hairpin loop near the ligand-binding pocket of the receptor. Similar to previously determined structures of SF-1 and its close relative LRH-1, an extended helix H2 present in the PC-SF-1 structure provides an additional, fourth layer on the surface of the LBD that is absent in most NR structures. As observed previously for other NR5A structures, the PC-bound SF-1 LBD adopts an active conformation with its helix H12 positioned for coactivator binding. The coactivator PGC-1α peptide is bound in the coactivator groove of SF-1, forming an extended network of intermolecular interactions at the buried protein-peptide interface of about 1000 Å2 (Fig. 2B), consistent with high affinity of the PGC-1α peptide for SF-1 observed in our binding assays (Fig. 1, C and D).
Figure 2.
Structure of the PC-bound SF-1 conforms to other SF-1 LBD structures. A, Cartoon representation of the PC-SF-1 structure. Polypeptide chains for the SF-1 LBD and bound PGC-1α peptide are in orange and purple, respectively. PC bound into the ligand-binding cavity is shown as a stick model. B, A magnified view of the PGC-1α peptide bound into the AF-2 site of SF-1. Helices H3, H4, and H12 forming the coactivator-binding groove of SF-1 are indicated. Hydrophobic side chains participating in docking interactions are shown as stick models. Two oppositely charged side chains of SF-1 (R282 and E455, indicated) form the electrostatic clamp registering the position of the bound PGC-1 peptide.
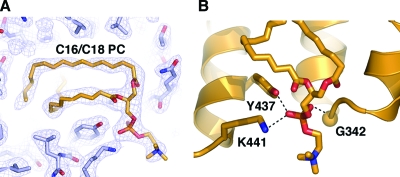
The difference Fourier (Fo − Fc) map for the SF-1 protein model revealed well-defined continuous electron density for two differently shaped tails within the ligand-binding cavity, with a clear tetrahedral-shaped density at the opening of the pocket and an extension of the density at the protein solvent boundary. The two acyl chains, the phosphate group, and the exposed head of the C16/C18 PC were unambiguously modeled in this electron density (Fig. 3A), consistent with results of mass spectrometry analysis that confirmed the presence of this ligand in the crystallized SF-1 protein. The phosphate group of the bound PC was found to be coordinated at the opening of the ligand-binding pocket, making contacts with conserved SF-1 residues G342 from helix H7 and Y437 and K441 from helix H11 (Fig. 3B).
Figure 3.
Analysis of the bound PC in the SF-1 hormone-binding pocket shows optimal binding. A, Electron density corresponding to the bound PC. Electron density from the simulated annealing composite omit map is shown as blue mesh. Modeled PC is shown as stick model. B, Coordination of the phosphate group from the bound PC at the entrance to the SF-1 ligand-binding pocket. Side chains of residues G342, K441, and Y437 participating in coordination are indicated.
Comparative structural analysis of different SF-1 LBDs
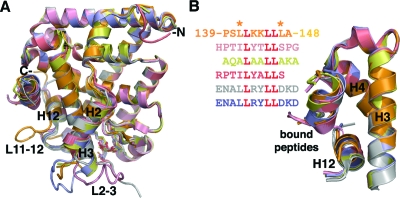
The PC-bound SF-1/PGC-1α structure (orange) was superposed with all independently determined structures of the SF-1 LBD (Fig. 4A). All these superposed SF-1 LBDs were bound by different peptides [PGC-1α, short heterodimer partner (SHP), nuclear receptor coactivator (NcoA)2, transcriptional intermediary factor (TIF)-2] and ligands (PG, PE, PC; see legend to Fig. 4 for details). Specifically, the bound phospholipids in these six SF-1 structures differ by both the chemical structure of the exposed head groups (glycerol, ethanolamine, or choline) and the lengths of associated fatty acyl chains (the lengths of the acyl chains varied between C16/C16 and C16/C18). Despite these major differences, all six superposed SF-1 LBDs align very closely, displaying root mean square deviations of approximately 0.5 Å between the 220 analogous Cα atoms of the described structures and the PC-bound SF-1. Whereas the major α-helices and β-strands superpose well for all SF-1 structures, two surface loops, L2-3 and L11-12, display noticeable conformational variations (Fig. 4A). We analyzed the B values (atomic temperature factors) of these SF-1 structures and compared the mean B values for these two loops and the core LBD helices. This analysis showed that B values for residues from loops L2-3 and L11-12 are at least 2 times higher than those for the rest of the structure, revealing that these loops are dynamic and thus able to adopt multiple conformations. Data and discussion on the functional relevance of these loops are presented later.
Figure 4.
Comparison of the PC-bound SF-1 with other available SF-LBD structures reveal remarkable similarities. A, Superposition of six SF-1 LBDs with different bound ligands. PC-bound mSF-1 with coactivator PGC-1α peptide is in orange; PG-bound mSF-1 with corepressor SHP peptide (1YMT) is salmon; PE-bound mSF-1 with corepressor SHP peptide (1YP0) is pink; PE bound hSF-1 with coactivator TIF-2 peptide (1YOW) is yellow; and two noncrystallographically related PE-bound hSF-1 LBDs with coactivator NCoA-2 peptide (1ZDT) are blue and silver, respectively. Conformationally variable loops L2-3 and L11-12 are indicated. B, A magnified view of the AF-2 sites of the superposed SF-1 structures. The coloring scheme is consistent with panel A. Helices H3, H4, and H12 forming the SF-1 coactivator groove and the bound peptides are indicated. The primary sequences for the bound peptides are shown with the conserved LXXLL motif highlighted in red. Two leucine residues flanking the LXXLL motif in PGC-1α are indicated and discussed further in Results and Discussion.
Regardless of whether a corepressor (SHP) or coactivator (PGC-1α, TIF2, or NCoA-2) peptide is bound, all six superposed SF-1 LBDs assume an active conformation. Indeed, 40 analogous Cα atoms from helices H3, H4, and H12 that form the AF-2 site deviate only by experimental error of approximately 0.3 Å between the PC-bound SF-1 and the other five available SF-1 structures (Fig. 4B), suggesting that the coactivator/corepressor binding site of the receptor is extremely stable. This structural feature likely underlies the constitutively active nature of SF-1 and suggests that the receptor-specific corepressors and coactivators might equally compete for binding to SF-1 with the net effect on transcriptional activity relying on the relative concentrations of these coregulators.
Although the number of SF-1 structures bound to regulatory peptides is relatively small, one can analyze how specific contacts at the peptide-receptor interface might translate into the relative binding affinities of SF-1 for various peptides and corresponding coregulators. For instance, the presence of additional hydrophobic side chains of Leu141 and Leu147 that flank the 142-LXXLL-146 motif of PGC-1α (Fig. 2B) might underlie the high affinity of PGC-1α peptide for the SF-1 LBD by enhancing hydrophobic interactions at the AF-2 site. Indeed, in other regulatory peptides, small polar or charged side chains of Ser, Thr, or Asp are present at the position equivalent to Leu147 of PGC-1α (Fig. 4B). The intermolecular hydrophobic interactions at the AF-2 site of SF-1 are also aided by the electrostatic clamp formed by the receptor residues Arg282 and Glu455, which register the position and orientation of the bound PGC-1α peptide by forming hydrogen bonds with its main chain atoms (Fig. 2B).
Comparing bound phospholipids
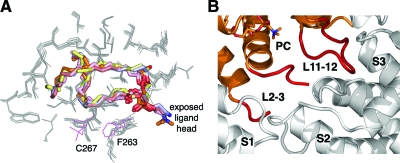
Closer examination of the positions and conformations of the bound ligands in the superposed SF-1 LBDs revealed remarkable similarities. Indeed, buried fatty chains of all ligands assume nearly identical configurations within the ligand-binding cavity, and the coordinated phosphate groups at the opening of the hormone-binding pocket are virtually superposable in all SF-1 structures (Fig. 5A). Thus, whereas C16/C18 phospholipids fill the interior cavity of the hormone-binding pocket completely, their shorter analogs (C16/C16) pack less tightly, leaving part of the pocket unoccupied. Because of the similar conformations of the buried phospholipid acyl chains and their tight interpacking contacts, there are few differences in the positions of the amino acid side chains forming the interior of the hormone-binding cavity in different SF-1 structures. In the PC-SF-1 structure, the most noticeable deviations are observed for side chains of Cys267, which assumes a noncharacteristic position because of the chemical modification by cacodylate that was present in crystallization buffer, and the neighboring Phe263, which shifts to accommodate bulky cacodylate (both residues are shown in magenta, Fig. 5A). In contrast to the buried regions, the solvent exposed head groups in the observed phospholipids vary significantly in different SF-1 structures (Fig. 5A).
Figure 5.
Comparative analysis of bound phospholipids from different SF-1 structures. A, A magnified view of the superposed bound ligands. The ligands are shown as stick models in colors consistent with Fig. 4: PC is in orange; PG (1YMT) is salmon; PE (1YP0) is pink; PE (1YOW) is yellow; and two noncrystallographically related PE molecules (1ZDT) are blue and silver, respectively. Side chains from the interiors of the ligand-binding pockets are shown as gray lines. Different conformations for F263 and C267 of the PC-bound SF-1 are shown in magenta. B, Intermolecular protein interactions stabilizing conformations of loops L2-3 and L11-12 in the PC-bound SF-1 structure. Variable loops L2-3 and L11-12 are highlighted in red. Symmetry molecules contacting loops L2-3 and L11-12 are shown in gray and indicated as S1, S2, and S3.
Based on these observations, we hypothesize that the buried part of the bound phospholipids, including the specifically coordinated phosphate group at the entrance to the pocket, mimic an analogous part of a true endogenous SF-1 ligand and its specific interactions with the receptor. The extensive contacts between the receptor and the longer C16/C18 pair of acyl chains suggest that this fatty acid chain configuration would be optimal for any putative SF-1 ligand. Indeed, phospholipids with acyl chains longer than C16/C18 would destabilize the receptor’s active conformation and compromise the transcriptional activity of SF-1, as suggested previously (10). Whether ligands with acyl chains shorter than C16/C18 might function as partial agonists, as predicted, remains to be determined at both the cellular and structural level.
Dynamic conformations observed in loops L2-3 and L11-12
Despite the overall similarity of the SF-1 LBDs, two surface loops, L2-3 and L11-12, connecting helices H2-H3 and H11-H12 deviate significantly between the compared structures (Fig. 4A). The key structural element that controls binding of transcriptional regulators at AF-2 site is helix H12, which rests against helix H3 (Fig. 4B). Because loops L2-3 and L11-12 might influence the positioning of the functionally important helix H12, and because both loops are positioned near the entrance to the ligand-binding cavity of SF-1 and in the vicinity of the solvent-exposed head groups of the phospholipids, we examined whether the nature of the bound ligand might control the conformations of these loops in different SF-1 structures. Unfortunately, available SF-1 structures are unable to provide strong evidence that the bound phospholipid ligand affects the conformational state of the LBD. Indeed, no direct contacts are found between the exposed phospholipid head and other regions of the receptor including loops L2-3 and L11-12. Furthermore, conformations of loops surrounding the entrance to the hormone pocket are identical in structures of mSF-1 bound by ligands that differ by both the exposed head and buried acyl chains (C16/C18 PG, 1YMT, salmon; and C16/C16 phosphatidyl ethanolamine, 1YP0, pink in Fig. 4A). In contrast, two noncrystallographically related human SF-1 LBDs with an identical bound phospholipid (PE, 1ZDT, silver and blue in Fig. 4A) exhibit divergent conformations of loop L2-3. Collectively, these structural observations imply that various configurations of loops L2-3 and L11-12 in existing SF-1 structures are influenced mostly by intermolecular protein contacts favored by specific crystallization conditions and different crystal symmetry, as illustrated by the PC-bound SF-1 structure (Fig. 5B).
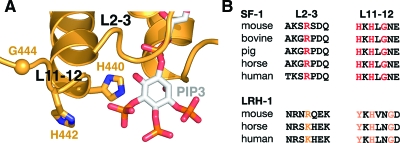
Despite the fact that our structural analysis of PC-bound SF-1 was unable to establish a convincing allosteric link between the nature of the bound ligand and the observed conformational state of the SF-1 LBD, the dynamic nature of the SF-1 loops L2-3 and L11-12 is notable when taken in the context of the overall similarity of the superposed molecules. Moreover, the proximity of L2-3 and L11-12 to the bound ligand in the SF-1 structures suggests that these loops could be restructured in the presence of a proper ligand by directly interacting with its solvent-exposed head. Consistent with this idea, modeling experiments with phospholipids PIP2 or PIP3 position the inositol di- or triphosphate groups at the distance of direct interaction with both loop L2-3 and L11-12 (Fig. 6A). In particular, in the model of PIP3 bound to SF-1 there are two NR5A-conserved histidine residues in loop L11-12, H440 and H442, which are positioned for direct interaction (∼3 Å) with the bound ligand through hydrogen bonding to the exposed phosphate groups of PIP3 (Fig. 6, A and B). Additionally, conserved flexible glycine G444 in the vicinity of these two histidine residues could accommodate different conformations of L11-12 for optimal contacts with the ligand. Based on these observations, we hypothesize that specific conformations of loops L2-3 and L11-12 might favor binding of a true regulatory ligand that would influence SF-1 activity.
Figure 6.
Architecture of loops surrounding the entrance to the ligand-binding pocket of SF-1. A, A fragment of the SF-1 LBD structure bound with modeled PIP3. Shown is the polypeptide chain for SF-1 (orange) with the modeled PIP3 in the ligand-binding pocket (stick model). Positions of loops L2-3 and L11-12 at the entrance to the pocket are indicated. Side chains of NR5A subfamily-conserved residues H440 and H442 in L11-12 (shown as stick models) are at distances required for direct interactions (∼3 Å) with the bound ligand. The NR5A subfamily conserved G444 is shown as an orange sphere. B, Multiple sequence alignment for loops L2-3 and L11-12 in SF-1. Subfamily conserved residues R256 from L2-3 and H440, H442, and G444 from L11-12 are shown in red. Analogous residues in nuclear receptor LRH-1 are shown in orange.
Impaired ligand exchange in SF-1 Loop L2-3 mutant
To test these structural predictions and to probe whether loops L2-3 and L11-12 participate in ligand binding, we carried out ligand exchange experiments with wild-type SF-1 LBD and variants containing mutations in loops L2-3 and L11-12. To begin with, we made use of a naturally occurring human missense point mutation R255L in loop L2-3. This mutation was identified in a heterozygous female patient exhibiting adrenal disease with apparent normal gonadal development (18). To date, the only other human SF-1 mutation found in the LBD is in helix H11 (L437Q) and is predicted to destabilize the LBD and prevent ligand binding (19). When mutation mR256L was introduced into mSF-1 (mR256L), this variant of SF-1 behaved similarly to wild-type SF-1 with respect to stability, solubility, and chromatographic behavior (data not shown). However, ligand exchange experiments showed that although both PIP2 and PIP3 ligands quantitatively replace the endogenously bound PG in wild-type SF-1 (Fig. 7, A, C, D, and E), binding of these phospholipids by the mR256L mutant is substantially reduced (as shown for PIP2 in Fig. 7B). As predicted from the SF-1 LBD structure, no ligand binding or exchange was observed for a double-pocket mutant A270W/L345F under the same experimental conditions (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). This particular SF-1 mutant is not able to bind ligands because of bulky hydrophobic side chains introduced into its ligand-binding cavity, and, therefore, it was used as a negative control in our ligand exchange assays.
Figure 7.
Loop mutant SF-1 proteins exhibit impaired ligand exchange compared with wild-type protein. A, Chromatographic ion exchange profile for wild-type (WT) SF-1 LBD after ligand exchange. The peak corresponding to the ligand-exchanged PIP2-bound wild-type SF-1 is shown in black, whereas the position of the peak for the original PG-bound wild-type SF-1 is indicated by dashed gray line. Quantification of the peak volumes at a 5:1 ligand-protein molar ratio suggests an exchange rate of approximately 90%. B, Analogous chromatographic profile for R256L SF-1 mutant. Peaks corresponding to the original PG-bound and PIP2-bound SF-1 are indicated. The ligand exchange rate for loop L2-3 SF-1 mutant is less than 30%. C and D, Chromatograms for ligand-exchanged wild-type and H440D/H442D mutant SF-1 proteins with either PIP2 (panel C), or PIP3 (panel D) bound. Differences between electrostatic properties of wild-type SF-1 and H440D/H442D variant allowed for a simultaneous comparative ligand exchange analysis. E, Results of three independent ligand exchange experiments are presented as bar graph. Ligand exchange rates for PIP2- and PIP3-bound H440D/H442D mutant protein (dark gray) are compared with the analogous rates for wild-type SF-1 (light gray). F, Wild-type and H440D/H442D mutant proteins exhibit equal stability and solubility as judged by SDS-PAGE analysis.
To address the role of the conserved residues in loop L11-12, the SF-1 LBD variant harboring mutations in the conserved histidine residues (H440D/H442D) was expressed and tested in the analogous ligand exchange experiments. Differences in the electrostatic properties and distinct elution profiles of wild-type and H440D/H442D mutant allowed us to directly compare the ligand exchange rates of these proteins with either PIP2 or PIP3 (Fig. 7, C and D). Indeed, one is able to easily separate PIP2- or PIP3-bound species of wild-type SF-1 and the analogous species of L11-12 mutant protein using ion exchange chromatography, as shown in Fig. 7, C and D. This direct comparison of wild-type and mutant protein revealed that introduction of negatively charged residues in loop L11-12 greatly diminished ligand exchange rates for both PIP2 and PIP3 (Fig. 7, C–E). We noted that the exchange rate for phosphatidylinositol 3, 4, 5-triphosphate [PI(3,4,5)P3] was consistently lower than for PI(4,5)P2 for the mutant protein. This result might reflect a potential steric clash or unfavorable electrostatic interactions between the 3′-phosphate group of PIP3 and the negative charges present in the H440D/H442D mutant (Fig. 6A).
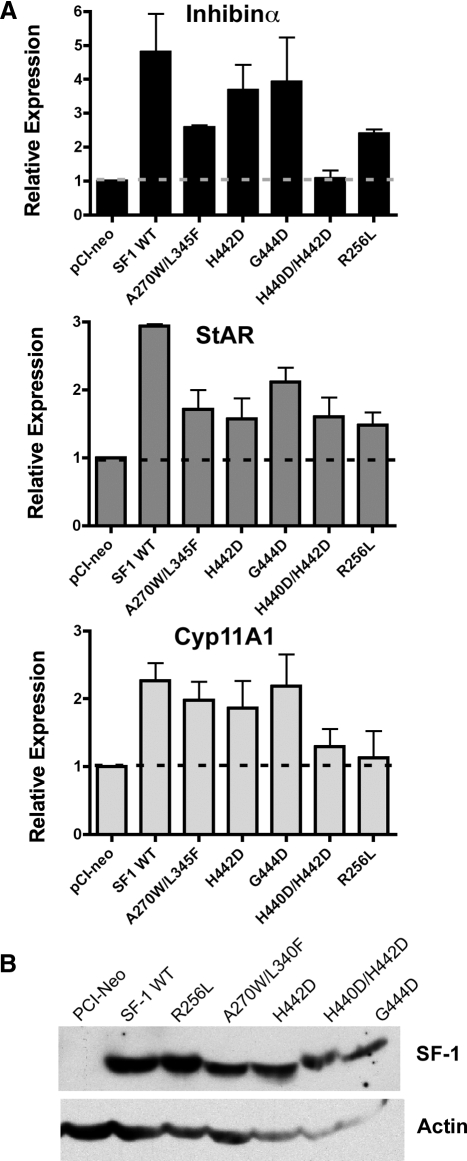
The function of these L2-3 and L11-12 mutant SF-1 proteins also appeared to be diminished as evidenced by their inability to fully activate endogenous inhibin-α, cytochrome P450 Cyp11A1, and steroidogenic acute regulatory (StAR) transcripts in JEG-3 cells (Fig. 8A). Indeed, the H440D/H442D and the R256L mutants exhibited the lowest transcriptional activities, even when compared with the A270W/L345F SF-1 pocket mutant. Taken together, these data imply that inefficient ligand binding/exchange correlates with compromised transcriptional activity of SF-1, as shown here. Consistent with our conclusion, loss of ligand binding is predicted to account for the diminished activity observed for other SF-1 LBD mutants (7,10) and may account for the endocrine disorder associated with the R255L human mutation (18).
Figure 8.
Transcriptional activity of L2-3 and L11-12 mutant SF-1 proteins is diminished. A, Induction of three SF-1 targets (inhibin α, Cyp11A1, and StAR) was determined by RT-qPCR after wild-type (WT) and mutant SF-1 expression vectors were transfected into human JEG-3 placental cells. The relative expression of all three target genes was normalized to the control parent vector (pCI-neo, indicated). B, Expression of all SF-1 variant proteins was equivalent as judged by Western blot analysis (upper panel); the control parent vector is shown in the first lane. The actin loading control is shown in the lower panel.
Summary
Here we provide biochemical and structural evidence that nonbacterial phospholipids readily exchange for bacterial ligands fortuitously bound to the SF-1 LBD. The ability to exchange exogenous phospholipids into the SF-1 hormone pocket and crystallize the ligand-exchanged SF-1 LBD is in contrast to other receptors, such as HNF4, which binds fatty acids constitutively (20). Comparative analysis of all known SF-1 LBD structures reveals the dynamic nature of loops L2-3 and L11-12 surrounding the entrance to the receptor ligand-binding pocket. This observation suggests that a bound ligand might control the conformations of loops L2-3 and L11-12. Furthermore, specific residues in these loops might have evolved to accommodate binding of distinct phospholipids including phosphatidyl inositol phosphate ligands. Our finding that mutations in loop L2-3 and L11-12 greatly impair ligand exchange and reduce SF-1 transcriptional activity suggests that SF-1 must bind ligand efficiently for achieving optimal activity. These correlative data, together with the fact that the bacterial SF-1 ligand is easily replaced by nonbacterial phospholipids, and that the ligand-exchanged PC-bound SF-1 LBD is stable and readily crystallizable, all strongly support the idea that regulatory, rather than fortuitous or structural ligands, exist for NR5A receptors.
Whereas the ligand-controlled stabilization of helix H12 in the AF-2 region is thought to be essential for achieving full agonist activity, recent studies suggest that partial agonists might affect other regions of the nuclear receptor LBD. In particular, structural and kinetic analyses of PPARγ showed that the receptor’s helix H3, which is adjacent to loop L2-3, dynamically responds to different bound ligands (21). Moreover, structural analysis of LRH-1, a close relative of SF-1, demonstrates the importance of loop L11-12 for stabilizing a regulatory complex between LRH-1 and its functional corepressor Dax-1 (22). Collectively, these findings show that other dynamic regions of the LBD must be considered when analyzing allosteric signaling by nuclear receptor ligands. Thus, we hypothesize that the different conformations observed for L2-3 and L11-12 of SF-1 reflect a built-in ability of these loops to change their structure in response to physiological signals such as sensing the presence/absence of a bound SF-1 hormone.
What remains to be determined is whether distinct phospholipids and their exposed head groups differentially affect SF-1 function. Notably, mutations that prevent ligand binding but presumably stabilize the LBD structure result in a partially competent receptor that is able to activate endogenous target genes, as shown for the A270W/L345F SF-1 pocket mutant (Fig. 8A). The fact that L2-3 and L11-12 SF-1 variants exhibit lower transcriptional activity than the described pocket mutant is consistent with the idea that loops at the entrance to the receptor pocket indeed control binding of ligands. The lower activity of these mutants could be explained by their compromised ligand binding in the absence of compensatory, stabilizing hydrophobic interactions within the ligand pocket that are present in the SF-1 pocket mutant A270W/L345F. These comparative data also suggest that the solvent-exposed portion of the phospholipid and/or the specific configurations of loops coordinating a bound ligand could modulate SF-1 activity beyond the basal level of a structurally stabilized receptor. Whether the configurations of loops L2-3 and L11-12 are simply required for efficient ligand binding/exchange or whether these loops participate in other aspects of SF-1 function (e.g. influencing recruitment of coregulators) remains to be established. Clearly, further structural studies of SF-1 bound to additional phospholipids including PIP2, PIP3, and phosphatidic acid should reveal whether distinct phospholipids alter the conformation of SF-1 LBD and affect its function as true regulatory ligands.
Materials and Methods
Protein purification and ligand exchange
mSF-1 LBD construct and purification protocol were described earlier (7). PC was purchased from Avanti Polar Lipids (Alabaster, AL); PI (4)P, PI(5)P, PI(4,5)P2, and PI(3,4,5)P3 were purchased from Cayman Chemical Co. (Ann Arbor, MI). Preparation of the PC liposome and liposome-mediated ligand exchange were performed as described earlier (7). For ligand exchange experiments comparing wild-type, R256L, and A270W/A345F mutant proteins, individual phospholipids [(PC, PI(4,5)P2 or PI(3,4,5)P3] were solubilized in water, and each phospholipid was mixed with 2 mg of purified wild-type or mutant mSF-1 LBD at a 1:5 molar ratio. For a more quantitative ligand exchange analysis comparing wild-type and H440D/H442D mutant proteins, a molar ratio of 1:2 was used. The SF-1/phospholipid mixtures were incubated with liposomes overnight at room temperature and then purified using G25 HiTrap column (GE Healthcare Bio-Sciences Corp., Buckinghamshire, UK). For the final purification, proteins were loaded on the MonoQ column in the buffer containing 20 mm HEPES (pH 7.4) and 1 mm EDTA and eluted with a gradient of ammonium acetate. Ligand content of all proteins was verified by nondenaturing mass spectrometry analysis as described earlier (7).
AlphaScreen-based peptide binding assay
All peptides (PGC-1α: EEPSLLKKLLLAPA; Dax1–3: QGSILYSLLTSAQ; SRC-2: LLEKHKILHRLLDKDDTKD) were purchased from Bio-Synthesis, Inc. (Lewisville, TX) The His6-tagged SF-1 LBD proteins with either bacterial PG or nonbacterial phospholipids bound were prepared as described above. Buffer consisting of 50 mm MOPS (pH 7.4), 50 mm NaCl, 10 mm dithiothreitol, 0.1 mm BSA, and 0.05 mm CHAPS was used throughout the procedure. Biotinylated Dax1–3 peptide was incubated overnight at 4 C with the His6-SF-1 protein (∼400 nm) at molar ratio of 1:1. Solutions of competing PGC-1α and SRC-2 peptides in dimethylsulfoxide (0.001–500 nm) were added to 50 nm of this preincubated protein; dimethylsulfoxide was used as a control. After incubation for 2 h, the mixture of donor and acceptor beads (PerkinElmer AlphaScreen His6-tag detection kit; PerkinElmer Corp., Wellesley, MA) was added to the samples, and the fluorescence was measured using the AlphaQuest-HTS (PerkinElmer) machine according to the manufacturer’s protocol.
Crystallization and structure determination of the PC-bound mSF-1 LBD
The purified SF-1 protein samples with different exchanged ligands were concentrated to about 6 mg/ml and used fresh for crystallization experiments. Only SF-1 with bound PC produced crystals suitable for high resolution x-ray diffraction analysis. The purified PC-bound mSF-1 LBD was mixed with the mPGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) peptide 137-EEPSLLKKLLLAPA-150 at a protein-peptide molar ratio of 1:3 and crystallized using the vapor diffusion method by mixing 1 μl of the protein-peptide complex (∼6 mg/ml) with 1 μl of precipitant solution containing 20% polyethylene glycol 3350K, 0.1 m sodium cacodylate (pH 6.5), 0.2 m sodium acetate, 2 mm Tris (2-carboxyethyl) phosphine, and 10% xylitol. Crystals reached full size within 5 d at 4C and were flash frozen in liquid nitrogen before data collection. The x-ray diffraction data were measured at −180 C and collected to 2.2 Å at Advanced Light Source (Lawrence Berkeley National Laboratory) beamline 8.3.1 (λ = 1.1 Å) using a single crystal. Data were integrated using DENZO and scaled with SCALEPACK. The crystal was of the orthorhombic space group P212121 with one SF-1/PGC-1α peptide complex in the asymmetric unit and cell dimensions of a = 46.90 Å, b = 67.23 Å, and c = 82.69 Å. The structure of the PC-bound SF-1 LBD was determined by the molecular replacement method using the CNS algorithms with a starting model derived from the atomic coordinates for PG-bound mSF-1 (PDB ID 1YMT). Electron-density maps based on coefficients 2Fo-Fc were calculated from the phases of the initial model. Subsequent rounds of model building and refinement were performed using program packages QUANTA (Molecular Simulations, Inc.) and CNS, respectively. At later stages of the refinement, the entire structure was checked using simulated annealing composite omit maps. The current structure is refined to R/Rfree values of 25.0/22.1 (25.0 − 2.2 Å) and includes amino acid residues 222–253 and 257–462 with bound PC for mSF-1 LBD, and residues 139–148 for the PGC-1α peptide. Data collection and refinement statistics are shown in Table 1.
Superposition and comparative analysis of different SF-1 LBD structures
The PC-bound structure of mSF-1 was superposed with four structures of SF-1 LBD bound to different ligands and regulatory peptides (PDB IDs 1YMT, 1YOW, 1ZDT and 1YP0) using the program PyMol. Only coordinates for Cα atoms of amino acid residues found within helices were used for the superposition. Coordinates for the bound ligands, peptides, and for residues constituting conformationally variable loops L2-3 and L11-12 were excluded from these calculations. Figures were produced using PyMol.
Cell transfections and qPCR
JEG-3 cells (American Type Culture Collection (ATCC) no. HTB-36) in 100-mm plates were transfected with 5 μg of different constructs including pCI-neo parent vector, pCI-neo mSF-1 wild type, pCI-neo mSF-1 mutant R256L, pCI-neo mSF-1 mutant A270W/L345F, pCI-neo mSF-1 mutant H442D, pCI-neo mSF-1 mutant H440D/H442D, or pCI-neo mSF-1 mutant G444D using FuGene HD reagent (Roche Chemical Co., Indianapolis, IN; no. 04709691001). Whole-cell extracts and the RNA were isolated 24 h after transfection. Western blot assay was conducted using an anti-SF1 (H. Ingraham, unpublished) and antiactin antibodies. RT-quantitative PCR (qPCR) was conducted using SYBR GreenER (Invitrogen, Carlsbad, CA; no. 11760-100) on ABI 7300 real-time PCR system (ABI Advanced Technologies, Inc., Columbia, MD). Validated forward and reverse qPCR primers are as follows: hIHA: forward (for), GGGAACGGTGGATCGTGTA, reverse (rev), CCCACAACCACCATGACAGT; hSTAR: for, CCCATGGAGAGGCTCTATGAA, rev-GTTCCACTCCCCCATTGCT; hCyp11a1: for-GGGTCGCCTATCACCAGTATT, rev, GCTGCCGACTTCTTCAACAG; hCyclophilin: for, TTTCATCTGCACTGCCAAGA, rev, TTGCCAAACACCACATGCT.
Supplementary Material
Acknowledgments
We thank Dr. Ed Ly for technical advice on preparation of liposomes for liposome mediated ligand exchange assays. We also thank Drs. Lucy Campbell and Miyuki Suzawa for useful technical advice and discussion of the manuscript.
Footnotes
This work was supported by National Institutes of Health RO1 grants to R.J.F. and H.A.I.
Disclosure Summary: E.S., R.B., I.N., J.I., F.C., R.F., and H.I. have nothing to disclose. J.W. is employed by GlaxoSmithKline and has stock options.
First Published Online November 6, 2008
Abbreviations: AF, Activation function; Cyp, cytochrome P450; for, forward; LBD, ligand-binding domain; LRH-1, liver receptor homolog 1; NcoA, nuclear receptor coactivator; NR, nuclear receptor; PC, phosphatidylcholine; PE, phosphatidylethanol; PG, phosphatidylglycerol; PGC, peroxisomal proliferator-activated receptor-γ coactivator; PI(3,4,5)P3; phosphatidylinositol 3,4,5-triphosphate; PIP, phosphatidylinositol monophosphate; PIP2, phosphatidylinositol diphosphate; PIP3, phosphatidylinositol triphosphate; qPCR, quantitative PCR; rev, reverse; SF-1, steroidogenic factor-1; SHP, short heterodimer partner; StAR, steroidogenic actute regulatory; TIF, transcriptional intermediary factor.
References
- Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez 2003 A SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Lee WC, Hsu NC, Huang F, Chung BC 2004 SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA 1999 Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526 [DOI] [PubMed] [Google Scholar]
- Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA 2005 The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol 25:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Juan LJ, Chung BC 2005 SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol 25:10442–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE 2005 Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore JT, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana VG, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willlson TM, Ingraham HA 2005 Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL 2005 The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA 102:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan Z, Tripathy A, Raetz CR, McDonnell DP, Moore DD, Redinbo MR 2005 Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat Struct Mol Biol 12:357–363 [DOI] [PubMed] [Google Scholar]
- Li Y, Choi M, Suino K, Kovach A, Daugherty J, Kliewer SA, Xu HE 2005 Structural and biochemical basis for selective repression of the orphan nuclear receptor liver receptor homolog1 by small heterodimer partner. Proc Natl Acad Sci USA 102:9505–9510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablin EP, Krylova IN, Fletterick RJ, Ingraham HA 2003 Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol Cell 11:1575–1585 [DOI] [PubMed] [Google Scholar]
- Hunt AN 2006 Completing the cycles; the dynamics of endonuclear lipidomics. Biochim Biophys Acta 1761:577–587 [DOI] [PubMed] [Google Scholar]
- Hunt AN, Clark GT, Neale JR, Postle AD 2002 A comparison of the molecular specificities of whole cell and endonuclear phosphatidylcholine synthesis. FEBS Lett 530:89–93 [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Redinbo MR 2005 Orphan nuclear receptors adopted by crystallography. Curr Opin Struct Biol 15:708–715 [DOI] [PubMed] [Google Scholar]
- Dammer EB, Leon A, Sewer MB 2007 Coregulator exchange and sphingosine-sensitive cooperativity of steroidogenic factor-1, general control nonderepressed 5, p54, and p160 coactivators regulate cyclic adenosine 3′,5′-monophosphate-dependent cytochrome P450c17 transcription rate. Mol Endocrinol 21:415–438 [DOI] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Kelly S, Wang E, Merrill Jr AH, Sewer MB 2007 Steroidogenic factor-1 is a sphingolipid binding protein. Mol Cell Endocrinol 265–266:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG 2000 The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- Biason-Lauber A, Schoenle EJ 2000 Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, Sultan C, Dattani MT, Achermann JC 2007 Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab 92:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisely GB, Miller AB, Davis RG, Thornquest Jr AD, Johnson R, Spitzer T, Sefler A, Shearer B, Moore JT, Miller AB, Willson TM, Williams SP 2002 Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure 10:1225–1234 [DOI] [PubMed] [Google Scholar]
- Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR 2007 Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15:1258–1271 [DOI] [PubMed] [Google Scholar]
- Sablin EP, Woods A, Krylova IN, Hwang P, Ingraham HA, Fletterick RJ 2008 The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc Natl Acad Sci USA 105:18390–18395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.