Abstract
Glucagon-like peptide-1 (GLP-1) plays a role in modulating neuroendocrine and autonomic function. The hypothalamic paraventricular nucleus (PVN) contains aggregations of GLP-1 fibers and expresses GLP-1 receptors, making it a likely site of action for GLP-1 signaling. The current study was designed to establish domains of GLP-1 action, focusing on axosomatic appositions on different neuroendocrine and autonomic cell populations in the PVN. The data indicate abundant GLP-1-immunoreactive terminal appositions on corticotropin releasing hormone neurons in the medial parvocellular PVN. GLP-1 positive boutons can also be observed in apposition to oxytocinergic neurons and on retrogradely-labeled pre-autonomic neurons projecting to the region of the nucleus of the solitary tract. In contrast, there were very few vasopressinergic neurons with GLP-1 appositions. Overall, the data indicate that the central GLP-1 system preferentially targets neurons in hypophysiotrophic zones of the PVN, consistent with excitatory actions of GLP-1 on adrenocorticotropin release. GLP-1 is also in position to influence oxytocin secretion and control outflow to brainstem cardiovascular relays.
Keywords: corticotrophin releasing hormone, arginine vasopressin, oxytocin, pre-autonomic, Fluorogold, nucleus of the solitary tract, retrograde tracing
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted from the intestine by nutrient signals, in particular, glucose ingestion (Kieffer and Habener, 1999). Like many ‘gut’ peptides, GLP-1 is also expressed in brain. Expression of GLP-1 is largely confined to groups of cells in the nucleus of solitary tract (NTS) and ventrolateral medulla of the brainstem (Larsen et al., 1997). These neurons send GLP-1-immunoreactive axons and terminals to numerous regions of the brain, including regions responsible for integration of neuroendocrine stress responses (paraventricular nucleus) and energy balance (arcuate nucleus) (Drucker, 1990, Larsen et al., 1997, Sarkar et al., 2003). The GLP-1 receptor (GLP-1R) is expressed in regions in receipt of GLP-1 fibers, including the PVN, the periventricular hypothalamus, the dorsomedial hypothalamus (DMH), and the arcuate nucleus (Arc) (Merchenthaler et al., 1999, Shughrue et al., 1996, Tang-Christensen et al., 2001).
Taken together, the distribution of GLP-1 and its receptor in brain suggests that central GLP-1 is functioning as a neurotransmitter/neuromodulator in neuroendocrine regulatory systems. This hypothesis is supported by functional data indicating a role for GLP-1 in ingestion and hypothalamo-pituitary-adrenocortical (HPA) axis function. Centrally infused GLP-1 elicits dose dependent suppression of food intake (Navarro et al., 1996, Tang-Christensen et al., 1996, Turton et al., 1996) that may be related to visceral signaling associated with food consumption (Vrang et al., 2003). In addition to its anorectic effects, GLP-1 also activates the hypothalamo-pituitary-adrenal (HPA) stress axis. Intracerebroventricular infusion of GLP-1 stimulates ACTH and/or corticosterone release (Kinzig et al., 2003, Larsen et al., 1997). Induction of visceral illness by peripheral injections of lithium chloride (LiCl) activates GLP-1 expressing neurons in the NTS and the dorsomedial parvocellular region of the PVN (presumably CRH expressing neurons) (Rinaman, 1999). Importantly, the HPA response to LiCl is blocked by pre-administration of the GLP-1 antagonist des-His1, Glu8-exendin-4 (dHG-exendin) (Kinzig et al., 2003), suggesting that NTS GLP-1 is responsible for HPA activation induced by this stimulus. Anatomical data indicate that GLP-1 neurons from the NTS innervate the PVN (Larsen et al., 1997, Rinaman, 1999), and form synaptic contacts with CRH-immunoreactive neurons (Sarkar et al., 2003), further consistent with a direct role for GLP-1 in HPA axis signaling. Finally, central GLP-1 increases blood pressure and heart rate, and activates brainstem neurons projecting to sympathetic preganglionic neurons, indicative of stimulatory effects of GLP-1 on the sympathetic nervous system.
The data to date imply an important connection between medullary GLP-1 neurons and CRH neurons in the PVN. However, the PVN is an anatomically heterogeneous region, including several subtypes of neurons. In addition to parvocellular CRH neurons, this nucleus contains 1) magnocellular arginine vasopressin neurons projecting to the posterior pituitary; 2) magnocellular oxytocin neurons projecting to the posterior pituitary; and 3) parvocellular pre-autonomic neurons projecting to brainstem and spinal cord sites controlling cardiovascular function and sympathetic/parasympathetic activation. Given diverse physiological actions of central GLP-1, it is important to determine the anatomical relationships between GLP-1 projections and the various functional components of PVN. Therefore, the current study was designed to assess anatomical interactions between GLP-1-immunoreactive fibers and various neural subtypes in the PVN and SON.
MATERIALS AND METHODS
Rats (Sprague Dawley or Long Evans) were acquired from Harlan Labs (Indianapolis, IN). All animals were triply housed in a temperature- and humidity-controlled facility at the University of Cincinnati, on a 6am to 6pm light-dark cycle with free access to standard chow and water. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Antibodies
Immunostaining protocols were performed using previously characterized primary antibodies/antisera. A monoclonal mouse anti-GLP-1 antibody was acquired from Dr. D’Alessio, used at a 1:10000 dilution, using biotinylated tyramide amplification (see below). Rabbit anti-AVP neurophysin (1:30000) and rabbit anti-oxytocin neurophysin (1:10000) antisera were gifts from Dr. Alan Robinson (Roberts et al, 1993). Rabbit anti-CRF (RC70) antiserum (1:10000) was a gift from Dr. Wylie Vale, Salk Institute. Rabbit anti-Fluorogold antiserum (1:10000) was acquired from Chemicon (Catalog# AB153).
Retrograde tracing
To determine whether GLP-1 axons innervate brainstem-projecting neurons in the PVN, the retrograde tracer Fluorogold (FG; Fluorochrome Inc., Denver, CO) was stereotaxically injected into the region of the NTS (Stern, 2001, Stern and Zhang, 2003). Male Long-Evans rats were anesthetized by intraperitoneal injection of a ketamine-xylazine cocktail (90 and 5 mg/kg, respectively). FG (2% in saline; 200 nl) was pressure-injected unilaterally into the dorsal vagal complex at the level of the obex. The injection point was 4.8 mm posterior to the interaural line, 1.0 mm lateral to the midline, and 8.0 mm below the dorsal surface of the brain, using the coordinate system of Paxinos and Watson (1998). After 5–7 days of FG injection, animals were sacrificed by overdose of Pentobarbital and perfused with 150ml phosphate buffered saline (PBS), followed by 200ml of 4% paraformaldehyde (generated from powdered paraformaldehyde). Brains were removed from the skull, post-fixed in 4% paraformaldehyde overnight, and cryoprotected by immersion in 30% sucrose in PBS. Brains were quickly frozen in powdered dry ice, and sectioned at 25µm on a sliding microtome (Leica Microsystems Inc., Bannockburn, IL). Slices were placed into cryoprotectant solution (0.1M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ehtyleneglycol) and stored at − 20°C until processed for immunohistochemistry.
Adrenalectomy
CRH is difficult to visualize by standard immunostaining procedures, since CRH is quickly transported to terminals after synthesis and does not accumulate in perikarya under unstimulated conditions. Therefore, we examined PVN CRH expression following adrenalectomy, a treatment that blocks feedback inhibition of the HPA axis. Adrenalectomy reliably increases CRH immunoreactivity in PVN perikarya (Sawchenko et al., 1984, Wolfson et al., 1985).
Male Sprague-Dawley rats were anesthetized by intra-peritoneal injection of an anesthesia cocktail (ketamine, 85–95 mg/kg; xylazine, 10–15 mg/kg). Incisions were made bilaterally and both adrenals were removed. Drinking water was replaced by saline after surgery. After 7 days of recovery, animals were overdosed with Pentobarbital and perfused with 4% paraformaldehyde as noted above.
Immunostaining
GLP-1, arginine-vasopressin (AVP), oxytocin (OT), CRH, and FG were visualized using a standard dual immunofluorescence labeling protocol on floating sections from non-treated animals (GLP-1 and AVP, GLP-1 and OT), FG injected animals (GLP-1 and FG), and adrenalectomized animals (GLP-1 and CRH) (Mueller et al., 2005). Briefly, free floating brain sections were incubated with blocking solution (0.1% bovine serum albumin and 0.2% triton X-100 in 50mM KPBS), and incubated in primary antibody solution (GLP-1) overnight. Sections used to visualize FG sites were treated with 0.3% H2O2 in 50mM potassium PBS (KPBS; 40mM potassium phosphate dibasic, 10mM potassium phosphate monobasic, and 0.9% sodium chloride) for 10 minutes to eliminate endogenous peroxidase activities prior to incubation in primary antiserum solution. Sections were then incubated with biotinylated secondary antiserum (1:500 dilutions)(Vector laboratories, Inc., Burlingame, CA), and signals were amplified by incubating in avidin-biotin-peroxidase complex solution (1:2000 dilution)(ABC; Vector laboratories, Inc.), followed by incubation in biotinylated tyramide (1:250 dilution)(BT; Perkin-Elmer Life Sciences, Inc., Boston, MA) thereafter. Signal was then visualized by Cy3-conjugated streptavidin (1:500 dilution)(Jackson ImmunoResearch Labs, West Grove, PA). Subsequently, brains were incubated with second primary antiserum (AVP, OT, CRH, or FG) overnight and visualized by Alexa-488-conjugated secondary antibodies (1:500 dilution)(Molecular Probes, Eugene, OR). Sections were rinsed well with 50mM KPBS between incubations.
Images were obtained using a laser scanning confocal microscope system (LSM 510; Zeiss, Thornwood, NY)). Appositions between GLP-1 terminals and AVP, CRH, OT or FG cells were assessed in 0.5 um sections through the PVN or SON. Appositions were verified by lack of separation between boutons (red) and cells (green) in any sequence of optical sections, or by overlap between boutons and cells (appear yellow), as per Mueller et al, 2005. For presentation purposes, low magnification images (10–20X) images (25–35 sections) were taken and processed as a single projected image using Axiovision 4.4 software.
RESULTS
As can be seen in Figure 1A–D, GLP-1 fibers were distributed in medial parvocellular and dorsal parvocellular zones of the PVN, corresponding to median eminence and brainstem/spinal cord projecting cell populations, respectively. GLP-1 positive fibers were also observed in oxytocin-rich divisions of the PVN, including the rostral posterior magnocellular division of the PVN and the ventral aspect of the medial parvocellular PVN. In contrast, GLP-1 fibers did not heavily invest AVP-enriched PVN regions of magnocellular divisions of the PVN. No differences were observed in PVN GLP-1 distribution between the Long-Evans and Sprague-Dawley strains (data not shown).
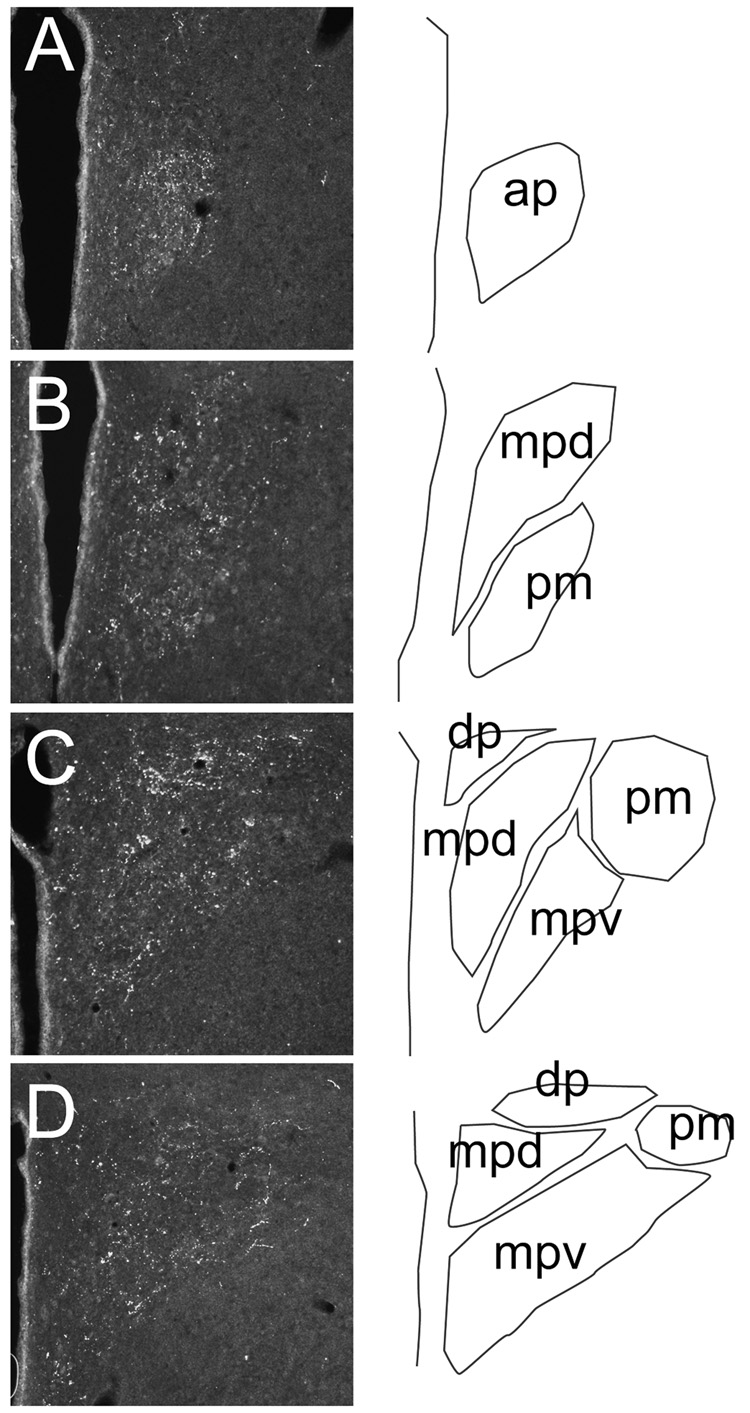
Fig. 1. Distribution of GLP-1 fibers at various rostrocaudal levels of the PVN.
Sections in A–D were taken at 180 um intervals through the PVN of a single animal, beginning at the level of the anterior parvocellular subdivision (approx. 1.32 mm posterior to Bregma, using the coordinate system of Paxinos and Watson (Paxinos and Watson, 1998)). Schematic delineations of PVN subdivisions are on the right of each image. Note rich fiber plexi in regions rich in hypophysiotrophic neurons, including the anterior parvocellular subdivision (ap) and dorsal region of the medial parvocellular subdivision (mpd). Innervation was also present in pre-autonomic subregions of the PVN (dorsal parvocellular (dp) cell group, ventral division of the medial parvocellular zone (mpv)). Note that innervation of the posterior magnocellular division (pm) was documented in the rostral component of this cell group (B) and at the periphery of this cell group at mid-PVN level (C), corresponding to regions rich in oxytocin neurons.
Dual immunofluorescence staining indicates that GLP-1 fibers formed terminal-like appositions in proximity to both oxytocinergic and CRH-containing neurons of the PVN (Fig. 2A–D). Oxytocin-immunoreactive neurons were distributed widely throughout the magnocellular regions of the PVN. At the mid-PVN level, OT expressing neurons were located at the rim of the posterior magnocellular division and in the ventral component of the medial parvocellular division of the PVN. Many of the GLP-1 fibers appeared to formed en passant appositions onto OT-immunoreactive perikarya (Fig. 2B). The anterior magnocellular region of the PVN contained clusters of OT expressing neurons that were also apposed by GLP-1-containing terminals. Finally, the periventricular portion of the anterior PVN (medial magnocellular region) also exhibited numerous OT-immunoreactive neurons, most of which were apposed by GLP-1 nerve fibers.
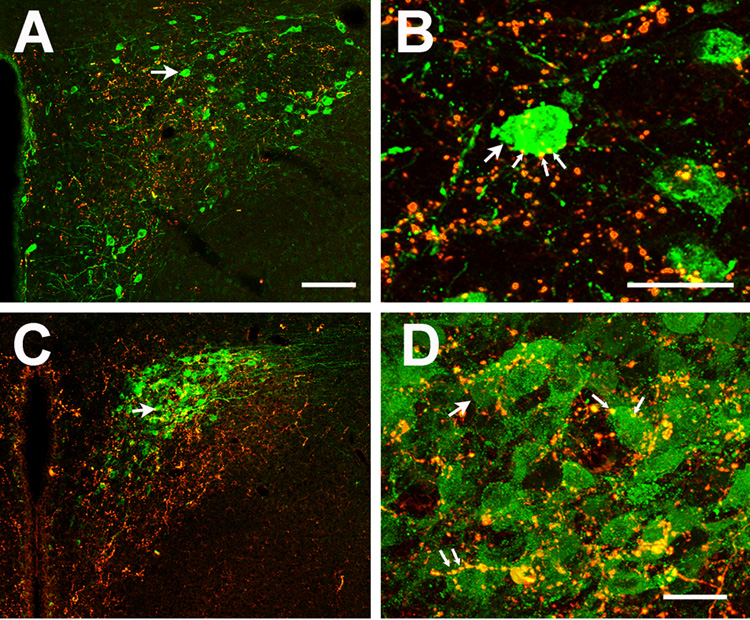
Fig. 2. Localization of oxytocinergic and CRH somata and GLP-1 -immunoreactive nerve fibers in the paraventricular nucleus.
OT expressing cells were located at the perimeter of the posterior magnocellular division of the PVN (A). A large proportion of OT neurons were apposed by GLP-1-immunoreactive boutons at this level. To permit visualization of the full extent of staining through the depth of a given section, a single projection image was generated by combining all images from a confocal stack (25–35 sections). B: Higher magnification example of GLP-1-immunoreactive appositions onto OT neurophysin-immunoreactive neuronal somata (corresponding cell indicated by arrow)(individual 0.5 um optical section). GLP-1- OT appositions are indicated by thin arrows. C. Projection image of CRH immunopositive cells localized in the medial parvocellular PVN, corresponding to the region of densest GLP-1 labeling. D. Higher power image of C, demonstrating evidence for GLP-1-CRH appositions on numerous cells in this region (corresponding cell indicated by arrow) (individual 0.5 um optical section). GLP-1-CRH appostions are indicated by thin arrows. Scale bars: 100µm (A,C) and 30µm (B,D).
Corticotropin-releasing hormone- immunoreactive neurons were located primarily at the dorsomedial parvocellular region of the PVN. This area received dense innervation by GLP-1. There were abundant GLP-1 bouton appositions on CRH positive cell bodies and dendrites (Fig. 2C–D) in this region.
Dual immunostaining of AVP neurophysin and GLP-1 is illustrated in Figure 3 A–D. In general, magnocellular AVP neurons received few GLP-1 contacts. However, there were scattered AVP-expressing neurons in the medial parvocellular division of the PVN that appeared to receive GLP-1 innervation (Fig. 3D), consistent with GLP-1 innervation of hypophysiotrophic AVP neurons.
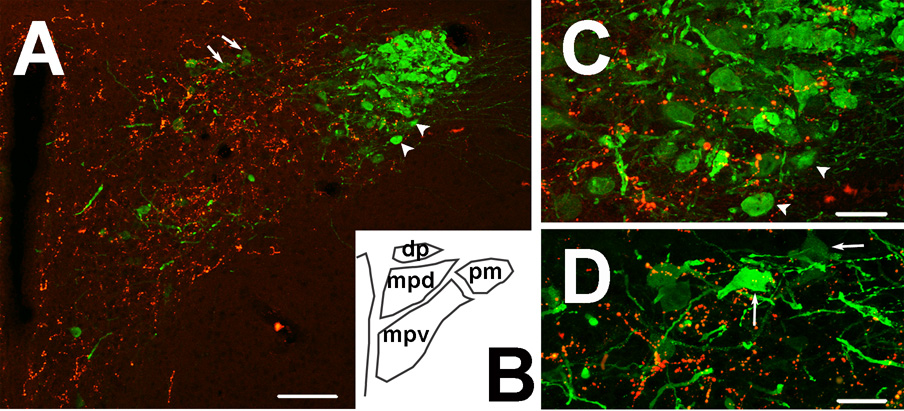
Fig. 3. AVP-immunoreactive neurons and GLP-1-immunoreactive nerve fibers in the paraventricular nucleus.
A: AVP expressing cells are packed in the core of the posterior magnocellular division of the PVN. GLP-1 immunoreactivity (red) is mainly expressed in the medial parvocellular division (projection image). B: Schematic diagram of the approximate area shown in panel A. C: Higher magnification figure of the posterior magnocellular division of the PVN, illustrating sparse GLP-1 fiber distribution in the AVP-rich core of this area, along with only occasional instances of bouton-soma appositions (individual 0.5 um optical section). Arrowheads in A correspond to neurons noted in C. D: Higher magnification figure of the medial parvocellular division of the PVN (individual 0.5 um optical section). Note the scattered AVP positive neurons receiving GLP-1 innervation in this region. Arrows in A correspond to indicated cells in D. Scale bars: 100µm (A) and 30µm (C, D).
The SON also received large numbers of GLP-1-immunoreactive fibers (Fig. 4 A–B). In the SON, OT expressing neurons show a rather distinct localization to the dorsal region. GLP-1 fibers were dense in this region, and formed appositions with OT expressing cell bodies. However, GLP-1-immunoreactive axons were mainly found in the dorsal part of the SON, whereas majority of AVP positive neurons were expressed in the ventral part of the SON. Once again, GLP-1 positive fibers did not appear to innervate SON AVP cell bodies to any substantial degree.
Fig. 4. GLP-1 positive fibers in the supraoptic nucleus, dual immunostained for AVP (A) and OXT (B).
Note that GLP-1 boutons rarely form appositions on AVP perikarya. In contrast, as was the case in the PVN, OXT neurons are apposed by GLP-1 boutons. Images were individual 0.5 um optical section.
Innervation of pre-autonomic PVN projection neurons was assessed following injection of Fluorogold into the nucleus of the solitary tract (NTS). A representative FG injection into the area of the NTS is illustrated in Fig. 5A, and the extent of injection depicted graphically in Fig. 5B. Overall, large injections of FG into to the NTS region labeled substantial numbers of neurons in the right and left PVN, as well as other known pre-autonomic regions (e.g., the central amygdaloid nucleus). As is evident from Fig. 5C–D, retrogradely labeled pre-autonomic neurons were visualized by FG immunostaining in anterior and posterior regions of the PVN. Dual-label analysis indicated that GLP-1-immunoreactive nerve fibers formed bouton-like arrangements on occasional FG-immunoreactive cell bodies in the dorsal, ventral, and lateral parvocellular cell groups of the PVN (Fig. 5C–D).
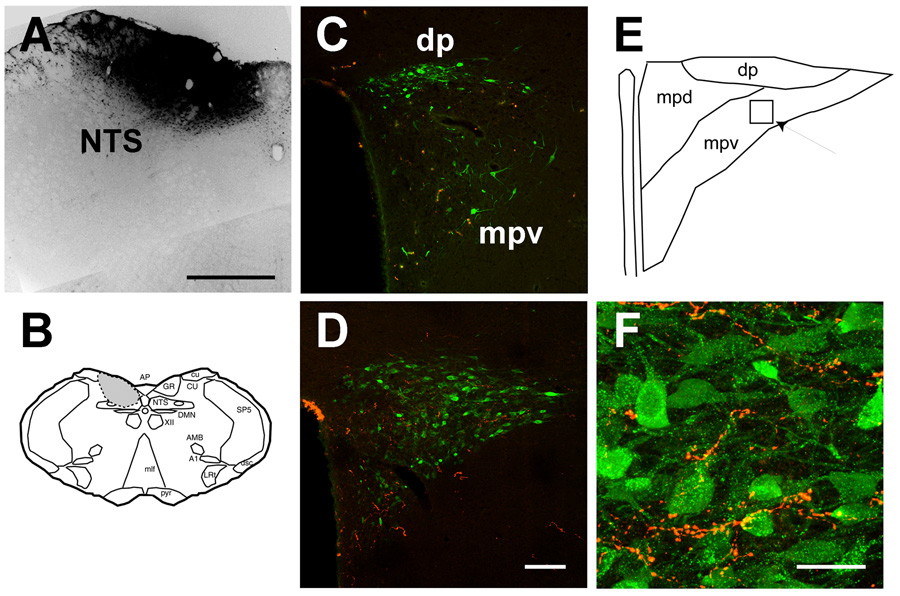
Fig. 5. Localization of FG-labeled somata and GLP-1-immunoreactive nerve fibers in the paraventricular nucleus.
A: Example of a large fluorogold injection encompassing the mid-level of the nucleus of the solitary tract (NTS). B. Schematic diagram of the approximate location of the Fluorogold injection in the brainstem, encompassing the NTS and surrounding region. C. Dual labeling for FG and GLP-1 at the mid-level of the PVN. Note that post GLP-1-immunoreactive fibers are located in the dorsal medial parvocellular zone, away from FG-immunoreactive neurons in the dorsal parvocellular (dp) and ventral medial parvocellular (mpdv) regions. D. Dual labeling for FG and GLP-1 in the caudal PVN. Note the limited intermingling of FG-immunopositive neurons and GLP-1 fibers at this level. E. Schematic diagram of the PVN level depicted in E, showing the approximate location of the field depicted in F. F. GLP-1-immunoreactive bouton appositions of FG-immunoreactive somata and proximal dendrites in the lateral parvocellular (posterior) region of the PVN (individual 0.5 um optical section). Scale bars: 500 µm (A), 100µm (C,D) and 30µm (F).
DISCUSSION
The current study documents close appositions between GLP-1 terminals and oxytocin neurons in all magnocellular PVN subdivisions and in SON, consistent with the potential for synaptic interactions. Medial parvocellular CRH neurons and to a lesser extent, vasopressin neurons also appeared to be apposed by GLP-1 boutons. In contrast, there were few GLP-1 appositions on magnocellular vasopressin neurons. Together, the data suggest that GLP-1 neurons innervate oxytocin as well as CRH neurons, and indicate a role for GLP-1 in modulation of functions regulated by magnocellular oxytocin (lactation and parturition) and parvocellular CRH (HPA axis).
The PVN also contains substantial populations of brainstem and spinal cord-projecting neurons. Assessment of GLP-1 appositions on pre-autonomic PVN neurons by GLP-1 was conducted following injections of retrograde tracer into the region of the NTS, which resulted in extensive labeling in the ipsilateral and contralateral PVN. Occasional GLP-1 appositions onto pre-autonomic neurons were observed, consistent with GLP-1 innervation of PVN brainstem-projecting cells.
Within both the PVN and SON, GLP-1 terminals appear to preferentially contact oxytocinergic magnocellular populations. In both regions, GLP-1 bouton appositions on magnocellular AVP neurons are sparse. The observed pattern of axosomatic interactions contrasts with that of the catecholaminergic system, which heavily innervates all subregions of the PVN and SON (Cunningham et al., 1990, Cunningham and Sawchenko, 1988). Previous work indicates that NTS and ventrolateral medullary GLP-1 neurons do not co-localize catecholaminergic markers (Larsen et al., 1997). In combination, these results support the hypothesis that these cell groups are anatomically and perhaps functionally distinct.
Previous studies indicate that like GLP-1 cells, inhibin beta-immunoreactive neurons in the NTS do not co-express catecholaminergic markers and preferentially innervate oxytocinergic neurons in magnocellular subdivisions of the PVN and SON (Sawchenko et al, 1988). A marked subpopulation of inhibin beta cells also express somatostatin and enkephalin (Sawchenko et al, 1990), suggesting that these co-localized peptides may similarly modulate function of oxytocinergic neurons. Moreover, inhibin beta neurons are localized in the same region of the NTS as GLP-1 cells (Sawchenko et al, 1988). It is plausible that NTS GLP-1 neurons may co-express one or more of these peptides, and represent the same functional cell population.
The PVN and SON innervation by GLP-1 corresponds well with both the localization of GLP-1 receptors and with assessments of GLP-1 induced PVN Fos induction. Expression of GLP-1 receptor mRNA expression is specifically enriched in CRH and oxytocin containing regions of the PVN (Shughrue et al, 1996). In addition, a study by Larsen and colleagues documents extensive induction of CRH neurons following GLP-1 administration, accompanied by a substantial but less extensive induction in oxytocin neurons and minimal induction in vasopressin neurons(Larsen et al., 1997), corresponding well with our co-localization data.
Our observation suggests a functional role for GLP-1 in release of CRH, OT, and to a lesser extent AVP in the PVN and SON. Our anatomical data are not completely consistent with previous literature on central actions of GLP-1. For example, our study finds minimal innervation of magnocellular AVP neurons, whereas previous studies document that central administration of GLP-1 elicits significant release of AVP (Bojanowska and Stempniak, 2000, Larsen et al., 1997). On the other hand, evidence for central release of OT by GLP-1 is inconsistent (Bojanowska and Stempniak, 2000, Larsen et al., 1997), which is not in keeping with data indicating GLP-1 fibers in contact with OT neurons. Taken together, the data suggest that central GLP-1 injections may be acting at a distance from the PVN to elicit vasopressin release (possibly via other GLP-1 receptive hypothalamic sites, such as the arcuate or dorsomedial nuclei (Shughrue et al, 1996), and may have complex actions on activation of oxytocinergic systems.
The dense innervation of CRH neurons by GLP-1 is consistent with previous electron microscopic data as well as known excitatory actions of GLP-1 on the HPA axis. Previous studies document that GLP-1 is involved in activation of the hypothalamo-pituitary-adrenocortical axis (Kinzig et al., 2003, Larsen et al., 1997). Central injections of GLP-1 cause Fos activation of PVN CRH neurons, whereas administration of a GLP-1 receptor antagonist can diminish HPA axis responses to acute stressors.
Pre-autonomic neurons are expressed throughout the PVN, with fewer cells in the rostral part, gradually increasing in number toward the mid- to caudal parts of the PVN (Stern and Zhang, 2003). GLP-1 nerve fibers were found more in the rostral to medial parts of the PVN (Larsen et al., 1997), thus only partially overlapping the distribution of NTS-projecting neurons. Consistent with this observation, GLP-1 axosomatic and axodendritic appositions were present on FG-labeled neurons, particularly at mid-levels of the PVN. Direct GLP-1 innervation of NTS-projecting cells groups suggests a substrate for known GLP-1 actions on the autonomic nervous system (Barragan et al., 1999, Yamamoto et al., 2002). Further, these data are consistent with the ability of central GLP-1 to induce Fos in pre-autonomic projections to the spinal cord from the dorsal and ventral parvocellular regions of the medial PVN (Yamamoto et al., 2002). Moreover, the direct GLP-1 projection to the PVN provides an opportunity for reciprocal neuronal interactions between the PVN and NTS. Reciprocal interactions are supported by immunohistochemical studies demonstrating appositions between oxytocinergic terminal boutons and GLP-1 somata in the NTS (Rinaman and Rothe, 2002).
The PVN also contains pre-autonomic neurons that project exclusively to the spinal cord (Swanson and Kuypers, 1980). Given the distribution of GLP-1 in the PVN, it is likely that spinal cord-projecting pre-autonomic neuronal populations will be innervated to a similar extent as NTS-projecting populations.
In summary, our evidence supports that hypothesis that GLP-1 innervates CRH and OT neurons in the PVN and the SON, and sends additional projections to pre-autonomic neurons in the PVN. The distribution pattern of GLP-1 nerve fibers suggests that GLP-1 modulates stress responses, and places GLP-1 in position to regulate multiple autonomic as well as neuroendocrine aspects of stress integration.
Acknowledgments
Funded by National Institute of Mental Health; Grant Number MH069680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon-like peptide-1 (7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept. 2000;91:75–81. doi: 10.1016/s0167-0115(00)00119-1. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J. Comp. Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp. Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon and the glucagon-like peptides. Pancreas. 1990;5:484–488. doi: 10.1097/00006676-199007000-00018. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Di S, Paden CM, Herman JP. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology. 2005;146:348–354. doi: 10.1210/en.2004-0539. [DOI] [PubMed] [Google Scholar]
- Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blazquez E. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem. 1996;67:1982–1991. doi: 10.1046/j.1471-4159.1996.67051982.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol. 2002;283:R99–R106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- Roberts MM, Robinson AG, Fitzsimmons MD, Grant F, Lee WS, Hoffman GE. c-fos expression in vasopressin and oxytocin neurons reveals functional heterogeneity within magnocellular neurons. Neurondocrinology. 1993;57:388–400. doi: 10.1159/000126384. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc. Natl. Acad. Sci. U S A. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Plotsky PM, Pfeiffer SW, Cunningham ET, Jr, Vaughn J, Rivier J, Vale W. Inhibin beta in central neuronal pathways involved in oxytocin secretion. Nture. 1988;334:615–617. doi: 10.1038/334615a0. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Arias C, Bittencourt JC. Inhibin beta, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 1990;291:269–280. doi: 10.1002/cne.902910209. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Zhang W. Pre-autonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;975:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;5(25 Suppl):S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- Wolfson B, Manning RW, Davis LG, Arentzen R, Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985;315:59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]