Abstract
Growth differentiation factor 3 (GDF3) is a member of the TGFβ superfamily. White adipose is one of the tissues in which Gdf3 is expressed, and it is the only tissue in which expression increases in response to high-fat diet. We generated Gdf3−/− mice, which were indistinguishable from wild-type mice and had normal weight curves on regular diet. However, on high-fat diet Gdf3−/− mice were resistant to the obesity that normally develops in wild-type mice. Herein we investigate the physiological and molecular mechanisms that underlie this protection from diet-induced obesity and demonstrate that GDF3 deficiency selectively affects white adipose through its influence on basal metabolic rates. Our results are consistent with a role for GDF3 in adipose tissue, with consequential effects on energy expenditure that ultimately impact adiposity.
GDF3 is a TGF beta superfamliy member expressed in white adipose. Gdf3-/- mice are resistant to diet-induced obesity due to effects on the metabolic rate.
The TGFβ superfamily is comprised of structurally similar, but functionally diverse, genes that play important roles in the growth, differentiation, and function of a variety of cell types (1). Some members of the superfamily, including the activins and myostatin, have dramatic influences on growth and body composition (2,3,4,5).
Growth differentiation factor 3/Vg1-related 2 (hereafter, GDF3) is a member of the TGFβ superfamily that shares greatest similarity (79%) with Vg1, a gene with important roles in mesoderm and axis induction in Xenopus laevis and chick embryos (6,7,8). Recently, we and others have shown that GDF3 acts in a similar fashion during the early stages of mouse embryogenesis (9,10,11). GDF3 also is postulated to influence the processes of stem cell maintenance based on its expression in embryonal carcinoma and yolk sac components of human testicular germ cell tumors, mouse F9 teratocarcinoma cells, and several other human primordial germ cell and tumor lines; in addition, Gdf3 mRNA expression is down-regulated when these cells differentiate (12,13,14,15).
Several TGFβ superfamily members have been implicated in regulating preadipocyte determination or differentiation, including bone morphogenetic protein (BMP)2 and BMP7, myostatin, and TGFβ (16,17,18,19,20,21,22). Roles in either stimulating or inhibiting adipogenesis have been demonstrated for these cytokines both in vitro and in vivo, thus indicating that an important balance of superfamily signaling must be maintained for normal adipogenesis and body adiposity.
Evidence is accumulating that GDF3 also has a prominent role in adipose tissue. Northern blot analysis has shown expression of Gdf3 in adipose (23), and mice deficient for fatty acid-binding protein 4 (Fabp4, a fatty acid transport protein in adipocytes) have increased Gdf3 mRNA and protein levels when fed a high-fat diet (24). Additionally, microarray experiments designed to detect changes in gene expression that correlate with body mass or adiposity identified Gdf3 (25). Under high-fat diet conditions, mice overexpressing GDF3 through adenoviral gene transfer display an augmentation of the increase in adiposity that typically is observed in wild-type mice (26), and recent studies strongly implicate ALK7, a candidate type I receptor for GDF3, as an important transducer of this signal in adipose tissue (27).
Our present study provides additional evidence supporting an adipogenic role for GDF3. In wild-type mice maintained on high-fat diet, Gdf3 expression is up-regulated selectively in white adipose when compared with mice on regular diet. We generated mice deficient in GDF3. The Gdf3−/− mice are healthy and fertile with no apparent gross or histological abnormalities, demonstrate normal weight gain on a regular diet, and have a modest reduction in adiposity. However, on a high-fat diet, the Gdf3−/− mice are protected from the obesity that normally develops in wild-type mice under these conditions and exhibit higher basal metabolic rates along with demonstrable differences in tissue mass, histology, and gene expression profiles that is restricted to white adipose. These results are consistent with a role for GDF3 in the development of diet- induced obesity through its selective actions on white adipose, as well as more globally on energy expenditure, thus indicating an important physiological role for GDF3 in the regulation of adiposity.
Results
The pattern of Gdf3 expression suggests possible roles in white adipose
We extended the analysis of Gdf3 expression to include fat from several anatomical locations and found that Gdf3 mRNA is detectable in all brown and white adipose depots. We next investigated whether Gdf3 expression changes under high-fat diet conditions and observed significantly increased levels of Gdf3 transcript in white adipose from mice with high-fat diet-induced obesity (Fig. 1A). This up-regulation was evident in several pooled samples and was further increased in older cohorts. The high-fat diet-induced effect on Gdf3 expression levels was solely observed in reproductive and retroperitoneal white adipose depots. Changes in expression in response to high-fat diet were not seen in spleen, thymus, or mesenteric white adipose, which are the tissues that exhibited the highest baseline levels of Gdf3 expression under regular diet conditions. Next, pooled reproductive fat from 6- to 12-month-old high-fat diet-fed wild-type mice was digested with collagenase to separate the stromal vascular fraction (containing preadipocytes, along with endothelial, nerve, inflammatory, and other cells) from the adipocytes, which were further separated by size as detailed in Materials and Methods. As shown in Table 1, Gdf3 expression in the stromal vascular fraction was consistently higher than in the adipocyte fraction, and more Gdf3 transcript could be detected in the small compared with the large adipocyte samples.
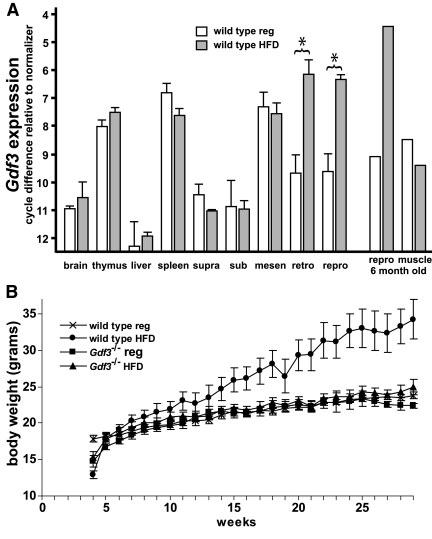
Figure 1.
Effect of high-fat diet on Gdf3 expression in wild-type tissues and on weight gain in Gdf3−/− mice. A, Real-time PCR quantifying Gdf3 expression in tissues from 12-wk-old mice that demonstrated an increase within white adipose in response to high-fat diet. Brown adipose samples were from suprascapular (supra) and subscapular (sub) fat depots. White adipose samples were from mesenteric (mesen), retroperitoneal (retro), and inguinal/parametrial (repro) fat depots. n = 3–6 for each group. An asterisk (*) indicates a statistically significant difference (P < 0.05). Depicted in the right portion of the graph is a representative quantification of Gdf3 expression in pooled tissues (in this instance, 6-month-old mice). B, Growth curves of wild-type and Gdf3−/− female mice fed either regular diet or high-fat diet. Note normal growth rate for Gdf3−/− mice irrespective of diet conditions and protection from diet-induced obesity. n = 10 for each group. HFD, High-fat diet; reg, regular.
Table 1.
Gdf3 expression in fractionated white adipose, fold difference relative to lowest expressor
| Age group | Stromal vascular fraction | Adipocyte fraction | Small adipocytes | Large adipocytes |
|---|---|---|---|---|
| 6 month | 18.4 | 9.8 | ||
| 6 month | 6.1 | 4.3 | 7.0 | 2.6 |
| 6–8 month | 2.5 | 1.6 | 4.3 | 1.0 |
| 9 month | 4.6 | 2.8 | ||
| 10 month | 5.7 | 4.6 | 11.3 | 5.3 |
| 12 month | 3.7 | 2.6 |
Gdf3−/− mice are protected from diet-induced obesity
Gdf3−/− mice were generated by targeted deletion of exon 2, which encodes the entire mature region of the GDF3 protein (supplemental Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org). In addition to confirming the successful knockout allele by Southern blot analysis, RT-PCR analysis of RNA from spleen and white adipose tissue detected no Gdf3 transcript (data not shown). An underrepresentation of homozygous null mice from heterozygous crosses led to the recognition of early embryonic lethality in approximately 35% of Gdf3−/− embryos (9). However, live-born Gdf3−/− pups were grossly and histologically indistinguishable from wild-type pups. Life span and reproductive function were normal. We monitored the growth of wild-type and Gdf3−/− mice, and weight curves on regular diet were the same. However, when fed high-fat diet, the Gdf3−/− mice gained weight at a rate similar to that of mice fed regular diet, rather than emulating the rapid weight gain of wild-type mice that exhibited diet-induced obesity (Fig. 1B).
To determine whether the protection from diet-induced weight gain was a general phenomenon, or a selective effect on the mass of specific tissues, dissections of several organs and individual fat depots were performed in 12-wk-old mice, the age at which the divergence in weight curves became clearly apparent. Organ weights were generally the same when comparing wild-type and Gdf3−/− mice irrespective of diet, except for white adipose tissues from Gdf3−/− mice, which tended to weigh less. However, the differences were not significant (Fig. 2A). In wild-type mice under high-fat diet conditions, the white adipose depots (reproductive and retroperitoneal) markedly increased in mass as expected whereas, in contrast, this diet-induced effect was not observed in the Gdf3−/− mice. Of the brown adipose depots, only suprascapular fat showed a blunted pattern of increase due to its mixed component of sc (white) adipose. Otherwise, the mass of brown adipose depots was not affected by either the diet condition or the GDF3 deficiency state.
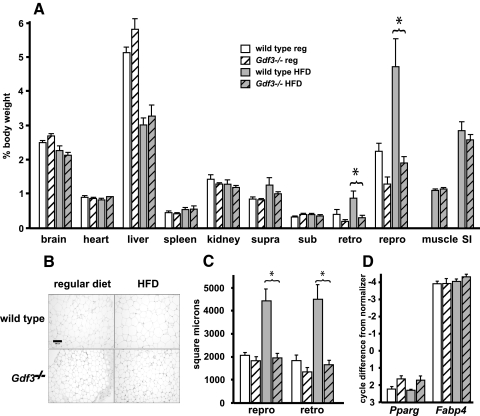
Figure 2.
Body composition, histological, and adipogenic gene expression analyses of 12-wk-old wild-type and Gdf3−/− mice. A, Mass of dissected organs and fat depots represented as a percentage of body weight. The normal diet-induced increase in white adipose depot mass seen in wild-type mice was not observed for the Gdf3−/− mice. Muscle and small intestines (SI) were weighed only from mice on high-fat diet. An asterisk (*) indicates a statistically significant difference (P < 0.05). n = 6–8 for each group. B, Representative histological appearance of retroperitoneal fat. In wild-type regular diet panel, bar is 100 μm. C, Quantification of adipocyte cell surface area. The typical finding of diet-induced adipocyte hypertrophy was not observed in Gdf3−/− mice fed high-fat diet (n = 6–8 for each group). D, Expression analyses of the effect of GDF3 deficiency on adipogenesis markers in whole white adipose tissue (n = 4). HFD, High-fat diet; repro, inguinal/parametrial; retro, retroperitoneal; sub, subscapular; supra, suprascapular.
The difference in white adipose mass was further analyzed histologically. Gdf3−/− white adipose tissues did not exhibit the adipocyte hypertrophy typically seen in wild-type tissues in response to caloric excess (Fig. 2B). Quantification of this difference revealed that under high-fat diet conditions, the cross-sectional areas of adipocytes in Gdf3−/− mice were significantly smaller than those of wild-type mice, and similar to the sizes observed in wild-type mice fed a regular diet (Fig. 2C).
Whole white adipose from Gdf3−/− mice exhibited similar adipogenic gene expression to wild type, as peroxisome proliferator-activated receptor γ (Pparg) and Fabp4 transcript levels were not significantly different (Fig. 2D). Analyses of pooled tissues from older mice also showed no differences in expression (data not shown). When comparing wild-type and Gdf3−/− mouse embryo fibroblasts that were induced in tandem, no significant differences in differentiation were appreciated, because lipid droplets appeared in both groups on d 3–d 4, and there were no differences in the expression of adipogenesis markers (data not shown). Taken together, the results from these experiments indicate that GDF3 is not required for adipocyte differentiation or the expression of adipogenic genes.
Gdf3 expression in 3T3-L1 preadipocytes
The results from the white adipose fractionation studies above, combined with published reports describing high expression levels in many other types of undifferentiated cells, suggested that GDF3 might be present in preadipocytes and during the early stages of adipocyte differentiation. This hypothesis was tested by analyzing the temporal expression of Gdf3 in differentiating 3T3-L1 cells (a mouse preadipocyte cell line). However, Gdf3 transcripts were essentially undetectable before induction (data not shown). The cells were then induced to differentiate utilizing standard protocols, and quantitative RT-PCR was performed on 3T3-L1 cells collected every 8 h during the first 3 d of differentiation, and then daily thereafter. Visual inspection and the expression of adipogenic markers confirmed that differentiation proceeded as expected, but the level of Gdf3 transcript continued to be very low or undetectable at all time points (data not shown).
Mouse physiological studies: GDF3-deficient mice have increased basal metabolic rates
We next investigated the physiological differences which could account for the observed effects of GDF3 deficiency on body mass and adiposity. Intake and output were measured in 6-wk-old mice, at a point before the divergence in weight that develops between wild-type and Gdf3−/− mice. Caloric intake did not differ between wild-type and Gdf3−/− mice while on high-fat diet and therefore was not the underlying cause for the subsequent weight difference (Fig. 3A). The amount of water ingested was the same (data not shown). Also, there was no statistically significant difference in the stool output [average 24-h grams of stool output/grams of food intake: wild-type regular diet: 0.464 ± 0.050, Gdf3−/− regular diet: 0.497 ± 0.010, P = 0.53; wild-type high-fat diet: 0.042 ± 0.002, Gdf3−/− high-fat diet: 0.046 ± 0.002, P = 0.10 (n = 8)]. Analysis of the fat composition of the stool and small intestines indicated that malabsorption was not a contributing factor to the differences in weight gain, because the fecal lipid content was similar between the two genotypes, and the small intestines from Gdf3−/− mice did not differ from the wild type except for modest reductions in mono-, di-, and triglyceride content (Fig. 3B).
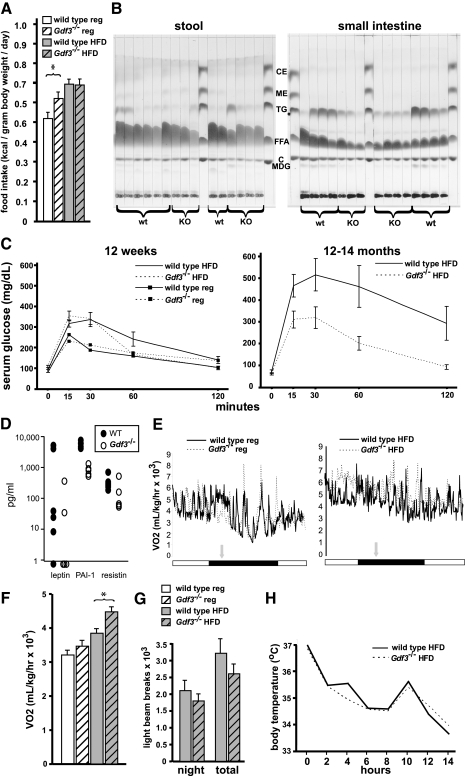
Figure 3.
Physiological analyses of wild-type and Gdf3−/− mice. A, Caloric intake in 6-wk-old mice. Intake of high-fat diet was identical between wild-type and Gdf3−/− mice, but Gdf3−/− mice ate more regular diet (n = 8). B, Fat composition analysis of individual stool (left) and small intestine (right) samples from 12-wk-old mice fed high-fat diet. wt, Wild type; KO, knockout for Gdf3; MDG, mono- and diglycerides; C, cholesterol; FFA, free fatty acids; TG, triglycerides; ME, methyl ester; CE, cholesterol ester. C, Glucose tolerance tests in 12-wk-old mice on regular and high-fat diet (left), and 12- to 14-month-old mice on high-fat diet (right). Error bars were not included for mice fed regular diet to improve visibility of data from the high-fat diet cohorts. For the 12-wk-old mice, note that wild-type mice under high-fat diet conditions were delayed in returning to normal glucose levels 60 min after the glucose challenge. The glucose levels of Gdf3−/− mice at this time point, irrespective of diet, were similar to those of the regular diet-fed wild-type mice (n = 6–8 for each group). In the older cohort, Gdf3−/− mice are protected from diet-induced glucose intolerance (n = 5–7 for each group). D, Serum levels of leptin, PAI-1, and resistin in wild-type (closed circles) and Gdf3−/− (open circles) mice on high-fat diet. PAI-1 levels were significantly lower in Gdf3−/− mice (P = 0.001; n = 5–6). E, Representative 24-h indirect calorimetry tracings for 12-wk-old mice fed regular diet (left) and high-fat diet (right). Horizontal bar under graphs depicts light phase (white) or dark phase (black). Vertical gray arrow indicates removal of food; mice were considered fasted after 4 h. F, Summary of basal metabolic rates in 12-wk-old mice. Oxygen consumption values were collected from fasted mice during the light (quiescent) phase. Increased rates were recorded for the Gdf3−/− mice irrespective of diet and were significantly different on high-fat diet. An asterisk (*) indicates a statistically significant difference (P < 0.05; n = 6–7) for each group. G, Summary of ambulatory activity for 6-wk-old high-fat diet-fed mice. The data was averaged from the last two full days of measurements. Fine activity levels also showed no significant difference between the genotypes (n = 6). H, Cold tolerance tests in 16-wk-old mice. Heavier Gdf3−/− mice were picked that had similar weights as the wild-type mice that were used in this study (n = 3–4). HFD, High-fat diet; reg, regular.
The possible effects of GDF3 deficiency on lipid and glucose metabolism were also studied. Gdf3−/− mice fed high-fat diet had serum lipid profiles that were less abnormal than those from wild-type mice maintained on the same diet; however, none of the differences were significant (Table 2). The response to a glucose challenge was similar in 12-wk-old wild-type and Gdf3−/− mice that were fed a regular diet (Fig. 3C). On high-fat diet, however, evidence of developing glucose intolerance was apparent in wild-type mice by 12 wk, but age-matched Gdf3−/− mice displayed a normal glucose response. By 12–14 months, wild-type mice had marked glucose intolerance, whereas Gdf3−/− mice continued to have near-normal glucose tolerance curves (Fig. 3C).
Table 2.
Lipid profile
| Genotype | Cholesterol | HDL | LDL | VLDL | Triglycerides |
|---|---|---|---|---|---|
| Wild type (n = 6) | 134 ± 7 | 129 ± 7 | 14 ± 1 | 13 ± 1 | 66 ± 5 |
| Gdf3−/− (n = 5) | 117 ± 4 | 111 ± 3 | 12 ± 1 | 12 ± 1 | 59 ± 3 |
Values are expressed in milligrams/dl. HDL, High-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein.
Levels of serum adipokines were also measured for potential differences between Gdf3−/− and wild-type mice. Plasminogen activator inhibitor 1 levels were significantly lower in Gdf3−/− mice (Fig. 3D). Leptin values varied widely, were overall lower in Gdf3−/− mice, and generally corresponded to adiposity irrespective of genotype; however, due to variance, the differences were not significant. Resistin values were similar between the two groups, whereas levels of IL-6, monocyte chemoattractant protein 1, TNFα, and insulin were undetectable.
The only physiological difference associated with the divergence in diet-induced weight gain in Gdf3−/− mice was elevated energy expenditure. Basal metabolic rates assessed by indirect calorimetry revealed greater oxygen consumption in Gdf3−/− mice relative to their wild-type counterparts that was further augmented by high-fat diet conditions (Fig. 3, E and F). The respiratory quotient was the same (0.65 for both genotypes), however, indicating similar substrate (carbohydrate vs. fat) utilization. The increased basal metabolic rate in Gdf3−/− mice was not due to hyperactivity (Fig. 3G), did not lead to significant differences in body temperature [12-wk-old high-fat diet-fed females, wild type: 36.9 ± 0.2 C, Gdf3−/−: 36.8 ± 0.3 C, P = 0.63 (n = 7–8)], and cold tolerance testing did not reveal significant differences in body temperature in response to a cold environment (Fig. 3H).
Increased expression of metabolic genes in white adipose from GDF3-deficient mice
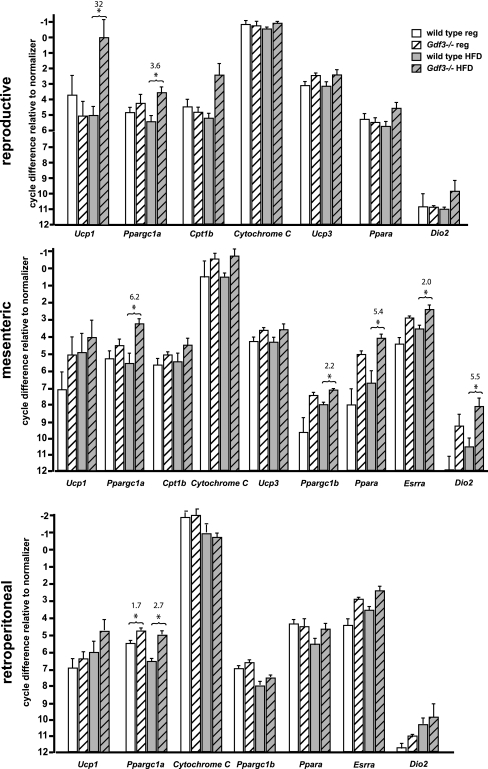
A panel of genes representing diverse metabolic processes was compiled and used to screen for changes in expression that would be consistent with the observed metabolic phenotype. Genes included in the panel affect the metabolic rate by influencing mitochondrial biogenesis and function, by altering the expression of the uncoupling proteins (UCPs) and other downstream genes, or by regulating the availability and oxidation of free fatty acids, which in turn have effects on mitochondrial UCP activity. Under high-fat diet conditions, an up-regulation of several genes, including Ucp1, Ppargc1a, Cpt1, and others, was noted in white adipose from Gdf3−/− compared with wild-type mice (Fig. 4). Interestingly, these increases were not uniform across all white adipose depots, although there was substantial overlap for the pattern of expression of specific genes when experimental groups were compared. In general, highest expression was observed in high-fat diet-fed Gdf3−/− mice, and the mesenteric depots showed the greatest number of genes affected.
Figure 4.
Real-time PCR analysis of metabolically active tissues. Metabolism-related genes up-regulated in white adipose (reproductive, mesenteric, retroperitoneal) from Gdf3−/− vs. wild-type mice. Ucp1, Uncoupling protein 1; Ppargc1a, peroxisome proliferator-activated receptor γ coactivator 1α; Cpt1b, carnitine palmitoytransferase 1β; Ucp3, uncoupling protein 3; Ppara, peroxisome proliferator-activated receptor α; Ppargc1b, peroxisome proliferator-activated receptor γ coactivator 1β; Esrra, estrogen-related receptor α, Dio2, type 2 iodothyronine deiodinase. An asterisk (*) indicates a significant difference (P < 0.05). Numerical values above compared groups indicate fold differences in mRNA levels (n = 4–5 in each group). HFD, High-fat diet; reg, regular.
Effects of GDF3 deficiency are selective to white adipose
Because increased metabolism was the only physiological difference detected in the Gdf3−/− mice, we examined the other tissues that contribute substantially to energy expenditure. Brown adipose, liver, and muscle had not shown an up-regulation of Gdf3 expression in response to high-fat diet conditions (Fig. 1A), and the weights of these tissues collected from wild-type and Gdf3−/− mice did not differ (Fig. 2A). Histological studies also showed no differences. Brown adipose and muscle exhibited cellular architecture that was similar when comparing the two genotypes, including absence of cellular hypertrophy in muscle and brown adipose, and comparable appearance of the lipid droplets in brown adipocytes (supplemental Fig. S2). Comparisons of liver from high-fat diet-fed wild-type and Gdf3−/− mice did not reveal differences in the age of onset or the extent of steatosis (supplemental Fig. S2 and data not shown).
The same panel of metabolic genes used in the expression profiling of white adipose was studied in brown adipose, muscle, and liver. However, none of these genes showed an increase in expression when compared with the same tissues collected from wild-type mice (data not shown).
Discussion
The initial suspicion of an important role for GDF3 in adipocyte biology and the regulation of adiposity was based on its expression in fat and the adipogenic roles of other TGFβ superfamily members. We present additional supporting evidence by characterizing and quantifying the changes in Gdf3 mRNA levels in response to high-fat diet. Gdf3 expression was consistently increased in white adipose (>3 cycle difference by real-time PCR, or approximately 8-fold) irrespective of the sample source (individual or pooled tissues) or the age of the mice. Interestingly, this diet-induced up-regulation was observed only in white adipose and did not occur in any of the other tissues that were examined, including metabolically active ones such as brown adipose, liver, and muscle, or in other tissues that have high Gdf3 expression such as spleen and thymus. Furthermore, not all white adipose depots up-regulated Gdf3. Its expression in mesenteric fat was higher at baseline, but in contrast to the other two white adipose depots examined, did not increase under high-fat diet conditions. The reason for this difference is unclear, but may reflect a constitutive vs. diet-related role for GDF3 in mesenteric as compared with other white adipose depots. Examples of differences in gene expression, protein production, and responses to physiological stimuli among white adipose depots from different anatomic locations have been described (28,29,30), and mesenteric fat is thought to contribute disproportionately to the development of diet-induced insulin resistance as part of the metabolic syndrome in humans (31,32). These collective results not only signify a prominent role for Gdf3 selectively in white adipose but also suggest that depot-selective differences in its up-regulation may be an important consideration in future studies to understand the physiological consequences of a high-calorie environment.
Additional evidence for the importance of GDF3 in the development of obesity is the protective phenotype conferred by the deficiency state. On a high-fat diet, the changes typically observed in wild-type mice, including rapid weight gain, increase in white adipose depot mass, and adipocyte hypertrophy, did not occur in Gdf3−/− mice. These results correspond well with recent similar observations of others (27) and the reciprocal observations of increased adiposity and exaggerated adipocyte hypertrophy in GDF3 overexpressor mice (26).
Only one physiological difference was identified to explain the protection from diet-induced obesity phenotype of Gdf3−/− mice, i.e. basal metabolic rates were increased, especially under high-fat diet conditions. Decreased oral intake was not a contributing factor to the protective phenotype because mice of each genotype ingested the same amounts of high-fat diet. However, an intriguing observation is that Gdf3−/− mice consumed greater amounts of regular diet compared with wild type. This behavior may be a compensatory response to the higher metabolic rates, allowing Gdf3−/− mice to maintain a normal body weight. In contrast, when fed high-fat diet, the increased basal metabolic rate would offset the excess calories that otherwise would have contributed to increased adiposity, resulting in the protection from diet-induced obesity. Other possible contributors to the regulation of energy balance such as physical activity, the thermogenic effect of food (increase in VO2 from fasted to fed state at night was 130 ± 2% for wild type and 128 ± 4% for Gdf3−/− mice; P = 0.65), body temperature differences, energy substrate utilization, and malabsorption were not significantly different between wild-type and Gdf3−/− mice. In some instances, the trend for the Gdf3−/− mice was the opposite of what might be expected for a lean phenotype, including slightly decreased physical activity and decreased content of acylglycerols and triglycerides in the small intestines (Fig. 3B). Lastly, we cannot exclude the possibility that the partial early embryonic lethality of GDF3 deficiency selects for a subset of mice whose metabolisms are somehow programmed to confer the protective phenotype. However, the basis for such a phenomenon at the molecular level is unclear, because the embryonic lethality of Gdf3−/− embryos is due to a perturbation of Nodal signaling, the functional role of which is restricted to events that govern early embryogenesis, including formation of mesoderm and left-right axis determination (9,33,34,35). In adult Gdf3−/− mice, no adverse effects on the weights, cytoarchitechture, or function of mesodermally derived tissues are evident, nor are there abnormalities of the left-right axis. Thus, disruption of Nodal signaling is unlikely to contribute to the antiobesity effects of GDF3 deficiency.
In addition to resisting diet-induced obesity, additional beneficial effects are provided by the GDF3-deficient state. Serum lipid profiles were improved in the Gdf3−/− mice when compared with wild type. Whereas impaired glucose-processing ability was apparent already in the wild-type mice at 12 wk of age, Gdf3−/− mice had normal glucose tolerance. However, this protection is likely a secondary consequence of their reduced adiposity (rather than a direct effect of GDF3 on glucose metabolism) because the mice that overexpressed GDF3, as described by Wang et al. (26), did not exhibit the reciprocal finding of an increased susceptibility to glucose intolerance. With the exception of type 1 plasminogen activator inhibitor (PAI-1), no significant genotype effects on adipokines were observed. The significance of the reduction of PAI-1 in Gdf3−/− mice is unclear; however, elevated PAI-1 levels are associated with an increased risk of thrombosis, cardiovascular disease, obesity, and the metabolic syndrome in humans (36), and at least some of the features of Gdf3−/− mice suggest that they are protected from these conditions.
One of our initial hypotheses was that the protection from diet-induced obesity of Gdf3−/− mice might be due to a direct effect of GDF3 deficiency on preadipocyte differentiation. We have shown that more Gdf3 transcript is present in the stromal vascular cells within white adipose, and that smaller (and presumably less mature) adipocytes express more Gdf3 than larger adipocytes. However, Gdf3 expression is barely detectable in undifferentiated 3T3-L1 and wild-type mouse embryo fibroblasts and remains low during differentiation (data not shown). One explanation for these observations is that the source of GDF3 may not be from preadipocytes as we had initially suspected, but instead from another cell type within the stromal vascular compartment (e.g. endothelial, pericyte, nerve, lymphatic, or immune). Consistent with this hypothesis, our preliminary studies indicate that a macrophage-like cell could be a major source of GDF3 in adipose tissue (data not shown).
Gdf3−/− mice on regular diet are not lipodystrophic and have normal adipocyte sizes and fat pad masses. These results indicate that GDF3 is not required for the differentiation of preadipocytes to mature adipocytes, in vivo. This does not exclude the possibility, however, that GDF3 may modulate the efficiency of the process through its interactions with other TGFβ superfamily members that contribute to normal adipocyte differentiation, i.e. BMPs and myostatin. Consistent with this model, GDF3 has recently been implicated as a BMP inhibitor (10). Additionally, GDF3 can use the same receptors as myostatin, which has been shown previously to have direct and indirect effects on adipogenesis (18,19). Although a direct test of this hypothesis is in order, by adding GDF3 protein directly to differentiating adipocytes, to our knowledge physiologically bioactive GDF3 protein is not available. We have tested the bioactivity of GDF3 protein from two sources: a commercial recombinant version expressed in bacteria, and human embryonic kidney 293T cells that were transfected with the bfGDF3 plasmid to produce conditioned media from which the GDF3 protein was affinity purified. This approach was shown previously to produce large amounts of processed GDF3 protein in conditioned media (9). Unfortunately, neither of these sources of GDF3 has shown convincing activity in three independent bioassays (data not shown), and only modest evidence of Sma- and Mad-related protein (SMAD) 3 phosphorylation (an indicator of receptor activation) is seen at pharmacological doses of the purified protein (500 ng/ml). Therefore, we are unable to make definitive conclusions regarding the effects of exogenous GDF3 protein on adipogenesis. Alternate strategies to address this question are in progress.
Based on the physiological and molecular evidence presented herein, white adipose is likely the primary tissue that produces GDF3 in response to changes in diet, and it is the primary target tissue on which GDF3 acts to modulate the physiological responses to a high-fat diet environment. Furthermore, GDF3 can act in a non-cell-autonomous fashion on white adipose based on the increased adiposity of mice overexpressing GDF3 from an ectopic source (26). However, we have shown that GDF3 is undetectable in differentiating preadipocytes in vitro, and that absence of GDF3 does not adversely affect adipogenesis under these conditions. It is conceivable that the results obtained from 3T3-L1, an aneuploid cell line, do not accurately reflect the cellular and molecular complexity of differentiating adipocytes in vivo, as previously suggested (reviewed in Ref. 37). Another explanation could be that although GDF3 is not absolutely required for adipogenesis, it influences this process at earlier (before the commitment of stem cells to the adipocyte lineage) or later (during adipocyte hypertrophy) stages. Failure of adipocytes from Gdf3−/− mice to hypertrophy under high-fat diet conditions is consistent with the second model, and studies are ongoing to address both possibilities.
Our experiments revealed increased mRNA levels of genes that regulate mitochondrial biogenesis and energy metabolism, two defining characteristics of brown adipose, yet the up-regulation occurred only in white adipose. However, neither immunoblotting, nor immunohistochemistry was sufficient to detect the UCP1 protein, the marker that increased the most in white adipose tissue of Gdf3−/− mice, suggesting that only a small subset of cells within white adipose tissue may be responsible for the changes in gene expression, rather than a more generalized transformation of the cellular phenotype. One possibility is that GDF3 affects the fate decisions of adipocyte precursors, influencing their gene expression in a fashion that favors white adipose differentiation, and that GDF3 deficiency results in the commitment of a subset of these cells to the brown adipose lineage, similar to observations in Eif4ebp1 knockout mice (38), and transgenic mice overexpressing Foxc2 (39) or Adrb1 (40). Alternatively, GDF3 could contribute at a later stage to the regulation of genes that maintain the function of mature white adipocytes; the consequences of GDF3 deficiency would be to change the properties of some of these cells to more closely resemble brown adipocytes, similar to Rb−/− cells (41) or white adipocytes overexpressing Ppargc1a (42). Considering the large contribution of white adipose to the total body mass relative to brown adipose, even a small shift in adipocyte determination or gene expression could have a substantial effect on the basal metabolic rate. The result would be a diet-induced increase in energy expenditure that counteracts the excess calories supplied by the high-fat diet, leading to the obesity resistance of Gdf3−/− mice.
Many mouse models have been described that are protected from diet-induced obesity, and a small subset of this group manifests no other major phenotypic consequences resulting from the null mutation. GDF3 also belongs to this subset but, additionally, may be unique as the only one demonstrated to be up-regulated within white adipose in response to high-fat diet conditions. Because GDF3 is a secreted factor that is present in the circulation with selective effects on white adipose, whereas the consequences of GDF3 deficiency in adult mice are otherwise minimal, it is a particularly attractive target for pharmacotherapeutic inhibition. Investigating mechanisms through which its absence or reduction protects against increased adiposity will lead to a greater understanding of the expanding roles of TGFβ superfamily signaling in the pathophysiology of obesity and its comorbid conditions, with these efforts leading to new interventions to combat the obesity epidemic.
Materials and Methods
Mice and diet
Mice of C57BL6/J and 129S6SvEv mixed genetic background were used and housed in an Institutional Animal Care and Use Committee approved facility in accordance with an approved animal protocol. Access to water and one of two diets was supplied ad libitum. Regular diet (Laboratory Autoclavable Rodent Diet 5010, Purina Mills, Inc., Richmond, IN) had a caloric content of 3.4 kcal/g (calories from fat, 13%; protein, 28%; carbohydrates, 59%); high-fat diet (Mouse Diet High Carbohydrate High Fat F3282; BioServ, Frenchtown, NJ) was 5.3 kcal/g (calories from fat, 59%; protein, 16%; carbohydrate, 24%). Wild-type and Gdf3−/− pups (2–5 d old) were transferred to cages containing lactating wild-type mothers fed either regular or high-fat diet, with consistent diet conditions maintained after weaning. Only female mice were used in this study.
Creation of Gdf3 mutant mice
Electroporation, embryonic stem cell selection and analysis, blastocyst injection, and embryo implantation were as described elsewhere (43,44). In brief, we used an embryonic stem cell line of 129S6SvEv origin derived from AB2.1, which was isogenic to the sequences of the targeting construct. After electroporation, several Gdf3 mutant clones that were correctly targeted were identified by Southern blot analysis and injected into mouse blastocysts to produce chimeras, and eventually Gdf3+/− and Gdf3−/− mice.
Physiological and serum studies
Metabolic rates were assessed by indirect calorimetry (Equal Flow Multi-Animal Air Supply System; Oxymax, Columbus, OH). Mice were placed in individual cages with food and water in the afternoon. The mice were allowed to adjust to the new surroundings for 1 h before the data collection was begun. Access to food was continued for a few hours into the dark phase after which the food was removed before midnight. After 4 h, the mice were considered to be in a fasted state, and further calorimetric measurements were collected for the dark (active) and light (resting) cycles. Basal metabolic rates were determined from the data gathered while the mice were in the fasted state for the following day (typically 7–9 h). Food intake was measured for isolated mice in metabolic cages (Lenderking Caging Products; Millersville, MD). Daily recordings of body weight, food consumption, water intake, and stool output were performed over 1 wk after allowing 48 h for acclimation to the new environment. Activity levels were measured in the Mouse Phenotyping Core at the Baylor College of Medicine utilizing a Photobeam Activity System, i.e. Home Cage (San Diego Instruments, San Diego, CA). The mice were individually housed and fed ad libitum, and activity was recorded for approximately 72 h. The data from d 2 and 3 were used to quantify fine motor and ambulatory activity during the light and dark phases. Cold tolerance tests were performed by measuring rectal temperature with a thermometer (Barnant, Barrington, IL) every 2 h for fasted and individually housed mice placed in a 4 C cold room. Glucose tolerance tests were conducted with tail vein blood and a glucometer (OneTouch Ultra; Lifescan, Milpitas, CA). After an overnight fast, the initial time point was collected and designated “0 min”, 2 g glucose/kg body weight was injected ip, and blood glucose levels were measured at 15, 30, 60, and 120 min. Fasting lipid profiles were obtained from the Comparative Pathology Laboratory at Baylor using a COBAS INTEGRA 400 plus analyzer (Roche Diagnostics, Basel, Switzerland), and fasting adipokine profiles were obtained from the Proteomics Core at Baylor using the LINCOplex mouse adipokine kit (Millipore Corp., Billerica, MA) run on a Bioplex 200 system (Bio-Rad Laboratories, Inc., Hercules, CA).
Tissue dissection, processing, and histology
Intraperitoneal injection of 0.02 ml/g body weight of 2.5% Avertin (Sigma-Aldrich, St. Louis, MO) was used for anesthesia, followed by cardiac puncture for collection of serum, and euthanization by cervical dislocation. The organs collected included brain, heart, liver, kidneys, spleen, small intestines, and skeletal muscle (gastrocnemius and soleus). Small intestines were dissected out from the pylorus to the ileocecal junction and flushed several times with PBS to clean out the intestinal contents. Suprascapular brown adipose depots were weighed en bloc with attached sc white adipose; the white adipose was then trimmed away before RNA extraction. The other brown adipose depot was from the subscapular region, which does not have associated white adipose. White adipose depots included mesenteric, reproductive (inguinal plus parametrial), and retroperitoneal; only the latter two depots were weighed because well-defined anatomic landmarks for consistent dissections were not possible for mesenteric adipose. The mass of the tissues and organs was represented as a percentage of body weight, with the denominator being an approximation of lean body mass that was calculated by subtracting out the weight of the intestines and reproductive fat from the total body weight. Histology specimens were fixed in 4% paraformaldehyde in PBS, embedded in Paraplast X-TRA (Kendall, Mansfield, MA), and mounted. Sections (8 μm) were stained with hematoxylin and eosin. For assessing adipocyte size, a representative microscope field view of white adipose was captured using SPOT Image analysis software (Diagnostic Instruments, Sterling Heights, MI) and analyzed with ImageJ software, version 1.32j (National Institutes of Health, Bethesda, MD; http://rsb. info.nih.gov/ ij/). Each cell surface area was measured and the extent of hypertrophy determined by averaging the top quintile of cell surface areas.
Stool and small intestinal fat composition
Stool collected over a 24-h period was dried at 60 C, weighed, suspended in 1 ml PBS, and then homogenized. Small intestines were homogenized in 3 ml PBS. Each homogenate was mixed with an equal volume of a 1:1 ratio of chloroform-methanol, vortexed vigorously for lipid extraction, and then centrifuged for 10 min at 4000 rpm. The upper organic phase was transferred to another tube and dried to completion under nitrogen, and the lipid residue was resuspended in chloroform; for stool, the volume (in microliters) of chloroform added was equivalent to the original dried stool weight (in micrograms), whereas for small intestines, 200 μl of chloroform was added. Each sample (10 μl) was spotted on to a thin layer chromatography plate, which was then placed in a chromatography chamber with the solvent consisting of a 85:20:1 ratio of petroleum ether-diethyl ether-acetic acid. The lipid standard was composed of cholesterol oleate, methyl oleate, triolein, oleic acid, and cholesterol (20% of each; Nu-Chek Prep, Elysian, MN). After the run was finished, the plates were dried briefly, exposed to sublimated iodine to visualize the separated lipid components, and scanned to preserve the image.
White adipose tissue fractionation
Reproductive fat was aseptically dissected and coarsely minced with scissors before being placed in 3–5 vol Krebs-Ringer HEPES containing 1 mm KH2PO4, 1% BSA, and 1 mg/ml collagenase (Sigma-Aldrich). After dissociation for 1 h at 37 C, the suspension was centrifuged at 2000 rpm for 2 min. The overlying adipocyte layer was removed, washed with 5 volumes of Krebs-Ringer HEPES containing 0.1 mm KH2PO4 and 1% BSA, and then centrifuged. This process was repeated twice more to obtain washed adipocytes for RNA extraction. The pelleted cells from all of the washes were combined together as the stromal vascular fraction. Size fractionation of the adipocytes was conducted by modifying the technique described by Guo et al. (45): the volume was brought up to 10 ml by the addition of buffer, the tube was inverted several times, and after 10 sec, a 1-ml sample of the most buoyant cells was obtained from the surface of the liquid and designated as “large adipocytes”; after 60 sec, another 2-ml sample was collected from beneath the bottom boundary of the adipocyte layer that consisted of cells that had not yet floated to the surface, and were designated “small adipocytes.”
3T3-L1 cells and mouse embryo fibroblasts (MEFs)
3T3-L1 cells were obtained from American Type Culture Collection (Manassas, VA). MEFs were harvested from pregnant wild-type and Gdf3−/− mice that were euthanized by cervical dislocation; the embryos were removed on embryonic d 13.5, homogenized using a P1000 pipetman with 1 ml of MEF growth medium [DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 1% penicillin/streptomycin, 1% l-glutamine, 0.2% β-mercaptoethanol (Sigma-Aldrich)], and then divided equally into several wells of a six-well plate. Individual lines were created from single embryos, and typically there was enough tissue for two (and occasionally three) wells. For all cell types, 2 d after confluency was reached, standard induction protocols were applied: d 0 to d 2, induction media (DMEM, 10% FBS, 1% penicillin/ streptomycin, 1% l-glutamine, 1 mm sodium pyruvate, 0.5 mm 3-isobutyl-1-methylxanthine (IBMX), 1 μm dexamethasone, 10 μg/ml insulin); d 2 to d 4, insulin media (induction media without IBMX or dexamethasone); d 5 to d 8, growth media (induction media without IBMX, dexamethasone, or insulin).
RNA extraction, RT-PCR, and real-time PCR
Freshly collected tissues or cells were flash frozen on dry ice and processed shortly thereafter, or immediately placed in RNA STAT (Leedo Medical, Houston, TX) for extraction according to the manufacturer’s instructions. Generation of cDNA was through established methods utilizing SuperScript III reverse transcriptase (Invitrogen). For real-time PCR, we used software, reagents, and equipment from Applied Biosystems (Foster City, CA). The primer sequences, with the final nanomolar primer concentrations per reaction, will be provided upon request.
Data presentation and statistics
Error bars in all graphs depict the sem. Real-time PCR expression data are graphically represented as the difference in the number of cycles (ΔCt) relative to the normalizer, cyclophilin B. Values included in Table 1 represent fold differences relative to the lowest level of expression (2−ΔΔCt). Cycle number values were normalized to cyclophilin B. Two-tailed t-tests, assuming unequal variance, were performed with Excel data analysis software (Microsoft, Redmond, WA).
Supplementary Material
Acknowledgments
We thank Dianne Houston-Hawkins, Caifen Tang, Steven Coleman, Keltoum Anflous, Keith Weiser, Lan Li, Corinne Spencer, and Barbara Incerti for their technical assistance, and Bruce Witthuhn, David Bernlohr, and Lawrence Chan for helpful discussions.
Footnotes
This work was supported in part by National Institutes of Health Grants HD01156, HD27823, DK073572 (to C.W.B.), HD41648 (to J.J.S.), HD32067 (to M.M.M.), and the Robert Wood Johnson Foundation. This work was also supported in part by Research Grant 5-FY01-482 from the March of Dimes Birth Defects Foundation. C.W.B. was the recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2008
Abbreviations: BMP, Bone morphogenetic protein; GDF, growth differentiation factor; IBMX, 3-isobutyl-1-methylxanthine; MEF, mouse embryo fibroblast; PAI-1, type 1 plasminogen activator inhibitor; UCP, uncoupling protein.
References
- Altmann CR, Chang C, Munoz-Sanjuan I, Bell E, Heke M, Rifkin DB, Brivanlou AH 2002 The latent-TGFβ-binding-protein-1 (LTBP-1) is expressed in the organizer and regulates nodal and activin signaling. Dev Biol 248:118–127 [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ 2002 Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- Carlson CJ, Booth FW, Gordon SE 1999 Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol 277:R601–R606 [DOI] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM 2000 Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25:453–457 [DOI] [PubMed] [Google Scholar]
- Brown CW, Li L, Houston-Hawkins DE, Matzuk MM 2003 Activins are critical modulators of growth and survival. Mol Endocrinol 17:2404–2417 [DOI] [PubMed] [Google Scholar]
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J 1997 Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124:5127–5138 [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD 2002 A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev 114:115–118 [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD 2001 Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128:2915–2927 [DOI] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW 2006 The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133:319–329 [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH 2006 GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development 133:209–216 [DOI] [PubMed] [Google Scholar]
- Andersson O, Bertolino P, Ibanez CF 2007 Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol 311:500–511 [DOI] [PubMed] [Google Scholar]
- Caricasole AA, van Schaik RH, Zeinstra LM, Wierikx CD, van Gurp RJ, van den Pol M, Looijenga LH, Oosterhuis JW, Pera MF, Ward A, de Bruijn D, Kramer P, de Jong FH, van den Eijnden-van Raaij AJ 1998 Human growth-differentiation factor 3 (hGDF3): developmental regulation in human teratocarcinoma cell lines and expression in primary testicular germ cell tumours. Oncogene 16:95–103 [DOI] [PubMed] [Google Scholar]
- Jones CM, Simon-Chazottes D, Guenet JL, Hogan BL 1992 Isolation of Vgr-2, a novel member of the transforming growth factor-β-related gene family. Mol Endocrinol 6:1961–1968 [DOI] [PubMed] [Google Scholar]
- Korkola JE, Houldsworth J, Chadalavada RS, Olshen AB, Dobrzynski D, Reuter VE, Bosl GJ, Chaganti RS 2006 Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res 66:820–827 [DOI] [PubMed] [Google Scholar]
- Ezeh UI, Turek PJ, Reijo RA, Clark AT 2005 Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 104:2255–2265 [DOI] [PubMed] [Google Scholar]
- Sottile V, Seuwen K 2000 Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett 475:201–204 [DOI] [PubMed] [Google Scholar]
- Ji X, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T 2000 Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab 18:132–139 [DOI] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L 2003 Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Streeper RS, Farese Jr RV, Yamamoto KR 2006 Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA 103:15675–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier DE, Comerford SA, Hammer RE 1997 Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-β1 transgenic mice. J Clin Invest 100:2697–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy L, Derynck R 2003 Transforming growth factor-β inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278:9609–9619 [DOI] [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R 2000 Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J Cell Biol 149:667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lee S-J 1993 GDF-3 and GDF-9: two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J Biol Chem 268:3444–3449 [PubMed] [Google Scholar]
- Witthuhn BA, Bernlohr DA 2001 Upregulation of bone morphogenetic protein GDF-3/Vgr-2 expression in adipose tissue of FABP4/aP2 null mice. Cytokine 14:129–135 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang Y, Meng Y, Shi Y 2004 GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem Biophys Res Commun 321:1024–1031 [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P 2008 Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci USA 105:7252–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Leibel RL, Edens NK, Kral JG 1993 Lipolysis in intraabdominal adipose tissues of obese women and men. Obes Res 1:443–448 [DOI] [PubMed] [Google Scholar]
- Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, Vidal H 1998 Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 47:98–103 [DOI] [PubMed] [Google Scholar]
- Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O'Rahilly S 1998 Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 47:1384–1391 [DOI] [PubMed] [Google Scholar]
- Montague CT 2002 Adipose depot-specific effects of PPARγ agonists: a consequence of differential expression of PPAR γ in adipose tissue depots? Diabetes Obes Metab 4:356–361 [DOI] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD 2005 Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 56:45–62 [DOI] [PubMed] [Google Scholar]
- Schier AF 2003 Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19:589–621 [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM 2000 Nodal signalling in vertebrate development. Nature 403:385–389 [DOI] [PubMed] [Google Scholar]
- Whitman M, Mercola M 2001 TGF-β superfamily signaling and left-right asymmetry. Sci STKE 2001:RE1 [DOI] [PubMed] [Google Scholar]
- De Taeye B, Smith LH, Vaughan DE 2005 Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol 5:149–154 [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS 1998 Understanding adipocyte differentiation. Physiol Rev 78:783–809 [DOI] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras A-C, Katsume A, Elchebly M, Spiegelman BM, Harper M-E, Tremblay ML, Sonenberg N 2001 Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 7:1128–1132 [DOI] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S 2001 FOXC2 is a winged helix gene that counteracts obesity, hypertriglyderidemia, and diet-induced insulin resistance. Cell 106:563–573 [DOI] [PubMed] [Google Scholar]
- Soloveva V, Graves RA, Rasenick MM, Spiegelman BM, Ross SR 1997 Transgenic mice overexpressing the β1-adrenergic receptor in adipose tissue are resistant to obesity. Mol Endocrinol 11:27–38 [DOI] [PubMed] [Google Scholar]
- Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, Petrovic N, Enerback S, Nedergaard J, Cinti S, te Riele H, Kristiansen K 2004 Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci USA 101:4112–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D 2003 Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278:33370–33376 [DOI] [PubMed] [Google Scholar]
- Bradley A 1987 Production and analysis of chimaeric mice. In: Robertson EJ, ed. Teratocarcinomas and embryonic stem cells: a practical approach. Oxford, UK: IRL; 131–151 [Google Scholar]
- Matzuk MM, Finegold MJ, Su J-GJ, Hsueh AJW, Bradley A 1992 α-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319 [DOI] [PubMed] [Google Scholar]
- Guo KY, Halo P, Leibel RL, Zhang Y 2004 Effects of obesity on the relationship of leptin mRNA expression and adipocyte size in anatomically distinct fat depots in mice. Am J Physiol Regul Integr Comp Physiol 287:R112–R119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.