Abstract
The neuropeptide GNRH 1 stimulates the secretion of the reproductive hormone LH in pituitary gonadotropes. Other secretory cell types depend on the unfolded protein response (UPR) pathway to regulate protein synthesis and protect against endoplasmic reticulum (ER) stress in response to differentiation or secretory stimuli. This study investigated the role of the UPR in GNRH action within the LβT2 gonadotrope model. Cells were treated with GNRH, and the activation of UPR signaling components and general translational status was examined. The ER-resident stress sensors, Atf6, Eif2ak3, and Ern1, are all present, and GNRH stimulation results in the phosphorylation of eukaryotic translation initiation factor 2A kinase 3 and its downstream effector, eukaryotic translation initiation factor 2A. Additionally, activation of the UPR was confirmed both in LβT2 as well as mouse primary pituitary cells through identifying GNRH-induced splicing of Xbp1 mRNA, a transcription factor activated by splicing by the ER stress sensor, ER to nucleus signaling 1. Ribosome profiling revealed that GNRH stimulation caused a transient attenuation in translation, a hallmark of the UPR, remodeling ribosomes from actively translating polysomes to translationally inefficient ribonucleoprotein complexes and monosomes. The transient attenuation of specific mRNAs was also observed. Overall, the results show that GNRH activates components of the UPR pathway, and this pathway may play an important physiological role in adapting the ER of gonadotropes to the burden of their secretory demand.
Gonadotropin-releasing hormone activates endoplasmic reticulum-resident stress sensors, causing a transient pause in translation and redistribution specific mRNAs, including those encoding gonadotropin subunits.
The reproductive axis is controlled by release of the decapeptide GNRH 1 (also LHRH) from the hypothalamus. GNRH binding to its G protein-coupled receptor on gonadotrope cells in the anterior pituitary activates and maintains the synthesis of gonadotropin hormones LH and FSH and stimulates release of preformed stores of LH (1). These hormones, in turn, act on the gonad to regulate folliculogenesis, ovulation, spermatogenesis, and steroidogenesis. LH and FSH are glycoprotein heterodimers, each comprised of a common α-glycoprotein subunit (Cga, also αGSU) and a unique β-subunit (Lhb or Fshb) (2). Studies in pituitary gonadotropes are made possible by the LβT2 cell model, created through immortalization of mouse gonadotropes by targeted tumorigenesis (3). LβT2 cells have been shown to respond to GNRH by raising intracellular calcium levels and stimulating exocytosis (4). The signal transduction pathways and transcriptional responses induced by GNRH, including induction of Lhb and Cga among other genes important to gonadotrope function, have been extensively characterized (2,5) in part by utilizing this and other gonadotrope models (6).
Whereas the transcriptional regulation is well described, very little work has focused on posttranscriptional regulatory pathways induced by GNRH. One important posttranscriptional pathway is the unfolded protein response (UPR), which incorporates both transcriptional and posttranscriptional mechanisms in a multifaceted response to minimize endoplasmic reticulum (ER) stress. The ER lumen is an oxidative environment where protein folding and posttranslational modification of proteins that are secreted or targeted to the membranes occurs. The UPR is a quality control pathway that monitors changes in the ER lumen that perturb protein-folding capacity. Disruption of the ER lumen can be a result of pathological conditions such as hypoxia, viral infection, starvation, or a result of normal physiological processes such as secretion or high-protein synthetic demand. ER stress can also be induced experimentally by the overexpression of misfolded proteins, which overwhelm the ER, or by pharmacological insults that target glycosylation, calcium, or oxidative balance. The UPR seeks to reestablish balance and decrease burden by attenuating translation and degrading misfolded proteins, as well as increasing synthetic capacity by increasing the size of the ER and the capacity of the protein-folding machinery. If balance is not reached, the UPR induces apoptosis.
Stress in the ER lumen is sensed by three ER-resident proteins, EIF2A kinase 3 (EIF2AK3, also known as PERK or PEK), ER to nucleus signaling 1 (ERN1, also known as IRE1), and activating transcription factor 6 (ATF6). Activation of EIF2AK3 leads to an immediate attenuation of general translation through phosphorylation of eukaryotic translation initiation factor 2A (EIF2A, also known as eIF2α), which reduces protein synthesis demand on the ER. Phosphorylation of EIF2A also causes translation stimulation of Atf4 (activating transcription factor 4), a bZIP transcription factor that stimulates genes that further the UPR program, including those involved in amino acid transport, synthesis, metabolism, and the antioxidant response. EIF2AK3 activation is followed temporally by proteolytic activation of the basic leucine-zipper transcription factor ATF6, which regulates genes with ER stress response elements (ERSEs), such as chaperones. Finally, the UPR activates the kinase/endoribonuclease ERN1, which splices Xbp1 (X-box binding protein 1) mRNA, another bZIP transcription factor important for UPR transcriptional responses. XBP1 acts on promoters at UPR elements and is thought to be responsible for regulating genes that mediate ER-associated degradation of misfolded proteins. The UPR signaling pathway has been studied extensively and reviewed recently (7,8,9,10). Another, recently discovered arm of the stress response is inhibition of translational complexes at the ER translocon (11).
Recently, the UPR was shown to be crucial for the function of secretory cells, including plasma cells, β-cells, hepatocytes, and osteoblasts (12), all of which have heavy protein synthesis demands and thus rely on the proper function of the ER to maintain secretory output. Loss of Xbp1 in B lymphocytes in mice results in failure to differentiate into immunoglobulin-secreting plasma cells and ultimately failure to mount an immune response to polyoma virus infection (13). Ern1 is also required for proper immunoglobulin production and plasma cell differentiation (14). Mice that lack functional EIF2AK3 in insulin-secreting pancreatic β-cells have elevated serum glucose levels compared with wild-type littermates and eventually experience β-cell apoptosis and diabetes (15,16). Transgenic mice harboring a mutation in Eif2a that does not allow phosphorylation show impaired insulin production and loss of insulin-positive β-cells. Most transgenic neonates die within 18 h of birth (17).
Because gonadotropes are endocrine secretory cells that experience heavy secretion and protein synthesis demand, it can be hypothesized that the UPR plays a role in regulating function in these cells. The UPR is currently unexplored in gonadotropes, although indirect evidence indicates that this may be the case. Microarray studies have shown that GNRH induces Atf3 (18,19,20), a transcription factor induced by the EIF2AK3/ATF4 arm of the UPR (21). GNRH has been shown to transcriptionally regulate genes involved in amino acid synthesis, metabolism, and oxidative stress (18,19), also consistent with activation of EIF2AK3. Finally, Xbp1 predicted that targets such as those of the DnaJ family (22) are induced by GNRH (19,20). To meet the demands of hormone secretion, it is likely that gonadotropes mount a UPR-like response to GNRH. This hypothesis is addressed in this study by examiniation of the activation of UPR effectors in the LβT2 gonadotrope cell line and primary pituitary gonadotropes and by ribosome profiling to examine the general status of translation. The results indicate that the UPR is a target of GNRH action within the gonadotrope.
Results
GNRH activates the ER stress sensor EIF2AK3
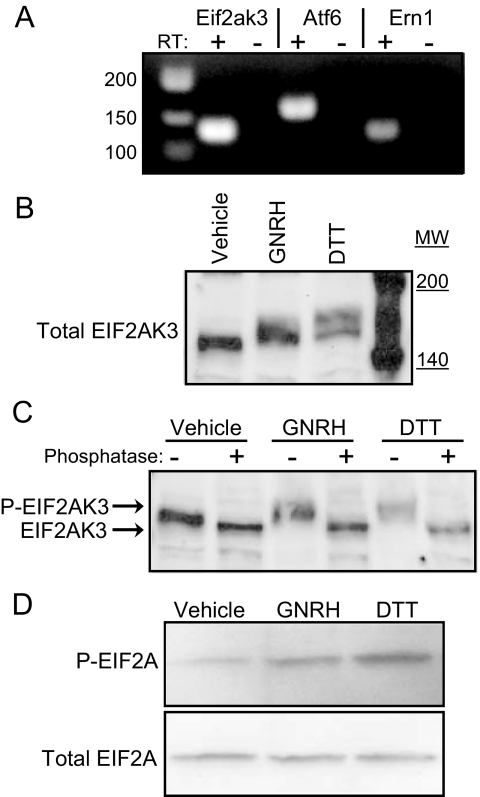
The main sensors of ER stress are three ER-resident proteins, ERN1, EIF2AK3, and ATF6. RT-PCR of mRNA isolated from LβT2 cells indicates that all three sensors are expressed in the cell line (Fig. 1A). An immediate effect of ER stress is activation of the UPR through EIF2AK3. Activated EIF2AK3 oligomerizes (23) and autophosphorylates at 10 different sites in its kinase domain (24). To determine whether GNRH exposure leads to the phosphorylation of EIF2AK3, LβT2 cells were treated with GNRH or dithiothreitol (DTT) for 30 min. DTT severely disrupts the ER’s oxidative environment required for proper protein folding (10) and is commonly used to disrupt translation and activate the UPR (11,25,26). Protein was harvested and subjected to Western blotting. A shift in EIF2AK3 mobility (Fig. 1B) is consistent with hyperphosphorylation of the protein, with DTT causing a slightly greater shift than seen with GNRH. To confirm that this shift is indeed due to phosphorylation events, protein extracts were treated with phosphatase and then subjected to Western blotting. The mobility shift of EIF2AK3 is abrogated by phosphatase treatment of extracts (Fig. 1C). Under basal conditions EIF2AK3 exists in a moderately phosphorylated state, with the level of phosphorylation increasing with GNRH or DTT treatment.
Figure 1.
Expression of ER stress sensors and activation of EIF2AK3 by GNRH in LβT2 gonadotropes. A, Total RNA was isolated from untreated cells and PCR was performed using transcript-specific primers to determine presence of the three known ER stress sensors Eif2ak3, Atf6, and Ern1. RT, Reverse transcriptase. B, Total protein was harvested from LβT2 cells treated with vehicle, 10 nm GNRH, or 2 mm DTT for 30 min and subjected to Western blotting. Antibody against total EIF2AK3 was used to detect EIF2AK3 in lysates. Hyperphosphorylation of EIF2AK3 is indicated by decreased electrophoretic mobility. C, The phosphorylation-dependent decrease in EIF2AK3 mobility with GNRH or DTT treatment was confirmed by treatment of protein extracts with λ-protein phosphatase before Western blotting. D, Phosphorylation of the EIF2AK3 target EIF2A was confirmed by Western blotting with antibodies specific to the phosphorylated and total forms of the protein. MW, Molecular weight.
Activated EIF2AK3 directly phosphorylates EIF2A, which is the α-subunit of the eIF2 translation initiation complex. Phosphorylation of EIF2A prevents initiation complex formation, leading to free ribosomal subunit accumulation and a decrease in general translation (27). To examine the phosphorylation status of EIF2A in response to GNRH, antibodies specific to total and phosphorylated EIF2A were used in Western blotting of GNRH and DTT-treated extracts. GNRH treatment resulted in an increase in EIF2A phosphorylation, and greater phosphorylation was observed after DTT treatment (Fig. 1D).
GNRH causes splicing of the UPR transcription factor Xbp1
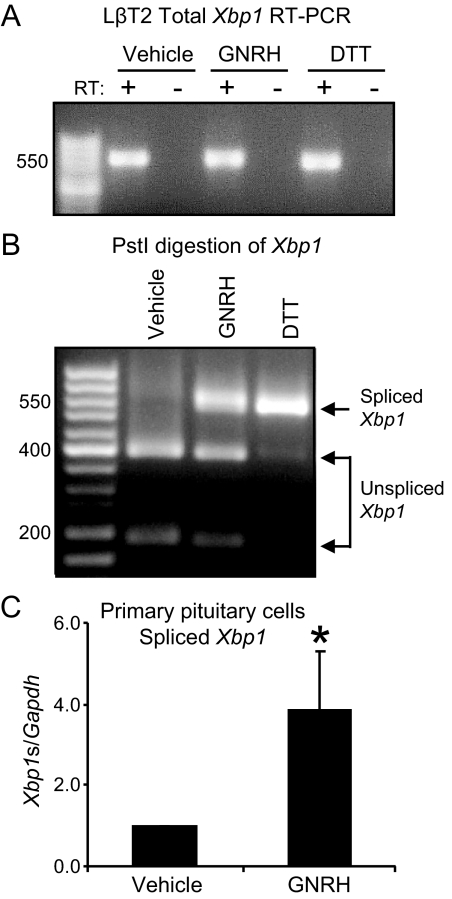
To confirm that GNRH is inducing ER stress, a different arm of the UPR was explored. Signaling to ERN1 leads to activation of Xbp1, which encodes a bZIP transcription factor that acts at UPR elements (8). Xbp1 is activated through a cytoplasmic mRNA splicing event, where removal of a small intron allows translation of an active transcription factor (28). The unspliced mRNA contains a PstI site in the intron that is removed by the splicing event (29). Xbp1 cDNA prepared from LβT2 cells treated with vehicle, GNRH, or DTT (Fig. 2A) was subjected to PstI digestion. GNRH caused splicing of Xbp1 mRNA, although to a lesser degree than DTT treatment (Fig. 2B), which resulted in splicing of all the available Xbp1 mRNA.
Figure 2.
GNRH causes splicing of the UPR transcription factor Xbp1 mRNA in LβT2 and mouse primary gonadotropes. A, LβT2 cells were treated with GNRH or DTT for 30 min and total RNA was harvested. Xbp1 mRNA was reverse transcribed and amplified using gene-specific primers. The resulting cDNA was subjected to agarose electrophoresis to confirm appropriate amplicon size. RT, Reverse transcriptase. B, Amplified Xbp1 cDNA was digested with PstI to confirm the loss of a PstI restriction site within the intron sequence. Spliced Xbp1 cDNA is insensitive to PstI digestion. C, Primary pituitary cells cultured from wild-type, 9-wk-old male mice were plated and treated with GNRH or DTT for 30 min. RNA was isolated and quantitative PCR was used to measure the spliced form of Xbp1 specifically (Xbp1s). Gapdh was used as an internal control. The reported values are the means ± sem of three independent experiments. Asterisks indicate statistical significance from a value of 1 as determined by Student’s t test (P ≤ 0.05).
To confirm that the UPR is activated by GNRH in primary gonadotrope cells, pituitaries were isolated from wild-type, sexually mature 9-wk-old male mice, dissociated, and cultured. Only gonadotropes express the receptor for GNRH (30) and thus should be the only cell type responding to GNRH. The cells were treated with GNRH for 30 min, and the splicing of Xbp1 was measured through quantitative PCR using primers specific to the spliced form of Xbp1 (Xbp1s). Splicing of Xbp1 increased 4-fold in response to GNRH (Fig. 2C).
GNRH causes an accumulation of ribonucleoprotein (RNP) complexes
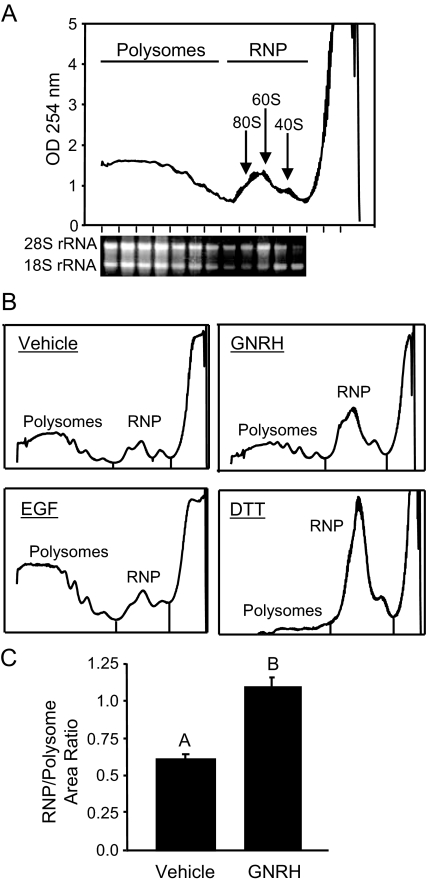
The observation that GNRH causes EIF2A phosphorylation (Fig. 1D) indicates that GNRH may attenuate translation. To determine the general translational status of the LβT2 cells, the cells were treated with GNRH for 30 min, and then ribosome and mRNA complexes were separated on sucrose gradients. Complexes were fractionated according to density while monitoring absorption at 254 nm. Presence of the 28S and 18S rRNAs was used to facilitate combining fractions to represent the actively translating polysome pool, consisting of mRNAs complexed with two or more ribosomes, or the RNP pool, consisting of mRNAs complexed with initiation factors or a single ribosome (monosome), and free 60S or 40S ribosomal subunits (Fig. 3A). The relative abundance of RNA in each pool was compared using the absorption profiles to evaluate the extent of translational regulation by GNRH. This comparison indicated a marked redistribution of RNA in response to acute GNRH, reflecting a general shift from actively translating polysomes to RNP complexes (Fig. 3B). The areas under the ribosome profile curves of the pools were integrated as a measure of the amount of RNA present in each pool. The RNP pool shifted from being about 60% of the polysome pool to being equivalent to the polysome pool with GNRH exposure (Fig. 3C). The degree of remodeling was dose dependent when comparing concentrations of 1, 10, and 100 nm (data not shown).
Figure 3.
GNRH exposure causes an accumulation of RNP complexes in LβT2 cells. A, Sample ribosomal profile from untreated LβT2 cells. RNA and ribosome complexes were harvested and fractionated on sucrose gradients while monitoring UV absorption at 254 nm. Fractions were pooled to represent polysomes (RNA with two or more ribosomes) and RNP complexes (ribosomal subunits, monosomes, initiation complexes), as shown. The peaks in the RNP fractions correspond to the individual ribosomal subunits and full 80S monosomes, as indicated by the relative proportions of 28S and 18S rRNA, representing the presence of the large and small ribosomal subunits, respectively. B, Representative ribosomal profiles of cells treated with GNRH, EGF, or DTT for 30 min. C, The ratio (RNP/polysome) of the integrated area under the curve from the profiles, as an indication of the level of redistribution of ribosomes. The reported values are the means ± sem of six independent experiments. Groups not connected by the same letter are significantly different (P ≤ 0.05) as determined by ANOVA and post hoc Tukey’s HSD test.
As a comparison, cells were treated with epidermal growth factor (EGF), a mitogen known to stimulate translation (31) but not induce the UPR. As expected, an increase in polysomes compared with RNP was observed (Fig. 3B). In contrast, treatment of the cells with DTT resulted in the expected depletion of polysomes (Fig. 3B), similar in direction but greater in magnitude than that of GNRH.
Disruption of intracellular calcium mimicks RNP accumulation by GNRH
GNRH is responsible for inducing secretion and de novo synthesis of gonadotropins in gonadotropes. GNRH mainly acts through a G protein-coupled receptor to activate Gαq and phospholipase C-β, mediating production of inositol 1,4,5 triphosphate (IP3) and diacylglycerol signaling intermediates. Diacylglycerol mediates activation of protein kinase C (PKC) (ε, δ) and all four MAPK cascades, ERK, c-Jun N-terminal kinase, p38, and MAPK7 (also known as BMK1) (5). ERK has been implicated in regulation of translation by GNRH (32). The IP3-mediated release of calcium from internal stores triggers secretion, whereas calcium influx from the extracellular environment renews internal stores (5). Modulation of protein synthesis in response to mitogenic signals is often regulated through phosphatidylinositol 3-kinase (PI3K) and FRAP (FK506-binding protein 12-rapamycin associated protein 1, also known as MTOR), the downstream targets of which include 4E-binding protein (EIF4EBP1, also known as 4EBP1 or PHAS-1) and ribosomal protein S6 kinase (p70s6k) (33). FRAP is a target of receptor tyrosine kinases and G protein-coupled receptors (34).
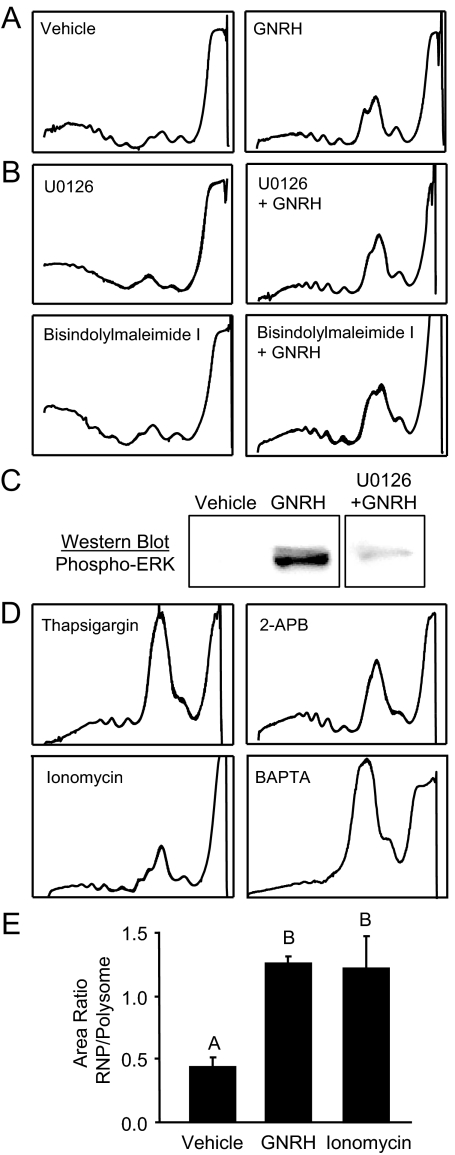
To understand the effect on translation mediated by GNRH, LβT2 cells were treated with pharmacological inhibitors of the various signaling pathways before GNRH exposure to determine which signaling pathways are required. The use of U0126 (Fig. 4B) to block ERK activation had no effect on GNRH-induced RNP accumulation. The efficacy of U0126 was confirmed by Western blotting for the phosphorylation status of ERK after GNRH treatment, in the presence of U0126 (Fig. 4C). Similar results were obtained using PD98059 to block ERK, SB203580 to block p38, SP600125 to block c-Jun N-terminal kinase (data not shown), or bisindolylmaleimide I to block PKC activation (supplemental data published as supplemental Fig. 1 on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). The results indicate that the PKC and MAPK signaling pathways are not required for ribosomal remodeling.
Figure 4.
Accumulation of RNP complexes is mimicked by disruption of intracellular calcium. LβT2 cells were treated and ribosomes were fractionated while monitoring UV absorption. A, Representative profiles from cells treated with vehicle or GNRH for 30 min. B, Representative profiles from cells treated with U0126 to inhibit MEK/ERK activation, before GNRH exposure for 30 min. C, The efficacy of U0126 was confirmed through Western blotting to determine ERK phosphorylation status. D, Representative profiles of cells treated with sarcoplasmic reticulum/ER Ca2+ adenosine triphosphatase antagonist thapsigargin, IP3-receptor antagonist 2-APB, calcium ionophore, and secretagogue ionomycin, or calcium chelator BAPTA. The profiles indicate that disruption of calcium homeostasis causes an accumulation of RNP complexes similar to GNRH. E, The ratio (RNP/polysome) of the integrated area under the curve from the profiles of GNRH and ionomycin-treated cells, as an indication of the level of redistribution of RNA. The reported values are the means ± sem of four independent experiments. Groups not connected by the same letter are significantly different (P ≤ 0.05) as determined by ANOVA and post hoc Tukey’s HSD test.
Similar to the inhibition of PKC/ERK, inhibiting calcium/calmodulin-dependent protein kinase II α activation via KN62 and voltage-gated calcium channel activation via nimodipine had no effect (data not shown). The use of translation inhibitors LY 294002 to block PI3K or rapamycin to block FRAP were not fruitful, because these agents alone caused translation attenuation (data not shown). Furthermore, although GNRH has been shown to activate PI3K via transactivation of the EGF receptor (35), signaling through PI3K and/or FRAP would result in a positive effect on translation, which is not consistent with an accumulation of RNP complexes. In LβT2 cells, PI3K appears to diverge from the classical receptor tyrosine kinase-signaling cascade, not utilizing AKT1 (and presumably, then, FRAP) in its direct downstream effects on cell survival (35), gonadotropin synthesis (36), or translation initiation factors (32). For these reasons, the PI3K/FRAP arm was not pursued further.
In contrast, ER calcium disrupters such as thapsigargin, which blocks calcium reuptake by the sarcoplasmic reticulum/ER Ca2+ adenosine triphosphatase pump, 2-aminoethoxydiphenyl borate (2-APB), which blocks IP3 receptors, calcium ionophore ionomycin, which increases intracellular calcium levels and is a known LH secretagogue (37), and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), which chelates calcium, were all sufficient to induce RNP remodeling, similar to GNRH (Fig. 4, D and E). Pharmacological agents that disrupt ER calcium stores have been shown to induce the UPR (38). Thus, ER and intracellular calcium levels appear to be involved in mediating RNP accumulation in response to GNRH stimulation.
Lhb, Cga, and Gapdh mRNAs redistribute to RNP complexes
To evaluate whether the overall ribosome changes seen after exposure to GNRH are reflected in the manner in which specific mRNAs relevant to gonadotrope function behave, Lhb and Cga mRNAs were examined. In the differentiated gonadotrope, GNRH stimulates transcription and synthesis of the LH subunits, which are modified and exported through the ER and are therefore dependent on proper ER function.
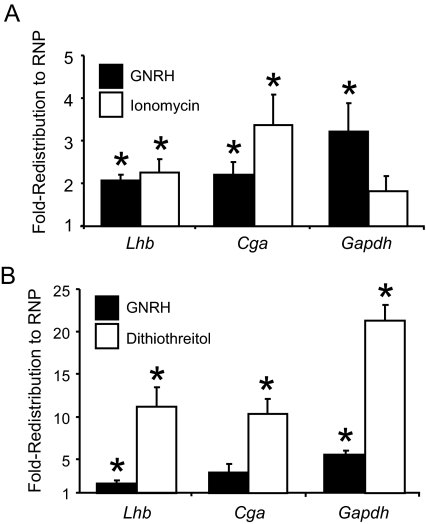
The behavior of Lhb and Cga mRNA was determined by measuring their mass ratio between the polysome and RNP pools. Redistribution of an mRNA species was calculated by comparing these ratios in GNRH-treated and vehicle-treated (control) samples. The calculated fold redistribution is thus a measurement of the change in transcript representation in each of the pools after GNRH treatment. In agreement with the overall shift of ribosomes (Figs. 3 and 4), Lhb, Cga, and Gapdh mRNAs all redistributed to RNP complexes after GNRH exposure (Fig. 5). This behavior was recapitulated by ionomycin (Fig. 5A) and DTT (Fig. 5B) treatment. The magnitude of redistribution of ionomycin mimicked GNRH, whereas DTT was much greater, consistent with the degree of ribosome movement induced by these agents (Figs. 3 and 4).
Figure 5.
Lhb, Cga and Gapdh mRNAs redistribute to RNP complexes in response to GNRH. LβT2 cells were treated as indicated, and ribosome/RNA complexes were fractionated. mRNA was isolated from the RNP and polysome pools of the fractionated extracts and measured using quantitative PCR. A fold-redistribution value was calculated as a measurement of the change in transcript representation in the pools after treatment, as compared with control (vehicle) treatment. The data are represented in the histogram such that a positive value indicates movement into the RNP pool, and a negative fold-redistribution value indicates movement into the polysome pool. A fold-redistribution value of 1 represents no change after treatment. A, The redistribution of mRNAs was calculated for cells exposed to 30 min of GNRH (black) or ionomycin (white). The reported values are the means ± sem of four independent experiments. B, The redistribution of mRNAs was calculated for cells exposed to 30 min of GNRH (black) or DTT (white). The reported values are the means ± sem of three independent experiments. Asterisks indicate statistical significance from a value of 1 as determined by Student’s t test (P ≤ 0.05).
GNRH attenuates translation initiation
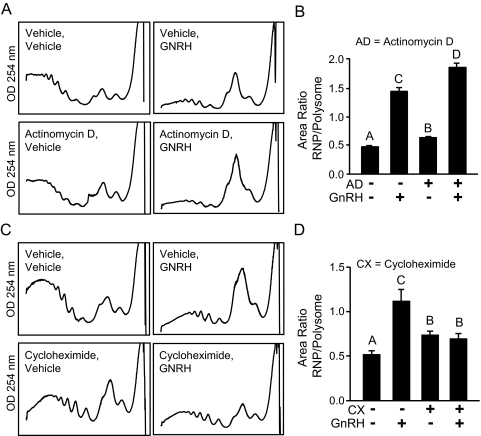
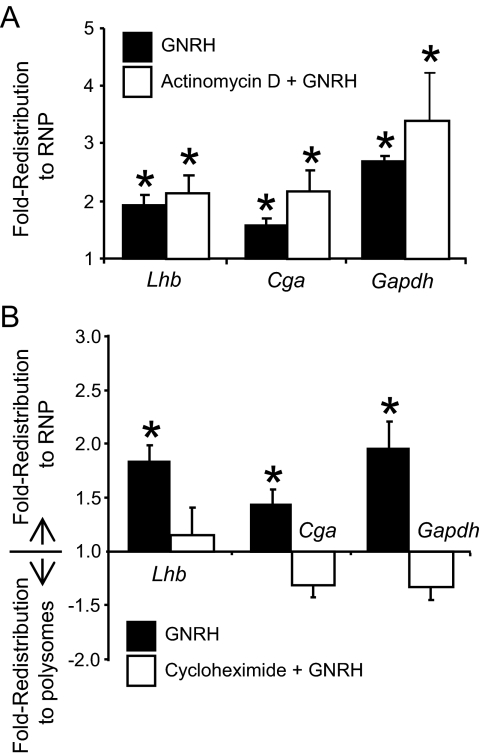
The observed accumulation of RNP complexes after treatment with GNRH may be a result of two distinct responses. First, the stimulatory effect of GNRH on transcription of various genes has been well described (18,19,20,39,40) and so GNRH could be stimulating production of new mRNA and ribosomes that are assembling in new translation initiation complexes, resulting in an increase of RNP complexes. In this scenario, inhibition of transcription would block any GNRH-induced redistribution. To test this possibility, LβT2 cells were treated with actinomycin D to block transcription before treatment with GNRH, and ribosomes were fractionated. Actinomycin D alone caused a slight redistribution of RNA into the RNP pool (Fig. 6, A and B). However, GNRH caused an additional influx, similar in magnitude to that seen in control samples. Measurement of the distribution of Lhb, Cga, and Gapdh mRNA was also consistent with this behavior (Fig. 7A). The efficacy of actinomycin D was confirmed by its ability to inhibit stimulation of Egr1 transcription by GNRH (data not shown). Because actinomycin D did not block the remodeling, this indicates that the rise in RNP complexes and accumulation of mRNAs within those complexes are independent of transcriptional events.
Figure 6.
GNRH attenuates translation by redistributing ribosomes to RNP complexes. LβT2 cells were pretreated with actinomycin D (AD) to block transcription or cycloheximide (CX) to block translation elongation and then treated with 10 nm GNRH or vehicle (PBS) for 30 min. Ribosome/RNA complexes were fractionated while monitoring UV absorption. A and C, Representative profiles. B and D, The ratio (RNP/polysome) of the integrated area under the curve from the profiles, as an indication of the level of redistribution of ribosomes. Cycloheximide, but not actinomycin D, blocks redistribution in response to GNRH, indicating that active translation, but not new mRNA or ribosome synthesis, is necessary for the accumulation of RNP complexes. The reported values are the means ± sem of four or more independent experiments. Groups not connected by the same letter are significantly different (P ≤ 0.05) as determined by ANOVA and post hoc Tukey’s HSD test.
Figure 7.
Translation of Lhb, Cga, and Gapdh mRNAs is attenuated. LβT2 cells were pretreated with actinomycin D to block transcription or cycloheximide to block translation elongation and then treated with 10 nm GNRH or vehicle (PBS) for 30 min. Ribosome/RNA complexes were fractionated. mRNA was isolated from the RNP and polysome pools of the fractionated extracts and measured using quantitative PCR. A fold-redistribution value was calculated as a measurement of the change in transcript representation in the pools after treatment, as compared with control (vehicle) treatment. The data are represented in the histogram such that a positive value indicates movement into the RNP pool, and a negative value indicates movement into the polysome pool. A fold-redistribution value of 1 represents no change after treatment. A, The redistribution of mRNAs was calculated for cells exposed to 30 min of GNRH (black) or pretreated with actinomycin D before GNRH (white). B, The redistribution of mRNAs was calculated for cells exposed to 30 min of GNRH (black) or pretreated with cycloheximide before GNRH (white). All the reported values are the means ± sem of four or more independent experiments. Asterisks indicate statistical significance from a value of 1 as determined by Student’s t test (P ≤ 0.05).
The second cause of RNP accumulation may be ribosome dissociation from polysomal mRNA after exposure to GNRH, thus redistributing mRNAs into the RNP pool, such as predicted by activation of the UPR and an attenuation of translation. In this scenario, inhibition of translation elongation would prevent ribosomes from dissociating, thus blocking any GNRH effect. To test this, LβT2 cells were pretreated with cycloheximide to block translation elongation before treatment with GNRH. Blocking translation elongation indeed blocked the redistribution of ribosomes (Fig. 6, C and D) as well as the redistribution of Lhb, Cga, and Gapdh mRNAs (Fig. 7B). Cycloheximide pretreatment alone caused a slight change in the distribution of RNA (Fig. 6, C and D). However, GNRH did not cause a further redistribution. Additionally, no changes in total mass of RNA were detected between vehicle and GNRH-treated cells, consistent with ribosome redistribution rather than influx of newly synthesized mRNA and ribosomes (data not shown). Overall, the results confirm that the RNP increase caused by GNRH is indicative of an attenuation of translation.
Attenuation of translation is transient
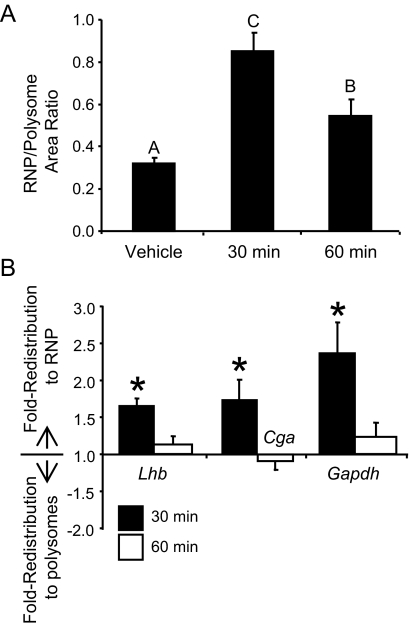
The primary function of pituitary gonadotropes is to produce LH and FSH, and thus it seems paradoxical that GNRH would decrease the translation of mRNAs that are important for the production of these hormones. It was of interest to determine whether this attenuation is permanent or transient. To test this, LβT2 cells were exposed to a 10-min pulse of GNRH and fractionated at various times after stimulation. Integration of the areas under curve of the resulting ribosome profiles indicates that a 10-min exposure to GNRH is sufficient to cause an accumulation of RNP complexes similar in magnitude to a 30-min exposure (Fig. 8). After 60 min, redistribution into RNP complexes subsides, but does not return to basal levels (Fig. 8A). Using quantitative PCR to follow the distribution of Lhb, Cga, and Gapdh mRNAs between polysomes and RNP complexes revealed that whereas the RNP accumulation does not completely resolve, the distribution of the specific transcripts examined does return to basal levels within 60 min (Fig. 8B).
Figure 8.
Attenuation of translation by GNRH is transient. LβT2 cells were treated with GNRH for 10 min and then GNRH was removed, cells were rinsed, and the media were replaced by fresh media. The cells were incubated for another 20 min or 50 min before harvest. Ribosome/RNA complexes were then fractionated. A, The ratio (RNP/polysome) of the integrated area under the curve from the profiles, as an indication of the level of redistribution of ribosomes. B, mRNA from the RNP and polysome pools of fractionated extracts was measured using quantitative PCR. A fold-redistribution value was calculated as a measurement of the change in transcript representation in the pools after GNRH treatment, as compared with control (vehicle) treatment. The data are represented in the histogram such that a positive value indicates movement into the RNP pool. A fold-redistribution value of 1 represents no change. The reported values are the means ± sem of six independent experiments. Groups not connected by the same letter are significantly different (P ≤ 0.05) as determined by ANOVA and post hoc Tukey’s HSD test. Asterisks indicate statistical significance from a value of 1 as determined by Student’s t test (P ≤ 0.05).
Discussion
The data show that in pituitary gonadotropes, GNRH, a central regulator of reproduction, induces the UPR pathway through activation of at least two of the three known ER stress sensors: EIF2AK3, which leads to translation attenuation through phosphorylation of EIF2A, and ERN1, which leads to splicing of transcription factor Xbp1 mRNA. Initial characterization of UPR signal transduction comes from experiments using agents that severely stress ER homeostasis and rapidly induce all three arms of the UPR. However, evidence is mounting that the UPR is crucial for normal physiological function, especially in the development or function of secretory cells (12). The data presented here support this hypothesis and are the first to examine activation of the UPR in gonadotropes.
Unlike pharmacological agents that severely disrupt ER function, physiological processes may be more subtle and utilize only specific UPR components or activate the UPR to a lesser degree or duration (12). This is observed in plasma cell differentiation, which requires activation of ERN1 but not EIF2AK3 or EIF2A phosphorylation (14). Loss of Eif2ak3 in mice results in loss of β-cell function (15), but loss of Xbp1 shows defects in liver growth (41) and lymphocyte differentiation (13). In myocytes, vasopressin at physiological concentrations activates the UPR but not to the same degree as pharmacological agents. It also does not result in chaperone synthesis or condition the cells to tolerate future ER stressors (42), as pharmacological UPR activation has been shown to do. Whereas GNRH appears to target EIF2AK3 and ERN1, it is unknown whether it targets ATF6. ATF6 acts on ERSEs to stimulate transcription of Ddit3 (DNA-damage inducible transcript 3, also known as Chop or Gadd153), Xbp1 (43) and chaperones, such as Hspa5 (heat shock 70 kDa protein 5, also known as Grp78 or BiP), Hsp90b1 (heat shock protein 90 kDa β member 1, also known as Grp94), and Calr (calreticulin) (44). GNRH has been shown to induce Xbp1 (19,20) and Ddit3 (19). Induction of Xbp1 or Ddit3, however, may also occur via XBP1 itself. Like ATF6, XBP1 can bind to ERSEs, although with less affinity (45). Therefore, GNRH may activate all three arms of the UPR, or just EIF2AK3 and ERN1, bypassing ATF6 and utilizing XBP1 instead to up-regulate Ddit3 and its own mRNA. This remains to be investigated in gonadotropes.
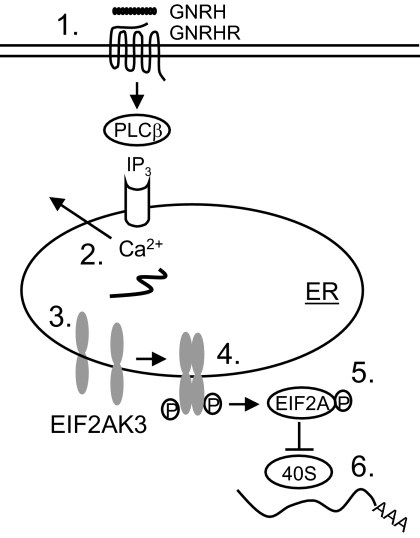
The model presented in Fig. 9 illustrates that GNRH, through activation of the IP3 receptor, causes calcium to leave the ER and thus signals to activate EIF2AK3 and attenuate translation. The involvement of intracellular calcium, but not other signal transducers of the known GNRH signaling pathways, is consistent with the hypothesis that the loss of calcium from the ER elicits the UPR. Ionomycin is an ionophore that carries Ca2+ ions across the plasma membrane into the cytosol and facilitates release of calcium from the ER, as demonstrated in a recent study (46). The data here are consistent with data from others that show exposure of cells to ionophores such as ionomycin inhibits translation and causes an increase in monosomes and free ribosomal subunits (42,47). How loss of calcium mechanistically causes activation of EIF2AK3 is unclear. Multiple signals may integrate to activate the UPR. First, the stimulus to secrete (β-cells) or differentiate (B cells) may be accompanied by an increase in demand for protein synthesis that the ER is not yet prepared for, resulting in accumulation of misfolded proteins that activate the UPR (12). The ER-resident chaperone HSPA5 associates with EIF2AK3 and ERN1 to prevent their dimerization and activation. Dissociation of HSPA5 from these sensors caused by HSPA5 association with unfolded proteins during stress allows EIF2AK3 and ERN1 dimerization and activation (23). Unfolded proteins may also bind directly to ERN1 or EIF2AK3 (7). Data showing that cycloheximide rescues tunicamycin- or thapsigargin-induced impairment of cell growth in cells lacking EIF2AK3 (48) are consistent with the idea that misfolded proteins trigger the UPR. Second, secretory protein production and secretion results in acute loss of amino acids and continued production of reactive oxygen species (ROS) generated by disulfide bond formation. The ER may act as a sensor to combat oxidative stress caused by accumulated ROS (49,50). Cells lacking UPR components accumulate higher levels of ROS that contribute to cell death (51). Finally, calcium may be the primary integrator of stress. Loss of calcium from the ER lumen during secretion may disrupt folding of proteins that require calcium to fold properly, particularly glycoproteins (42). Calcium loss may also directly disrupt chaperones, such as HSPA5, which bind calcium and are involved in maintaining ER calcium levels (52). EIF2AK3 itself has been implicated in integrating calcium-mediated ER stress responses (26). Loss of ER calcium has been linked to UPR activation in myocytes upon vasopressin stimulation (42). It is of interest to determine the mechanism of EIF2AK3 activation, especially in the context of physiological processes such as secretion. Calcium facilitates secretion (4) and transcriptional responses (53) in gonadotropes, and now it appears to play a role in translational control, giving greater credence to its importance in normal pituitary function.
Figure 9.
Proposed model for transient attenuation of translation induced by GNRH. GNRH acting on its receptor (step 1) to induce calcium efflux from the ER (step 2) leads to the loss of calcium and/or accumulation of unfolded proteins (step 3) that allows dimerization and activation by autophosphorylation of EIF2AK3 (step 4). Activated EIF2AK3 phosphorylates translation initiator EIF2A (step 5), leading to translation attenuation through inhibition of initiation complex formation and binding to mRNA (step 6). GNRHR, GNRH receptor; PLC, phospholipase C; AAA, poly-adenylate tail.
GNRH regulates several posttranscriptional processes. GNRH induces Lhb and Cga mRNA 3′-polyadenylation, leading to increased message stability (54). In the presence of GNRH, Cga message half-life increases from 1.2 h to 8 h (55). Interestingly, microarray data in HeLa cells show that mRNAs that are translationally attenuated during ER stress are also stabilized (56). This is consistent with the known increase in stability and now observed translational attenuation of Lhb and Cga mRNAs upon GNRH exposure. The receptor for GNRH (Gnrhr) may also be translationally regulated. GNRH receptor is down-regulated with exposure to high concentrations of GNRH, and its mRNA is bound by fewer ribosomes without a change in overall mRNA levels (57). Recent evidence specifically addresses the regulation of translation by GNRH through examination of cap-binding initiation factors. GNRH causes inactivation of EIF4EBP1, a negative regulator of the EIF4E cap-binding protein required for cap-dependent protein synthesis (32,58). In LβT2 cells, GNRH induces activation (phosphorylation) of several cap-dependent translation initiation factors (32). The stimulation of translation initiation factors can be reconciled with a UPR to attenuate translation. First, whereas the activation or increased availability of initiation factors may serve to contribute to translation of transcripts, the UPR may play an important role in regulating the fidelity of their translation. Second, although this remains to be addressed, the translation attenuation induced by GNRH may not result in attenuation of all mRNAs, because Xbp1 and Atf4 translation is induced by the UPR in other systems. The translation of a particular mRNA during conditions of global regulation such as the UPR may depend on the translational efficiency of that mRNA, which in turn is determined by its abundance, structure, or sequence in untranslated regions (59). Coupling this with changes in activation status, availability of initiation factors or other trans-acting factors may provide a balance that determines which mRNAs are sensitive to translation attenuation or changes in the general translational machinery. This type of specificity has been characterized in the well-studied stimulation of translation of Atf4 mRNA (9) and the related yeast gene GCN4 (60) during the UPR. In yeast, under conditions of general translational inhibition due to glucose deprivation (61), amino acid deprivation (62), or rapamycin-induced stress (63), various transcripts sediment with heavier polysomes, increasing in translational efficiency. The activation of cap-binding initiation factors by GNRH within the context of the UPR may lend specificity to the translational response.
It remains unknown what function the UPR serves in GNRH action. On one hand, activation of the UPR could be considered an acute developmental response to adapt the capacity of the ER of gonadotropes. In culture, the LβT2 cells can be considered naïve to GNRH, and activation of the UPR may be necessary to activate expansion of the ER, as is the case in plasma cells (10). Recently, generation of β-cell-specific Eif2ak3 knockout mice has led to the hypothesis that Eif2ak3 is critical for β-cell proliferation and differentiation during fetal development, rather than for preventing overburdening of the ER and apoptosis postnatally (64). The requirement of Eif2ak3 and the UPR for development of the mature gonadotrope may not be the case here, however, because the primary pituitary cells used in this study were from sexually mature mice that have already experienced GNRH in vivo and yet still up-regulate a marker of the UPR upon GNRH exposure in culture.
On the other hand, the UPR may serve to regulate translational fidelity and protect gonadotropes from apoptosis. The protective effect of the UPR has been observed in other secretory cell types. Global knockout of Eif2ak3 (15,16) or of the ability to phosphorylate EIF2A (17) in mice leads to pancreatic β-cell loss and the development of diabetes. Other secretory cells such as hepatocytes and osteoblasts also show defects with the loss of Eif2ak3 or other UPR components (12). Induction of the UPR protects cells from injury by future stress (65,66). This would fit in well with the pulsatile action of GNRH. As measured in rats (67) and sheep (68), GNRH is released once every 30 min to 1 h, and pulses last for less than 10 min. Whereas perturbations in the ER by pharmacological agents can be categorized as acute insults, physiological processes such periodic hormonal input for secretion can be viewed as chronic ER stress. Activation of the UPR by GNRH is transient in LβT2 cells and is induced at a lower magnitude than by DTT. This is consistent with the idea that GNRH is not activating an acute, extreme stress response but rather modulating the level of protein production in the ER to match synthetic capacity, adapting to physiological ER stress. Similarly, myocytes rapidly recover protein synthesis after vasopressin stimulation of the UPR (42). Paradoxically, the UPR can also signal to apoptotic pathways, such as through induction of Ddit3, which is thought to be induced by all three arms of the UPR (8). GNRH highly induces Atf3 (18,19,20), an inhibitor of Ddit3 (69). Microarray studies using various concentrations and durations of GNRH treatment do not reveal Ddit3 as a target of GNRH action, except in one study where Ddit3 was induced when chronic GNRH was given in conjunction with activin (19). It is possible that Atf3 prevents induction of Ddit3, thus conferring protection from apoptosis. In agreement with this, whereas pharmacological exposure of LβT2 cells to GNRH agonist for 96 h increases apoptosis (70), GNRH has been shown to be protective in vivo (71). Thus, the UPR might serve to prevent or limit the degree of apoptosis, both from GNRH as well as from the apoptotic signals that are induced by the UPR itself.
Although GNRH transiently attenuates translation, pulse-labeling experiments have not shown general changes in protein synthesis in response to GNRH treatment (data not shown). Steady-state levels of Lhb mRNA increases approximately 1.5-fold with GNRH stimulation (72), and this may serve to counter the 2-fold increase of Lhb mRNA in RNP complexes. Any observed decreases in overall translation may also be balanced by stimulation of translation of mRNAs not explored in this study. The transitory nature of translational attenuation induced by GNRH via the UPR is not necessarily targeted at reducing overall protein synthesis, but rather at maintaining the fidelity of translation. Gonadotrope-specific loss of components of the UPR in vivo may result in apoptotic loss of gonadotropes or loss of LHB production due to increased levels of protein misfolding and degradation, similar to the case described for islet cells (15). Future studies using in vivo models of UPR disruption will be necessary to fully characterize the physiological role of the UPR in gonadotropin synthesis and secretion.
The data here show that GNRH, a neuropeptide hormone that regulates reproduction, utilizes a regulatory mechanism previously associated with starvation or stress, and more recently shown to be important for normal secretory function, to modulate gene expression. GNRH signals to the UPR to target the translation of specific mRNAs important to differentiated gonadotrope cell function. This supports the idea that signaling to the UPR by ER-disrupting agents can be thought of as an extension of the UPR’s physiological role in sensing calcium and maintaining the integrity of the ER and the quality of proteins leaving it. This study leads to a more complete understanding of the integration of multiple levels of gene regulation by GNRH and adds to the physiological relevance of the UPR in maintaining cellular homeostasis.
Materials and Methods
LβT2 and primary pituitary cell culture
The LβT2 (3) mouse gonadotrope line was maintained in high-glucose HEPES-buffered DMEM supplemented with penicillin/streptomycin and 10% fetal bovine serum. The cells were incubated at 37 C in a humidified atmosphere of 5% CO2.
For primary pituitary culture, pituitaries were isolated from 9-wk-old wild-type male mice (C57BL/6). Mice were killed using CO2 asphyxiation followed by cervical dislocation, in accordance with UCSD Institutional Animal Care and Use Committee regulations. Whole pituitaries were isolated into ice-cold PBS and then dissociated through incubation in trypsin containing collagenase in a shaking 37 C bath for 15 min. Afterward, the pituitaries were gently pipetted to facilitate dissociation and then returned to the bath for another 15 min. Finally, deoxyribonuclease was added for 10 min, and then the cells were pelleted at 2000 × g for 5 min. The pelleted cells were resuspended and plated on polylysine-coated dishes in the same conditions described for the LβT2 cells.
Hormone and drug treatments
Cells were plated, grown overnight for 36 h before the media was changed to DMEM without serum, and then incubated for an additional 16–18 h. Cells were then treated with vehicle, 10 nm GNRH, 1 μm ionomycin (Calbiochem, La Jolla, CA) for 30 min, 50 nm thapsigargin for 1 h, 75 μm 2-APB (Calbiochem) for 10 min, 50 μm BAPTA (Calbiochem) for 30 min, 2 mm DTT for 30 min, or 10 nm EGF for 30 min. Where appropriate, 5 μm actinomycin D (Calbiochem) was added to the media for 1 h, 100 μg/ml cycloheximide for 5 min, or 1 μm U0126 (Calbiochem) for 30 min, before GNRH treatment. All reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. GNRH at 10 nm was previously shown to induce calcium flux and exocytosis in LβT2 (4). The drugs/hormones were left in the media for the duration of treatment. For the GNRH time course, GNRH was added to the media for 10 min, after which the media were removed, the cells were washed with PBS, and fresh media were added back until harvest.
RNA isolation and RT-PCR
LβT2 and primary pituitary cells were plated at a density of 1 × 107 cells per 10-cm dish or 300,000 cells per 48-well plate, respectively. Total RNA was harvested using Trizol LS (Invitrogen, Carlsbad, CA). The RNA was deoxyribonuclease treated and then reverse transcribed using QuantiTect Reverse Transcription Kit (QIAGEN, Valencia, CA). PCR was performed using HotStarTaq Mastermix (QIAGEN) and transcript-specific primers, as outlined below. Parallel control PCRs were performed using all components except for reverse transcriptase. PCR products were subjected to agarose electrophoresis in the presence of ethidium bromide.
Primer sequences were designed against murine mRNA sequences as available through PubMed. The sequences of the primers used were as follows. Atf6 forward (fwd), 5′-AAAGTCCCAAGTCCAAAGC-3′; Atf6 reverse (rev), 5′-CTGAAAATTCCAAGAGATGC-3′; Ern1 fwd, 5′-AACTCCTCTGTCTGCATCC-3′; Ern1 rev, 5′-GCCAACTATGTTGATAACTTCC-3′; Eif2ak3 fwd, 5′-AGCACGCAGATCACAGTCAGG-3′; Eif2ak3 rev, 5′-TGGCTACGATGCAAAGCAGG-3′.
Ribosome fractionation and mRNA isolation
Two 10-cm plates were used for each experimental condition with 2 × 107 LβT2 cells seeded per plate and treated as described. After treatment, 100 μg/ml cycloheximide was added to all the plates (except for any plates already pretreated with cycloheximide), and the cells were incubated on ice for 5 min. The cells were then harvested in 500 μl of ice-cold polysome extraction buffer (PEB) containing 140 mm KCl, 5 mm MgCl2, 1 mg/ml heparin sodium salt, and 20 mm Tris-HCl (pH 8), supplemented with fresh 0.5 mm DTT and 100 μg/ml cycloheximide on the day of the experiment. Cells from two plates of the same experimental condition were collected and pooled at this step. The cells were spun down at 1000 × g for 3 min, and then each pellet was resuspended in 200 μl PEB containing 1% Triton-X. The pellets were allowed to lyse on ice for 20 min with gentle inversion by hand every few minutes. Finally, the cellular debris was collected by centrifugation at 10,000 × g for 10 min. The supernatants were layered onto 5 ml 10–50% sucrose gradients made with PEB lacking Triton-X. The samples were centrifuged in a SW-55 swing-bucket rotor for 1.5 h at 150,000 × g.
A 21-gauge needle was used to collect fractions from the bottom of the gradient. Fractions were collected using a peristaltic pump while monitoring real-time absorption at 254 nm through a UVM-II monitor (GE Healthcare, Fairfield, CT). Fractions of 250 μl volume each were collected using a FC 203B fraction collector (Gilson, Middleton, WI). Monitoring of UV absorption and fraction collection was controlled through a Labview virtual instrument (National Instruments, Austin, TX) and a KPCI 3108 DAQ card (Keithley Instruments, Cleveland, OH). Fractions were dripped directly into sodium dodecyl sulfate for a final sodium dodecyl sulfate concentration of 1%. Each fraction was then treated with 0.2 mg/ml proteinase K at 37 C for 1.5 h.
RNA was purified from each fraction by phenol-chloroform extraction and ethanol precipitation. An aliquot (1 μl) of RNA from each fraction was subjected to agarose electrophoresis in the presence of ethidium bromide to verify the presence of the large and small ribosomal subunits through presence of the 28S and 18S rRNAs, respectively. The rest of the RNA from the fractions was pooled into polysome and RNP pools according to the gradient’s UV absorption profile. PolyA RNA was then isolated using Oligotex mRNA Mini Kit oligo-dT columns (QIAGEN) according to the manufacturer’s recommendations and eluted into a final volume of 40 μl for each pool. A 10 μl aliquot of mRNA was used for reverse transcription for quantitative PCR analysis.
Quantitative real-time PCR
For quantitative PCR analysis of fractionated, ribosome-bound mRNA, cDNA was synthesized using Omniscript Reverse Transcriptase (QIAGEN) and 10 μm of random hexamer primers (Applied Biosystems, Foster City, CA). For analysis of primary pituitary RNA, cDNA was synthesized using QuantiTect Reverse Transcription Kit (QIAGEN). Quantitative real-time PCR was carried out using the MyIQ Single-Color Real-Time Detection System (Bio-Rad Laboratories, Hercules, CA). The PCR products were amplified in the presence of SYBR Green using the QuantiTect SYBR Green PCR kit (QIAGEN) supplemented with 300 nm of transcript-specific primers and 1 μm fluorescein for proper instrument calibration. The cycling conditions used were those recommended by the PCR kit manufacturer. A melt curve was performed after each PCR run to ensure that just a single product was amplified in each assay, and the size of the products was verified using agarose gel electrophoresis.
Primer sequences were designed against murine mRNA sequences as available through PubMed. The Xbp1s primer set only recognizes the spliced Xbp1 mRNA. The primer sequences were as follows. Cga fwd, 5′-GGTTCCAAAGAATATTACCTCG-3′; Cga rev, 5′-GTCATTCTGGTCATGCTGTCC-3′; Gapdh fwd, 5′-TGCACCACCAACTGCTTAG-3′; Gapdh rev, 5′-GATGCAGGGATGATGTTC-3′; Lhb fwd, 5′-CTGTCAACGCAACTCTGG-3′; Lhb rev, 5′-ACAGGAGGCAAAGCAGC-3′; Xbp1s fwd, 5′-GAGTCCGCAGCAGGTG-3′; Xbp1s rev, 5′-GAATCTGAAGAGGCAACAGTG-3′.
Primers were designed to generate an 80–150 bp amplicon that crossed intron/exon boundaries where possible to avoid amplification of any contaminating unspliced RNA or genomic DNA. All PCRs were performed in triplicate, except for those involving cDNA from primary pituitary cells, which were performed in duplicate. A larger cDNA fragment of Cga, Gapdh, and Lhb was inserted into pCR2.1 (Invitrogen) and used to generate a standard curve and assess PCR efficiency for each run. The mass values for each transcript were extrapolated from the standard curve. For Xbp1s PCRs, cDNA from DTT-treated LβT2 cells were diluted serially and run alongside primary pituitary reactions to assess efficiency. In this case, the Pfaffl method (73) was used to calculate relative mass levels of Xbp1s with respect to Gapdh as an internal control.
To calculate a fold-redistribution value for an mRNA’s movement within the ribosome profile, the mass of each transcript in each ribosome pool (polysome or RNP) was measured, and a ratio was calculated for the mass of that transcript in the RNP pool compared with the polysome pool. A ratio was calculated for both the treated and control (vehicle treated) conditions. A fold-redistribution value was then generated by taking a ratio of the ratios: the RNP/polysome ratio in treated compared with the RNP/polysome ratio in control cells. Statistics were performed as indicated in each figure legend on these values. For ease of representation in the histograms only, the negative inverse was calculated and reported for fold-redistribution values between 0 and 1, such that movement of an mRNA into the polysome pool would report a negative redistribution value, and movement into RNP complexes would report a positive value.
Xbp1 splicing assay
Xbp1 splicing was detected through PCR amplification of Xbp1 by primers that recognize both the spliced and unspliced mRNA, followed by PstI digestion. The primers used were as follows: fwd, 5′-TACGGGAGAAAACTCACG-3′; rev, 5′-TCTGAAGAGCTTAGAGGTGC-3′. After PCR amplification, Xbp1 cDNA (10 μl of a 50 μl PCR) was treated with 20–40 U of PstI (New England BioLabs, Ipswich, MA) for 1 h and then resolved by agarose electrophoresis in the presence of ethidium bromide. The expected PCR band sizes were 549 bp for the unspliced and 523 bp for the spliced form. PstI digestion of unspliced Xbp1 yields fragments of 172 and 381 bp whereas the spliced mRNA is resistant to PstI.
Western blotting
Cells were plated (1 × 107) in 10-cm dishes. After treatment, protein was harvested using standard radioimmune precipitation assay lysis buffer supplemented with phosphatase inhibitors. Standard SDS-PAGE and semidry transfer method (Bio-Rad Laboratories, Hercules, CA) was used to transfer the extracts onto polyvinylidenedifluoride membranes (Bio-Rad Laboratories). Species-specific antibodies were used for all subsequent blotting.
For detecting phosphorylated and total EIF2A, membranes were blocked with 5% nonfat dry milk and the primary antibodies (Cell SignalingTechnology, Inc., Danvers, MA) delivered in 5% BSA. For EIF2AK3, blocking and primary antibody (Rockland Immunochemicals, Gilbertsville, PA) delivery was performed in 5% nonfat dry milk. For detecting phosphorylated ERK, blocking and primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) delivery was performed in 1× casein (Vector Laboratories, Burlingame, CA). All primary antibodies were diluted 1:1000 and incubated overnight. Blots were developed using 1:2000 dilution of biotinylated secondary antibodies (Santa Cruz Biotechnology) and Chemiglow chemiluminescence (Alpha Innotech, San Leandro, CA). Chemiluminescence was visualized using the GeneSnap BioImaging System (Syngene, Frederick, MD).
For confirmation of the phosphorylation status of EIF2AK3, extracts were lysed in radioimmune precipitation assay buffer supplemented with protease inhibitors (Roche, Basel, Switzerland) but not phosphatase inhibitors. Extracts were then treated with 2000 U of λ Protein Phosphatase (New England BioLabs, Ipswich, MA) for 15 min before SDS-PAGE.
Statistical analysis
The areas under the curves of the ribosome profiles were integrated using SigmaPlot version 9.01 (Systat Software, Richmond, CA). Any ratio or fold-redistribution data were transformed using log2, and a value of 10 was added to those values to ensure positive values before analysis. All subsequent analysis was conducted using JMP (SAS Institute, Cary, NC) on untransformed (other than log2 + 10 for ratio or fold-redistribution values) or optimally Box-Cox transformed values. All experiments were repeated at least three independent times, and reported values are the means ± sem.
Supplementary Material
Acknowledgments
We thank Lisa Gallegos for performing the fluorescence resonance energy transfer assays to assess PKC activation.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD043758, K02 HD040803, and U54 HD12303 (to M.L) and Grant T32 GM008666 (to M.T.D.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: 2-APB, 2-Aminoethoxydiphenyl borate; ATF6, activating transcription factor 6; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; DTT, dithiothreitol; EGF, epidermal growth factor; EIF2A, eukaryotic translation initiation factor 2A; EIF2AK3, EIF2A kinase 3; ER, endoplasmic reticulum; ERN1, ER to nucleus signaling 1; ERSE, ER stress response element; FRAP, FK506-binding protein 12-rapamycin associated protein 1; fwd, forward; HSPA5, heat shock 70-kDa protein 5; IP3, inositol 1,4,5 triphosphate; PEB, polysome extraction buffer; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; rev, reverse; RNP, ribonucleoprotein; ROS, reactive oxygen species; UPR, unfolded protein response.
References
- Pawson AJ, McNeilly AS 2005 The pituitary effects of GNRH. Anim Reprod Sci 88:75–94 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Thomas P, Mellon PL, Turgeon JL, Waring DW 1996 The LbT2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 [DOI] [PubMed] [Google Scholar]
- Kraus S, Naor Z, Seger R 2001 Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GNRH) receptor. Arch Med Res 32:499–509 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW 1997 Studies of gonadotropin-releasing hormone (GNRH) action using GNRH receptor-expressing pituitary cell lines. Endocr Rev 18:46–70 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P 2007 Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ 2006 Divergent roles of IRE1α and PERK in the unfolded protein response. Curr Mol Med 6:5–36 [DOI] [PubMed] [Google Scholar]
- Kaufman RJ 2004 Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci 29:152–158 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ 2004 A trip to the ER: coping with stress. Trends Cell Biol 14:20–28 [DOI] [PubMed] [Google Scholar]
- Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS 2006 Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127:999–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ 2006 From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13:374–384 [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH 2001 Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300–307 [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ 2005 The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest 115:268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D 2001 Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7:1153–1163 [DOI] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR 2002 The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ 2001 Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176 [DOI] [PubMed] [Google Scholar]
- Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S 2003 Identification of distinct gene expression profiles associated with treatment of LβT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene 308:67–77 [DOI] [PubMed] [Google Scholar]
- Zhang H, Bailey JS, Coss D, Lin B, Tsutsumi R, Lawson MA, Mellon PL, Webster NJ 2006 Activin modulates the transcriptional response of LβT2 cells to GNRH and alters cellular proliferation. Mol Endocrinol 20:2909–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ 2007 Pulse sensitivity of the luteinizing hormone β promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol 21:1175–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC 2004 Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24:1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH 2003 XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D 2000 Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332 [DOI] [PubMed] [Google Scholar]
- Ma Y, Lu Y, Zeng H, Ron D, Mo W, Neubert TA 2001 Characterization of phosphopeptides from protein digests using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and nanoelectrospray quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 15:1693–1700 [DOI] [PubMed] [Google Scholar]
- Hollien J, Weissman JS 2006 Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107 [DOI] [PubMed] [Google Scholar]
- Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR 2006 PERK (eIF2α kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem J 393:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss T, M WH 2003 Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays 25:1201–1211 [DOI] [PubMed] [Google Scholar]
- Yoshida H 2007 Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid Redox Signal 9:232323–232333 [DOI] [PubMed] [Google Scholar]
- Hirota M, Kitagaki M, Itagaki H, Aiba S 2006 Quantitative measurement of spliced XBP1 mRNA as an indicator of endoplasmic reticulum stress. J Toxicol Sci 31:149–156 [DOI] [PubMed] [Google Scholar]
- Rispoli LA, Nett TM 2005 Pituitary gonadotropin-releasing hormone (GNRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci 88:57–74 [DOI] [PubMed] [Google Scholar]
- Thomas G, Martin-Perez J, Siegmann M, Otto AM 1982 The effect of serum, EGF, PGF2 α and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell 30:235–242 [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA 2004 Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LβT2. Mol Endocrinol 18:1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PB, Fumagalli S, Thomas G 1999 Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev 9:49–54 [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras A-C, Sonenberg N 2000 Regulation of ribosomal recruitment in eucaryotes. In: Sonenberg N, Hershey JWB, Mathews M, eds. Translational control of gene expression. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 245–294 [Google Scholar]
- Rose A, Froment P, Perrot V, Quon MJ, LeRoith D, Dupont J 2004 The luteinizing hormone-releasing hormone inhibits the anti-apoptotic activity of insulin-like growth factor-1 in pituitary αT3 cells by protein kinase Cα-mediated negative regulation of Akt. J Biol Chem 279:52500–52516 [DOI] [PubMed] [Google Scholar]
- Mutiara S, Kanasaki H, Harada T, Oride A, Miyazaki K 2008 The involvement of phosphatidylinositol 3-kinase in gonadotropin-releasing hormone-induced gonadotropin α- and FSHβ-subunit genes expression in clonal gonadotroph LβT2 cells. Mol Cell Endocrinol 283:1–11 [DOI] [PubMed] [Google Scholar]
- Liu F, Ruiz MS, Austin DA, Webster NJ 2005 Constitutively active Gq impairs gonadotropin-releasing hormone-induced intracellular signaling and luteinizing hormone secretion in LβT2 cells. Mol Endocrinol 19:2074–2085 [DOI] [PubMed] [Google Scholar]
- Hendershot LM 2004 The ER function BiP is a master regulator of ER function. Mt Sinai J Med 71:289–297 [PubMed] [Google Scholar]
- Yuen T, Wurmbach E, Ebersole BJ, Ruf F, Pfeffer RL, Sealfon SC 2002 Coupling of GNRH concentration and the GNRH receptor-activated gene program. Mol Endocrinol 16:1145–1153 [DOI] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC 2001 Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem 276:47195–47201 [DOI] [PubMed] [Google Scholar]
- Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH 2000 An essential role in liver development for transcription factor XBP-1. Genes Dev 14:152–157 [PMC free article] [PubMed] [Google Scholar]
- Brostrom MA, Brostrom CO 2003 Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium 34:345–363 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K 2000 ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20:6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K 1998 Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273:33741–33749 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K 2004 Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem 136:343–350 [DOI] [PubMed] [Google Scholar]
- Elzi DJ, Bjornsen AJ, MacKenzie T, Wyman TH, Silliman CC 2001 Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils. Am J Physiol Cell Physiol 281:C350–C360 [DOI] [PubMed] [Google Scholar]
- Brostrom CO, Chin KV, Wong WL, Cade C, Brostrom MA 1989 Inhibition of translational initiation in eukaryotic cells by calcium ionophore. J Biol Chem 264:1644–1649 [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D 2000 Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ 2003 All roads lead to ATF4. Dev Cell 4:442–444 [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS 2004 Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D 2003 An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 [DOI] [PubMed] [Google Scholar]
- Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J 1997 BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem 272:30873–30879 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH 2004 Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 25:521–542 [DOI] [PubMed] [Google Scholar]
- Weiss J, Crowley Jr WF, Jameson JL 1992 Pulsatile gonadotropin-releasing hormone modifies polyadenylation of gonadotropin subunit messenger ribonucleic acids. Endocrinology 130:415–420 [DOI] [PubMed] [Google Scholar]
- Chedrese PJ, Kay TW, Jameson JL 1994 Gonadotropin-releasing hormone stimulates glycoprotein hormone α-subunit messenger ribonucleic acid (mRNA) levels in α T3 cells by increasing transcription and mRNA stability. Endocrinology 134:2475–2481 [DOI] [PubMed] [Google Scholar]
- Kawai T, Fan J, Mazan-Mamczarz K, Gorospe M 2004 Global mRNA stabilization preferentially linked to translational repression during the endoplasmic reticulum stress response. Mol Cell Biol 24:6773–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Laws SC, Rodic V, Sealfon SC 1995 Translational regulation of the gonadotropin-releasing hormone receptor in α T3-1 cells. Endocrinology 136:1128–1136 [DOI] [PubMed] [Google Scholar]
- Sosnowski R, Mellon PL, Lawson MA 2000 Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol Endocrinol 14:1811–1819 [DOI] [PubMed] [Google Scholar]
- Dever TE 2002 Gene-specific regulation by general translation factors. Cell 108:545–556 [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW 2004 Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5:827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KM, DeRisi JL, Brown PO, Sarnow P 2001 Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol Cell Biol 21:916–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova JB, Selley JN, Sanchez-Cabo F, Carroll K, Eddy AA, McCarthy JE, Hubbard SJ, Pavitt GD, Grant CM, Ashe MP 2005 Global gene expression profiling reveals widespread yet distinctive translational responses to different eukaryotic translation initiation factor 2B-targeting stress pathways. Mol Cell Biol 25:9340–9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss T, Baron-Benhamou J, Ansorge W, Hentze MW 2003 Homodirectional changes in transcriptome composition and mRNA translation induced by rapamycin and heat shock. Nat Struct Biol 10:1039–1047 [DOI] [PubMed] [Google Scholar]
- Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR 2006 PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4:491–497 [DOI] [PubMed] [Google Scholar]
- Hung CC, Ichimura T, Stevens JL, Bonventre JV 2003 Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem 278:29317–29326 [DOI] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP 2004 Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 23:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Richardson HN, Chappell PE, Levine JE 2001 In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 142:2929–2936 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130:503–510 [DOI] [PubMed] [Google Scholar]
- Wolfgang CD, Chen BP, Martindale JL, Holbrook NJ, Hai T 1997 gadd153/Chop10, a potential target gene of the transcriptional repressor ATF3. Mol Cell Biol 17:6700–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles LE, Hanyaloglu AC, Dromey JR, Pfleger KD, Eidne KA 2004 Gonadotropin-releasing hormone receptor-mediated growth suppression of immortalized LβT2 gonadotrope and stable HEK293 cell lines. Endocrinology 145:194–204 [DOI] [PubMed] [Google Scholar]
- Yin P, Arita J 2002 Proestrous surge of gonadotropin-releasing hormone secretion inhibits apoptosis of anterior pituitary cells in cycling female rats. Neuroendocrinology 76:272–282 [DOI] [PubMed] [Google Scholar]
- Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC 2002 GNRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GNRH and follistatin. Endocrinology 143:3243–3249 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.