Abstract
Transcriptional coactivators and corepressors are emerging as important regulators of energy metabolism and other biological processes. These factors exert their effects on the transcription of target genes through interaction with selective transcription factors and the recruitment of chromatin-remodeling complexes. Recent genetic and biochemical analyses of the peroxisomal proliferator-activated receptor-γ coactivator 1 networks provide novel mechanistic insights regarding their role in the control of mitochondrial oxidative metabolism. These coactivators integrate tissue metabolic functions in response to nutritional signals as well as circadian timing cues. In contrast to coactivators, transcriptional corepressors have been demonstrated to play an opposite role in the control of mitochondrial biogenesis and respiration. The balance of these coactivator and corepressor proteins and, more importantly, their access to specific transcriptional partners are predicted to dictate the epigenetic states of target genes as well as the metabolic phenotype of the cells. This review highlights the biological role and mechanistic basis of the peroxisomal proliferator-activated receptor-γ coactivator 1 networks in the regulation of chromatin-remodeling and mitochondrial oxidative metabolism.
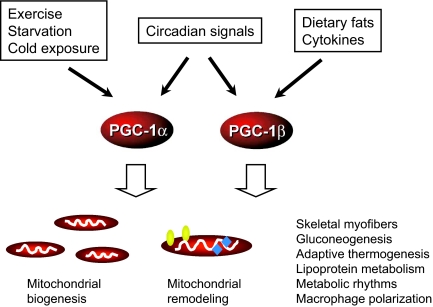
PGC-1 coactivators control mitochondrial metabolism through chromatin-remodeling.
Mitochondrial oxidative phosphorylation (OXPHOS) serves a central role for energy homeostasis in mammals. Mitochondrial volume density and bioenergetic properties are regulated by physiological signals as well as environmental stimuli to meet the demand for energy in tissues. Not surprisingly, mitochondrial mass is elevated in tissues with high ATP consumption, such as cardiac and skeletal muscle, the central nervous system, and brown adipose tissue. Further, the coupling status and fuel preference of mitochondria are influenced by nutritional status in the body. Impaired mitochondrial OXPHOS contributes to the pathogenesis of a wide range of disease conditions, including metabolic disorders, neurodegeneration, and heart failure. Genetic control of mitochondrial biogenesis and function has been an active area of research in recent years, in particular with regard to the role of the peroxisomal proliferator-activated receptor (PPAR) coactivator 1 (PGC-1) family of transcriptional coactivators (1,2,3,4,5). This review focuses on recent studies that highlight the role of PGC-1α and PGC-1β in mitochondrial energy metabolism, its integration with other biological processes, and the molecular analyses of the PGC-1 transcriptional networks.
Regulation of Mitochondrial Energy Metabolism by the PGC-1 Family of Transcriptional Coactivators
PGC-1α was originally identified as a transcriptional coactivator for nuclear hormone receptors that is highly inducible by cold exposure in brown fat (6,7). Overexpression of PGC-1α in cultured adipocytes activates the expression of mitochondrial genes as well as uncoupling protein 1 (UCP1), an important regulator of adaptive thermogenesis. Subsequent analyses in cultured cells and in transgenic mice have established the biological function of PGC-1α in the regulation of mitochondrial oxidative metabolism in diverse cell types. PGC-1α stimulates mitochondrial biogenesis in C2C12 myotubes, which results in increased mitochondrial mass and respiration (8). PGC-1β shares similar molecular structure and function with PGC-1α, including nuclear receptor binding and transcriptional activation as well as several conserved domains. Like PGC-1α, PGC-1β also activates mitochondrial biogenesis; however, PGC-1β increases a larger fraction of coupled respiration (9). These findings suggest that PGC-1α and PGC-1β may have distinct functions in fine tuning mitochondrial metabolism. The expression of PGC-1 and mitochondrial OXPHOS genes is significantly reduced in skeletal muscle from diabetic patients, implicating a potential role for these factors in the pathogenesis of insulin resistance (10,11). In addition to their role in mitochondrial biology, the PGC-1 coactivators also control diverse biological processes, including hepatic gluconeogenesis and lipoprotein metabolism, skeletal muscle fiber determination, circadian clock function, and angiogenesis, as well as macrophage polarization (Fig. 1).
Figure 1.
Metabolic regulation by the PGC-1 coactivators. PGC-1α and PGC-1β are highly responsive to nutritional and circadian signals as well as environmental stimuli. They enhance mitochondrial biogenesis and, in some cases, the remodeling of the bioenergetic properties of mitochondria. These coactivators also regulate glucose and lipid metabolism as well as other biological processes, serving as a molecular integrator of energy metabolism and tissue function.
Several transgenic mouse models have been generated to investigate the metabolic functions of PGC-1 in vivo. Transgenic expression of PGC-1α in skeletal muscle or heart results in robust activation of mitochondrial biogenesis (12,13). Remarkably, muscle-specific transgenic expression of PGC-1α, to the endogenous levels of PGC-1α present in oxidative muscle fibers, drives the formation of slow-twitch muscle fibers (13). Compared with fast-twitch muscle fibers, slow-twitch muscle fibers have high mitochondrial content and can sustain prolonged muscle contraction through oxidation of glucose and fatty acids. Transgenic muscles acquire functional characteristics of slow-twitch muscle and are more resistant to contraction-induced fatigue. PGC-1α expression is induced in skeletal muscle by exercise in rodents as well as in humans (14,15,16). These studies strongly suggest that PGC-1α likely mediate, to a significant degree, the benefits of exercise, particularly with regard to the effects of physical activity on skeletal muscle fiber composition and metabolic characteristics. The induction of PGC-1α in skeletal muscle is regulated by calcium signaling through calcium/calmodulin-dependent protein kinase IV and the myocyte enhancer factor 2 family of transcription factors (17,18). Remarkably, transgenic expression of calmodulin-dependent protein kinase IV in skeletal muscle leads to the induction of PGC-1α and slow-twitch muscle fiber program (18). Recent studies have demonstrated that PGC-1α protects skeletal muscle from starvation- and denervation-induced atrophy (19,20). Consistently, PGC-1α significantly improves muscle function in the context of muscle dystrophy. These beneficial effects of PGC-1α on muscle biology are likely mediated through improved bioenergetic function as well as the amelioration of inflammation in skeletal muscle (2). Transgenic expression of PGC-1β in skeletal muscle also stimulates mitochondrial biogenesis, whereas it activates type IIx muscle fiber markers (21).
The physiological significance of PGC-1α and PGC-1β in mitochondrial energy metabolism has been demonstrated in mouse strains lacking PGC-1α, PGC-1β, or both. PGC-1α null mice have reduced expression of mitochondrial genes in multiple tissues, particularly brown fat, skeletal and cardiac muscles, and the brain (22,23). Their ability to mount adaptive metabolic responses to stresses, such as cold exposure and starvation, is severely impaired in mice lacking PGC-1α. These defects underscore the role of PGC-1α in the control of mitochondrial OXPHOS as well as other metabolic pathways, such as hepatic gluconeogenesis, adaptive thermogenesis, and heme biosynthesis. Subsequent analyses of liver and skeletal muscle-specific PGC-1α null mice further support these conclusions (24,25,26). PGC-1α deficiency profoundly perturbs cardiac energy homeostasis and leads to impaired contractile function and heart failure after aortic constriction (22,27,28). An intriguing finding in these studies was that PGC-1α null mice develop spongioform neurodegeneration in selective brain areas. Given that only a subset of neurons is affected by PGC-1α deficiency, it is likely that neurons have different sensitivity to the deficiency of mitochondrial OXPHOS. Alternatively, PGC-1β may be sufficient to maintain the energetic function of mitochondria in some, but not all, neuronal populations. The significance of PGC-1α in neuronal energy metabolism was further illustrated in mouse models of Huntington’s disease and Parkinson’s disease (29,30).
In PGC-1β null mice, mitochondrial gene expression is also decreased in several tissues, including the liver and brown fat as well as skeletal and cardiac muscle (31,32,33). RNA interference (RNAi) knockdown of PGC-1β in PGC-1α null brown adipocytes further reduces mitochondrial gene expression, suggesting that both factors contribute to normal expression of mitochondrial genes (34). Recent work with mice deficient in both PGC-1α and PGC-1β clearly demonstrate the essential role of these factors in the control of mitochondrial biogenesis (35). Cardiac-specific PGC-1 deficiency causes an arrest in the induction of mitochondrial oxidative program and growth inhibition during the late fetal stage. These results illustrate that both PGC-1 coactivators are required for maintaining normal mitochondrial OXPHOS, potentially in response to distinct physiological signals. In fact, activation of cAMP signaling has been demonstrated to induce PGC-1α expression in brown adipocytes and hepatocytes as well as muscle cells (17,36,37). In contrast, PGC-1β expression is stimulated by certain fatty acids and cytokines (38,39,40).
Several recent studies have focused on identifying novel molecular pathways and chemical compounds that activate the PGC-1α pathway and mitochondrial OXPHOS. TORC2 (transducer of regulated CREB activity 2) has been demonstrated to be an important regulator of PGC-1α expression through its coactivation of cAMP response element binding protein transcription factor (41,42). TORC2 overexpression in C2C12 myotubes induces mitochondrial biogenesis in a PGC-1α-dependent manner (43). Interestingly, uncoupling of mitochondrial respiratory chain leads to the induction of PGC-1α expression, which contributes to the maintenance of ATP homeostasis in cells (44). Thus, partial mitochondrial uncoupling may enhance fuel oxidation through proton leak as well as the induction of mitochondrial biogenesis by PGC-1α. Unexpectedly, large-scale chemical screens have identified several classes of compounds that regulate PGC-1α expression and mitochondrial function, including microtubule inhibitors (45,46). The mechanistic details of how these compounds activate PGC-1α expression and mitochondrial biogenesis remain unknown.
Transcriptional Coactivators in the Integration of Diverse Biological Processes
Transcriptional coregulators are emerging as physiological integrators of energy metabolism and other biological processes (47,48). The expression of PGC-1α and PGC-1β is regulated by nutritional and hormonal signals as well as by circadian pacemakers. A common theme of these coactivator regulatory networks is that, by coactivating multiple transcription factor targets, they are able to coordinate diverse biological processes that constitute biological responses (3,49). For example, the liver adapts to short-term starvation by activating metabolic functions that are critical for systemic nutrient and energy homeostasis. After starvation, genes involved in hepatic gluconeogenic and fatty acid β-oxidation are induced to enhance glucose production and fat oxidation, respectively. These apparently independent metabolic pathways are controlled by different transcription factors. PGC-1α augments the transcriptional function of hepatocyte nuclear factor 4α, FOXO1, and glucocorticoid receptor, which are known to regulate gluconeogenic genes, whereas it coactivates PPARα in the stimulation of peroxisomal and mitochondrial fat oxidation (37,50,51). Interestingly, the function of PGC-1α in this context is negatively regulated by insulin signaling pathway and requires leucine-rich protein 130 kDa (LRP130), a binding partner for PGC-1α (52,53).
On the other hand, PGC-1β regulates triglyceride synthesis and lipoprotein secretion by the liver in response to dietary fat intake. Hepatic expression of PGC-1β using recombinant adenoviral vectors enhances triglyceride synthesis and significantly elevates plasma triglyceride levels (38,54). Through its coactivation of sterol regulatory element-binding protein and liver X receptor, as well as Foxa2 transcription factors, PGC-1β stimulates the program of hepatic very low-density lipoprotein secretion (38,55). Consistent with a role in lipoprotein secretion, PGC-1β null mice have increased triglyceride accumulation in the liver (31,32,33). Recent studies indicate that PGC-1β is a target of cytokine signaling and is induced in response to interferon and IL-4 through the canonical cytokine-signaling pathway (39,40). In macrophages, PGC-1β activates the alternative pathway that enables macrophages to acquire antiinflammatory properties (40). This functional specification of macrophage phenotype is accompanied by the stimulation of oxidative metabolic program. The molecular partners for PGC-1β in the regulation of macrophage phenotype, however, remain to be explored. In addition to its role in macrophages, PGC-1β also cooperates with estrogen-related receptor (ERR)α and regulates downstream genes that are critical for pathogen clearance (39).
In addition to metabolic regulation by nutrient and hormonal signals, the PGC-1 coactivators play a role in the temporal organization of metabolic functions by circadian pacemakers (56,57). The phenomenon of diurnal metabolic rhythm was observed several decades ago (58,59). In addition to the oscillations of circulating nutrients and hormones, the enzymatic activity and flux through major pathways, such as hepatic glucose production and lipogenesis, exhibit robust daily cycles. The restriction of metabolic functions to specific times during the day is believed to provide unique advantages for organisms as they anticipate and synchronize their feeding and activity cycles to the environment. In mammals, the circadian rhythms of physiology and behaviors are coordinated by biological clocks residing in the brain and also in the peripheral tissues (60,61,62,63,64,65). These molecular clocks consist of transcriptional activators and repressors assembled into autoregulatory loops that generate cyclic transcriptional activation with a period of approximately 24 h. Large-scale transcription profiling has revealed extensive transcriptional rhythms of the genes involved in glucose, lipid, and mitochondrial metabolism (66,67,68). Remarkably, the expression of PGC-1α and PGC-1β is under the regulation of circadian clocks. Moreover, rhythmic PGC-1α activation is required for normal circadian rhythms of locomotor activity, body temperature, and metabolic rate (57). Consistently, PGC-1α null mice have aberrant temporal regulation of metabolic gene expression in the liver and skeletal muscle.
Mechanistically, PGC-1α coordinates energy metabolism and the pacemaker functions through direct regulation of the expression of core clock genes (57). PGC-1α stimulates the expression of Bmal1 and Reverbα through its coactivation of the ROR family of orphan nuclear receptors (Fig. 2A). In this case, PGC-1α functions as a component of the clock oscillator through its direct regulation of clock gene transcription. Interestingly, Reverbα suppresses the induction of Bmal1 transcription by PGC-1α, thereby serving as a negative feedback mechanism to turn off the stimulatory effects of PGC-1α. Because PGC-1α expression itself is diurnally regulated, it is possible that the circadian pacemaker also signals to PGC-1α. The molecular mechanisms that transduce the circadian timing cues to PGC-1 expression and/or activity are currently unknown. Interestingly, two groups have recently demonstrated that Sirtuin 1 (SIRT1), an NAD(+)-dependent histone deacetylase, plays an important role in the regulation of clock gene expression (69,70). Because SIRT1 deacetylates PGC-1α and augments its transcription activity (4,71), it is possible that the cyclic induction of SIRT1 may lead to rhythmic activation of PGC-1α. By linking the metabolic and clock transcriptional programs, PGC-1α serves as a molecular switch that synchronizes metabolic functions to the circadian timing cues in mammalian tissues. Mice deficient in PGC-1β have altered locomotor activity patterns (32), suggesting that it may also play a role in the regulation of circadian biological rhythms.
Figure 2.
Transcriptional networks in the control of mitochondrial gene expression. A, PGC-1α and the regulation of molecular clock. Note that PGC-1α activates the expression of Bmal1 and Reverbα, which function as positive and negative regulators of the clock transcriptional network, respectively. B, The PGC-1 coactivators induce mitochondrial gene expression through docking transcription factors and recruiting chromatin-remodeling complexes. The balance between coactivators and corepressors determines the transcriptional output of mitochondrial genes and tissue metabolic phenotype. CBP, cAMP response element binding protein-binding protein; ROR, retinoid-related orphan receptor; Per, period; Cry, chryptochrome.
Transcription Factor Partners for PGC-1α and PGC-1β in Mitochondrial Gene Expression
The transcriptional partners for PGC-1, particularly those that recruit these coactivators to the promoters of mitochondrial genes, have been the focus of recent studies (5,72). PGC-1α interacts with nuclear respiratory factor 1 (NRF1) and NRF2, which bind to their respective sites present on numerous nuclear-encoded mitochondrial genes. Computational analyses of the proximal promoter sequences of mitochondrial genes have identified several novel motifs that may regulate their expression (73,74,75). Among these, binding sites for the ERR family of nuclear receptors, NRF2, and Yin Yang-1 (YY1) were highly enriched for the PGC-1α target genes (74). ERRα expression is strongly induced by PGC-1α, which also functions as a target for PGC-1α coactivation (74,76,77). Overexpression of ERRα in primary rat neonatal cardiac myocytes induces the expression of genes involved in fatty acid uptake, oxidation, and mitochondrial respiration (78). In this context, PPARα also serves as a downstream target gene of ERRα signaling and is required for the induction of the oxidative program by ERRα.
A critical role for ERRα in maintaining mitochondria bioenergetics in the heart was demonstrated using ERRα null mice. ERRα-deficient hearts have reduced OXPHOS gene expression, ATP synthesis rate, and rapid depletion of phosphocreatine after hemodynamic stress (79). Mice lacking ERRα develop signatures of heart failure in response to left ventricular pressure overload. Recently, genome-wide binding and expression analyses elegantly demonstrate a direct role for ERRα as well as ERRγ in orchestrating a broader program of cardiac energy metabolism and contractile functions (80). In C2C12 myotubes, PGC-1α stimulates the expression of PDK4 through coactivation of ERRα, leading to a switch from glucose to fatty acid oxidation (81). PDK4 expression in skeletal muscle and the liver exhibits robust diurnal rhythms in a PGC-1α-dependent manner (57). Hence, ERRα may also mediate the circadian regulation of PDK4 expression by PGC-1α in these tissues. The physical and functional interaction between PGC-1α and ERRα appears to go beyond mitochondrial oxidative metabolism. Recent studies indicate that PGC-1α induces the expression of vascular endothelial growth factor through the ERRα bindings sites present on the vascular endothelial growth factor promoter (82). The induction of angiogenesis by PGC-1α could, in principle, couple oxygen delivery and mitochondrial oxidative metabolism and protects skeletal muscle from ischemic injury.
NRF2 and YY1 are also predicted to regulate the expression of nuclear-encoded mitochondrial genes. The binding sites for these two transcription factors are significantly overrepresented on the promoters of mitochondrial genes, and interestingly, YY1 appears to serve as a common target for the PGC-1α and mammalian target of rapamycin signaling pathways (73,83). Reporter gene assays indicate that YY1 directly activates the promoters of mitochondrial genes, including Ndufs8 and subunits of the mitochondrial F0F1-adenosine triphosphatase (84,85,86). Further, RNAi knockdown of YY1 reduces mitochondrial gene expression as well as respiratory function. These studies implicate YY1 as a key component of the regulatory pathway that mediates the effects of nutrient status on mitochondrial energy metabolism. In the context of mitochondrial gene regulation by growth and nutrient signaling, the E2F4 transcriptional repressor complex appears to play a role in the suppression of mitochondrial metabolism during growth arrest (87). Global chromatin immunoprecipitation studies indicate that E2F4 binding sites are frequently adjacent to the NRF1 sites on mitochondrial genes, suggesting potential functional cross talk between these factors.
In addition to the transcription factors that regulate core mitochondrial gene expression and organelle biogenesis, several transcription factors have been demonstrated to modulate the bioenergetic properties of mitochondria. For example, PGC-1α induces UCP1 expression through coactivating PPARγ in brown adipocytes in response to cold exposure (7). Similarly, PGC-1α induction of Alas1, a rate-limiting enzyme for heme synthesis that resides in the mitochondrial matrix, enhances hepatic heme biosynthesis during starvation (26). The induction of Alas1 by PGC-1α is mediated, at least in part, through the coactivation of FoxO1 in the liver. Along this line, short-term starvation increases the expression of pyruvate dehydrogenase kinase 4 (PDK4) in skeletal muscle and the liver, in part, through the coactivation of ERRα by PGC-1α (81). PDK4 serves as a gatekeeper for the entry of pyruvate into mitochondria for oxidation. The induction of PDK4 results in a switch from glucose to fatty acid oxidation in the mitochondria. Hence, elevated PDK4 is expected to suppress the oxidation of glucose, a physiological response that is necessary for maintaining glucose homeostasis during starvation. The ability to control mitochondrial bioenergetics without affecting mitochondrial mass is emerging as an important mechanism that regulates the metabolic properties of this organelle.
Role of PGC-1α in the Recruitment of Chromatin-Remodeling Complexes
PGC-1α and PGC-1β function through recruiting chromatin-remodeling complexes, including the enzymatic complexes responsible for histone modification and nucleosome remodeling. PGC-1α physically interacts with p300/cAMP response element-binding protein-binding protein and GCN5 histone acetyltransferases (36,88). The recruitment of these histone acetyltransferase complexes to the proximity of PGC-1α target genes increases histone acetylation, a key step in transcriptional activation. Interestingly, GCN5 also acetylates PGC-1α and represses its transcriptional activity, potentially serving as a feedback mechanism. PGC-1α contains multiple lysine residues that undergo acetylation, although the biological function of individual acetylation sites remains to be defined. In contrast, the SirT1 histone deacetylase plays the opposite role and deacetylates PGC-1α in response to cellular nutrient status. Deacetylation of PGC-1α by SirT1 at these lysine residues enhances its function in the regulation of hepatic gluconeogenic and mitochondrial gene expression (4,71,88). In the context of metabolic regulation, PGC-1α appears to serve as an important target of SirT1 and the beneficial effects of resveratrol, an activator compound for SirT1 (89,90).
Recent studies demonstrate that PGC-1α increases histone 3 lysine 4 trimethylation, a hallmark for transcriptional activation, whereas at the same time it reduces H3K9 dimethylation, a marker associated with transcriptional repression (57). The exact histone methyltransferase and demethylase complexes that function in cooperation with PGC-1α remain to be identified. However, both PGC-1 coactivators interact with host cell factor 1, a protein that associates with the mixed lineage leukemia (MLL) family of histone 3 lysine 4 methyltransferase complexes (91,92). It is possible that host cell factor 1 may direct histone methyltransferases to the PGC-1α target loci.
In a genome-wide coactivation study, PGC-1α was found to interact with BRG1/BRM-associated factor (BAF60a), a subunit of the SWI/SNF nucleosome-remodeling complex (93). Adenoviral-mediated expression of BAF60a in primary hepatocytes activates the expression of peroxisomal and mitochondrial fatty acid oxidation genes and lowers hepatic triglyceride levels in mouse models of hepatic steatosis. The BAF60a/PGC-1α complex is required for the transcriptional function of PPARα in the context of hepatic lipid metabolism. Interestingly, BAF60a and its homolog BAF60c also interact with several other nuclear hormone receptors (94,95). As such, BAF60a may function as a targeting subunit for the SWI/SNF complexes through its interaction with PGC-1α and PPARα, leading to the stimulation of specific transcriptional programs. The induction of hepatic fat oxidation genes by PGC-1α is further modulated by Lipin 1, a gene mutated in fatty liver dystrophy (96). BAF60a also induces the expression of PEPCK as well as several clock genes, raising the possibility that the BAF60a/PGC-1α interaction may play a more general role in the transcriptional regulation by PGC-1α.
Transcriptional Repressors in the Regulation of Mitochondrial Oxidative Metabolism
Several transcriptional corepressors, most notably receptor-interacting protein 140 (RIP140) (NRIP1), have been implicated in the regulation of mitochondrial biogenesis (97). RIP140 was initially identified as a nuclear receptor interacting protein that has repressor activities (98,99). Although RIP140 is ubiquitously present in many tissues, its expression is regulated by various hormones and strongly induced during adipocyte differentiation (100,101). Moreover, RIP140 is abundantly expressed in EDL and gastrocnemius, which contain glycolytic and mixed muscle fibers, respectively. In contrast, the mRNA levels of RIP140 are significantly lower in oxidative muscles, such as soleus and diaphragm (102). Recent studies with RIP140 null mice indicate that RIP140 deficiency results in increased mitochondrial gene expression and oxidative capacity. The most striking effects were observed in the EDL muscle with a strong increase of succinate dehydrogenase activity in RIP140 null mice. These metabolic changes are accompanied by increased abundance of oxidative type IIa and IIx muscle fibers in the EDL. Mice deficient in RIP140 are lean and resistant to diet-induced obesity due to increased energy expenditure (100). Consistent with these loss of function analyses, transgenic expression of RIP140 in skeletal muscle has the opposite effects on mitochondrial and muscle fiber gene expression. Elevated RIP140 levels result in more glycolytic muscle phenotype (102). These findings indicate RIP140 and PGC-1α have opposing effects on the metabolic and contractile properties of skeletal myofibers.
Similar effects of RIP140 in the regulation of mitochondrial oxidative metabolism were observed in adipocytes (103,104). RIP140 null adipocytes have increased mitochondrial biogenesis and fatty acid oxidation rate as well as UCP1 gene expression (103). RNAi knockdown of RIP140 in adipocytes enhances insulin-stimulated glucose uptake, likely due to increased glucose oxidation (104). In contrast, reexpression of RIP140 in RIP140 null adipocytes suppresses the expression of a large number of mitochondrial genes and decreases cellular respiratory function. These studies clearly demonstrate that RIP140 regulates the oxidative metabolic program in a cell-autonomous manner. RIP140 contains four repression domains as well as multiple LXXLL motifs that mediate its interaction with nuclear receptors. The repressor activity of RIP140 is mediated, at least in part, through its interaction with histone deacetylase complexes (105,106). In reporter gene assays, RIP140 inhibits the transcriptional activity of all ERR members (107). ERRα also appears to mediate the derepression of UCP1 in RIP140-deficient adipocytes (108). Because ERRα and ERRγ interact with PGC-1 coactivators and are required for normal mitochondrial gene expression, a possible mechanism may involve competitive interaction of ERRα with RIP140 and the PGC-1 coactivators. Similar antagonistic action may occur in the context of other nuclear receptors, particularly PPAR and thyroid hormone receptor, which also regulate the expression of certain mitochondrial genes.
Because both RIP140 and PGC-1α interact with their nuclear receptor partners in a ligand-dependent manner, it remains unclear how the recruitment of these factors respond to hormonal signals. The stoichiometry of coactivators and corepressors in the cell may play an important role in determining the biological outcome. Deficiency of Sin3A, a core component of a multiprotein corepressor complex, significantly increases the expression of genes involved in oxidative metabolism (109,110). The role of Sin3A corepressor in the regulation of mitochondrial genes appears to be conserved in Drosophila and in mice. How the Sin3A transcriptional complex controls mitochondrial gene expression remains unclear. Nevertheless, Sin3A may modulate nuclear receptor function through its interaction with the nuclear receptor corepressor and silencing mediator of retinoid and thyroid hormone receptor corepressor complexes (111,112,113). Together, these data strongly suggest that the relative level and/or activity of coactivators and corepressors may serve an important function in maintaining cellular oxidative metabolism (Fig. 2B).
In summary, the regulatory networks that control mitochondrial oxidative metabolism in mammals comprise transcription factors and coregulators that act in concert to bring about organelle biogenesis as well as fine tuning of its bioenergetic properties. It is somewhat unexpected that transcriptional coactivators and corepressors are emerging as core regulators of the program of oxidative metabolism. A potential advantage here is that coregulators have the capability of modulating the activity of multiple target transcription factors, thereby coordinating different aspects of complex biological programs. In the case of PGC-1 coactivators, it has become clear that several transcription factors are involved, including NRF1, NRF2, ERRs, and YY1. In addition, certain transcription factors appear to mediate the regulation of unique mitochondrial functions in response to physiological signals, such as adaptive thermogenesis and heme biosynthesis. In contrast to PGC-1, RIP140 and Sin3A corepressor complexes inhibit the expression of mitochondrial genes, raising an intriguing possibility that the balance of coactivators and corepressors may determine cellular metabolic phenotype with regard to mitochondrial OXPHOS. Hence, molecular and/or chemical approaches that alter this balance are predicted to be an effective strategy for targeting mitochondrial metabolism and systemic physiology.
Acknowledgments
I thank members of the laboratory for discussions.
Footnotes
This work was supported by the National Institutes of Health (Grant R01DK077086) and the American Diabetes Association.
Disclosure Statement: The author has nothing to disclose.
First Published Online November 13, 2008
Abbreviations: ERR, Estrogen-related receptor; NRF, nuclear respiratory factor; OXPHOS, oxidative phosphorylation; PDK4, pyruvate dehydrogenase kinase 4; PGC, PPARγ coactivator; PPAR, peroxisomal proliferator-activated receptor; RIP140, receptor-interacting protein 140; RNAi, RNA interference; TORC2, transducer of regulated CREB activity 2; UCP1, uncoupling protein 1; YY1, Yin Yang-1.
References
- Finck BN, Kelly DP 2006 PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM 2008 The role of exercise and PGC1α in inflammation and chronic disease. Nature 454:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM 2005 Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P 2008 Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett 582:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC 2008 Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88:611–638 [DOI] [PubMed] [Google Scholar]
- Knutti D, Kaul A, Kralli A 2000 A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol 20:2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM 1998 A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839 [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM 2003 Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278:26597–26603 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP 2000 Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM 2002 Transcriptional co-activator PGC-1 α drives the formation of slow-twitch muscle fibres. Nature 418:797–801 [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO 2002 Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886 [DOI] [PubMed] [Google Scholar]
- Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T 2000 cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274:350–354 [DOI] [PubMed] [Google Scholar]
- Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T 2004 PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 96:189–194 [DOI] [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM 2003 An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci USA 100:7111–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS 2002 Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296:349–352 [DOI] [PubMed] [Google Scholar]
- Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM 2007 PGC-1α regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev 21:770–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM 2006 PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM 2007 The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5:35–46 [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP 2005 PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM 2004 Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM 2007 Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J Biol Chem 282:30014–30021 [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM 2007 Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell crosstalk. J Clin Invest 117:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM 2005 Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 122:505–515 [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM 2005 Transcriptional coactivator PGC-1 α controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271 [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM 2006 Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-γ coactivator 1α. Proc Natl Acad Sci USA 103:10086–10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D 2006 Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM 2006 Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig 2006 A Ablation of PGC-1β results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4:e369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM 2007 PGC-1β controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104:5223–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB 2006 Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM 2006 Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 3:333–341 [DOI] [PubMed] [Google Scholar]
- Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP 2008 Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev 22:1948–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM 1999 Activation of PPARγ coactivator-1 through transcription factor docking. Science 286:1368–1371 [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM 2001 Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM 2005 Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120:261–273 [DOI] [PubMed] [Google Scholar]
- Sonoda J, Laganiere J, Mehl IR, Barish GD, Chong LW, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F, Lee CH, Giguere V, Evans RM 2007 Nuclear receptor ERR α and coactivator PGC-1β are effectors of IFN-γ-induced host defense. Genes Dev 21:1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A 2006 Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab 4:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M 2005 The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437:1109–1111 [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates III JR, Takemori H, Okamoto M, Montminy M 2004 The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119:61–74 [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC 2006 Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA 103:14379–14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohas LM, St-Pierre J, Uldry M, Jager S, Handschin C, Spiegelman BM 2007 A fundamental system of cellular energy homeostasis regulated by PGC-1α. Proc Natl Acad Sci USA 104:7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Wagner BK, Ma Y, Chinsomboon J, Laznik D, Spiegelman BM 2008 Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1α and oxidative phosphorylation. Proc Natl Acad Sci USA 105:4721–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK 2008 Large-scale chemical dissection of mitochondrial function. Nat Biotechnol 26:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, White R, Parker MG 2006 Metabolic regulation by the nuclear receptor corepressor RIP140. Trends Endocrinol Metab 17:243–250 [DOI] [PubMed] [Google Scholar]
- Feige JN, Auwerx J 2007 Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol 17:292–301 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Heinrich R 2004 Biological control through regulated transcriptional coactivators. Cell 119:157–167 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM 2003 Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP 2000 The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MP, Qu L, Rohas LM, Lin J, Yang W, Erdjument-Bromage H, Tempst P, Spiegelman BM 2006 Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1α/LRP130 complex. Genes Dev 20:2996–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ 2007 Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 447:1012–1016 [DOI] [PubMed] [Google Scholar]
- Lelliott CJ, Ljungberg A, Ahnmark A, William-Olsson L, Ekroos K, Elmgren A, Arnerup G, Shoulders CC, Oscarsson J, Linden D 2007 Hepatic PGC-1β overexpression induces combined hyperlipidemia and modulates the response to PPARα activation. Arterioscler Thromb Vasc Biol 27:2707–2713 [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Stoffel M 2006 Coactivation of Foxa2 through Pgc-1β promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab 3:99–110 [DOI] [PubMed] [Google Scholar]
- Lin JD, Liu C, Li S 2008 Integration of energy metabolism and the mammalian clock. Cell Cycle 7:453–457 [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD 2007 Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447:477–481 [DOI] [PubMed] [Google Scholar]
- Hollister LE, Wright A 1965 Diurnal variation of serum lipids. J Atheroscler Res 5:445–450 [DOI] [PubMed] [Google Scholar]
- Seaman GV, Engel R, Swank RL, Hissen W 1965 Circadian periodicity in some physicochemical parameters of circulating blood. Nature 207:833–835 [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS 2004 Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5:407–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Takahashi JS 2000 Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci 23:713–742 [DOI] [PubMed] [Google Scholar]
- Morse D, Sassone-Corsi P 2002 Time after time: inputs to and outputs from the mammalian circadian oscillators. Trends Neurosci 25:632–637 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR 2002 Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, McKnight SL 2002 Metabolism and the control of circadian rhythms. Annu Rev Biochem 71:307–331 [DOI] [PubMed] [Google Scholar]
- Wijnen H, Young MW 2006 Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet 40:409–448 [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB 2002 Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–320 [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ 2002 Extensive and divergent circadian gene expression in liver and heart. Nature 417:78–83 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S 2002 A transcription factor response element for gene expression during circadian night. Nature 418:534–539 [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U 2008 SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328 [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P 2008 The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118 [DOI] [PubMed] [Google Scholar]
- Huss JM, Kelly DP 2005 Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115:547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P 2007 mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450:736–740 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM 2004 Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101:6570–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK 2008 A mitochondrial protein compendium elucidates complex I disease biology. Cell 134:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP 2002 Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J Biol Chem 277:40265–40274 [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A 2003 The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J Biol Chem 278:9013–9018 [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguere V, Kelly DP 2004 Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP 2007 The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6:25–37 [DOI] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V 2007 Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab 5:345–356 [DOI] [PubMed] [Google Scholar]
- Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP 2005 PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25:10684–10694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM 2008 HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451:1008–1012 [DOI] [PubMed] [Google Scholar]
- Xi H, Yu Y, Fu Y, Foley J, Halees A, Weng Z 2007 Analysis of overrepresented motifs in human core promoters reveals dual regulatory roles of YY1. Genome Res 17:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen GA, Vander Zee CA, Jordan EM 1996 Nuclear factor YY1 activates the mammalian F0F1 ATP synthase α-subunit gene. Gene Expr 5:181–191 [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Coombs C, Martin ME 2000 Isolation of a bi-directional promoter directing expression of the mouse GABPα and ATP synthase coupling factor 6 genes. Gene 261:311–320 [DOI] [PubMed] [Google Scholar]
- Lescuyer P, Martinez P, Lunardi J 2002 YY1 and Sp1 activate transcription of the human NDUFS8 gene encoding the mitochondrial complex I TYKY subunit. Biochim Biophys Acta 1574:164–174 [DOI] [PubMed] [Google Scholar]
- Cam H, Balciunaite E, Blais A, Spektor A, Scarpulla RC, Young R, Kluger Y, Dynlacht BD 2004 A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell 16:399–411 [DOI] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P 2006 GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab 3:429–438 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, et al 2006 Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J 2006 Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122 [DOI] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM 2002 Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277:1645–1648 [DOI] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W 2003 Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3–K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev 17:896–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu C, Li N, Hao T, Han T, Hill DE, Vidal M, Lin JD 2008 Genome-wide coactivation analysis of PGC-1α identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab 8:105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debril MB, Gelman L, Fayard E, Annicotte JS, Rocchi S, Auwerx J 2004 Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J Biol Chem 279:16677–16686 [DOI] [PubMed] [Google Scholar]
- Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK 2003 BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol 23:6210–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence Jr JC, Kelly DP 2006 Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab 4:199–210 [DOI] [PubMed] [Google Scholar]
- White R, Morganstein D, Christian M, Seth A, Herzog B, Parker MG 2008 Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett 582:39–45 [DOI] [PubMed] [Google Scholar]
- Cavailles V, Dauvois S, Danielian PS, Parker MG 1994 Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci USA 91:10009–10013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chinpaisal C, Wei LN 1998 Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol Cell Biol 18:6745–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG 2004 Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA 101:8437–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM 2001 Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem 276:34167–34174 [DOI] [PubMed] [Google Scholar]
- Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, Poutanen M, White R, Parker M 2007 The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab 6:236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG 2005 RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25:9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP 2006 Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest 116:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN, Farooqui M, Hu X 2001 Ligand-dependent formation of retinoid receptors, receptor-interacting protein 140 (RIP140), and histone deacetylase complex is mediated by a novel receptor-interacting motif of RIP140. J Biol Chem 276:16107–16112 [DOI] [PubMed] [Google Scholar]
- Wei LN, Hu X, Chandra D, Seto E, Farooqui M 2000 Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J Biol Chem 275:40782–40787 [DOI] [PubMed] [Google Scholar]
- Castet A, Herledan A, Bonnet S, Jalaguier S, Vanacker JM, Cavailles V 2006 Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol 20:1035–1047 [DOI] [PubMed] [Google Scholar]
- Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M, White R 2007 Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor α. Mol Endocrinol 21:1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA 2005 mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev 19:1581–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pile LA, Spellman PT, Katzenberger RJ, Wassarman DA 2003 The SIN3 deacetylase complex represses genes encoding mitochondrial proteins: implications for the regulation of energy metabolism. J Biol Chem 278:37840–37848 [DOI] [PubMed] [Google Scholar]
- Alland L, Muhle R, Hou Jr H, Potes J, Chin L, Schreiber-Agus N, DePinho RA 1997 Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG 1997 A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387: 43–48 [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM 1997 Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373–380 [DOI] [PubMed] [Google Scholar]