Abstract
Platelet-activating factor (PAF) is a potent, bioactive phospholipid that acts on multiple cells and tissues through its G protein-coupled receptor (GPCR). PAF is not stored but is rapidly generated via enzymatic acetylation of the precursor 1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine (lysoPAF). The bioactivity of PAF is effectively and tightly regulated by PAF acetylhydrolases, which convert PAF back to lysoPAF. Previous studies report that lysoPAF is an inactive precursor and metabolite of PAF. However, lysoPAF has not been carefully studied in its own context. Here we report that lysoPAF has an opposing effect of PAF in the activation of neutrophils and platelets. Whereas PAF potentiates neutrophil NADPH oxidase activation, lysoPAF dose-dependently inhibits this function. Inhibition by lysoPAF is not affected by the use of a PAF receptor antagonist or genetic deletion of the PAF receptor gene. The mechanism of lysoPAF-mediated inhibition of neutrophils involves an elevation in the intracellular cAMP level, and pharmacological blockade of adenylyl cyclase completely reverses the inhibitory effect of lysoPAF. In addition, lysoPAF increases intracellular cAMP levels in platelets and inhibits thrombin-induced platelet aggregation, which can be reversed by inhibition of protein kinase A. These findings identify lysoPAF as a bioactive lipid with opposing functions of PAF and suggest a novel and intrinsic regulatory mechanism for balance of the potent activity of PAF.
Platelet-activating factor (PAF, 1-O-hexadecyl-2-acetoyl-sn-glycero-3-phosphocholine) is one of the most active lipid species identified to date. It was first discovered in the early 1970s as a bioactive lipid generated by basophils capable of inducing platelet aggregation and anaphylaxis (Benveniste et al., 1972). In particular, levels of PAF have been shown to directly correlate with the severity of anaphylaxis (Vadas et al., 2008). In addition, PAF plays important roles in inflammation. PAF is produced by a variety of cells, including neutrophils. These effects are believed to be mediated by a G protein-coupled PAF receptor (Hwang et al., 1983; Honda et al., 1991).
Because of the potent and sometimes deleterious biological effects, PAF synthesis is tightly regulated. PAF is not stored in large amounts in the cell, and its production is induced by proinflammatory factors (Szabo et al., 1993). The induced synthesis of PAF involves the de novo pathway and remodeling pathway, the latter being responsible for the majority of PAF induced by inflammatory stimuli (Reinhold et al., 1989). In this process, phospholipase A2 cleaves membrane phospholipids at the sn-2 position and yields several lipid products, including lysoPAF (1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine). The highly specific PAF-acetyltransferase (PAF-AT) then adds an acetyl group to the sn-2 position to yield PAF (Shindou et al., 2007). PAF is subsequently degraded through the removal of the acetyl group by PAF-acetylhydrolases (PAF-AH) (Karasawa et al., 2003). The PAF-AH-mediated conversion to lysoPAF is a primary pathway for PAF inactivation, and there have been efforts in the exploration of PAF-AH as a potential therapeutic agent (Quarck et al., 2001; Arakawa et al., 2005; Gomes et al., 2006). The levels of PAF-AH are inversely correlated with the severity of anaphylaxis (Vadas et al., 2008), and the administration of human recombinant PAF-AH can inhibit several mouse models of anaphylaxis (Fukuda et al., 2000).
Although lysoPAF is believed to lack bioactivity and is often used as a control in experiments exploring PAF function, it has not been carefully studied in its own context. Given that lysoPAF levels are inversely related to those of PAF and that it is a metabolite and a precursor of PAF, there seems to be a need to understand any potential activity that this lipid may have. Furthermore, the potential therapeutic use of recombinant PAF-AH in humans requires a comprehensive understanding of the cellular functions of the lipid produced by this enzyme. In the course of a study of neutrophil activation by bioactive lipids, we found that lysoPAF exhibits an inhibitory effect on neutrophil superoxide production. Further investigation has led to the discovery of lysoPAF-induced intracellular cAMP production as a potential mechanism for its inhibitory effect. Moreover, treatment of platelets with lysoPAF reduces their aggregation after low-dose thrombin stimulation. These data suggest that lysoPAF balances some of the bioactivities of PAF, and the beneficial effects of PAF-AH may be attributed in part to this negative regulatory function of lysoPAF.
Materials and Methods
Materials. Synthetic lysoPAF (16:0, 18:0) and PAF (16:0) were obtained from Avanti Polar Lipids (Alabaster, AL). The lipids were dissolved in 50% (v/v) EtOH/H2O. Percoll was purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). The protein kinase A (PKA) inhibitors H-89 and KT5720 and the adenylyl cyclase inhibitor SQ22536 were purchased from Calbiochem (San Diego, CA). The PAF receptor (PAFR) antagonist SR27417 was a generous gift from Dr. J. M. Herbert (sanofi-aventis, Bridgewater, NJ). Isoluminol, C5a, fMLF, and cholera toxin were obtained from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase was acquired from Invitrogen (Carlsbad, CA).
Preparation of Neutrophils. Human peripheral blood was collected from healthy donors by venipuncture using a protocol approved by the Institutional Review Board at the University of Illinois at Chicago. Acid citrate dextran was used as an anticoagulant. Neutrophil isolation was carried out using discontinuous Percoll gradient (Lin et al., 2005). For preparation of mouse neutrophils, mice were anesthetized with CO2 and sacrificed by cervical dislocation. Femurs and tibias of wild-type and PAFR-/- litter mate mice, on a C57BL6 background, were isolated and placed in HBSS-Prep (HBSS, 0.5% fetal bovine serum, 10 mM HEPES, and 1% glucose). Bone marrow was then harvested, marrow was disaggregated, and then cells were separated by centrifugation using a discontinuous density gradient consisting of 3 ml of NycoPrep 1.077 (Axis-Shield, Oslo, Norway) underlaid with 72% Percoll (Amersham Biosciences). Neutrophils were collected at the NycoPrep-Percoll interface and washed 2× with HBSS-Prep. Final resuspension was in 0.5% bovine serum albumin in RPMI medium. Purity of neutrophils was consistently at 80% or greater, as determined by fluorescence-activated cell sorting scatter, Wright staining, and Hemavet (Drew Scientific, Dallas, TX).
Preparation of Human Platelets. Blood was taken as described above with approval. Acid citrate dextran was used as an anticoagulant. Platelet isolation was performed as described previously (Li et al., 2006).
Superoxide Generation. Human PMN superoxide generation was measured by isoluminol-enhanced chemiluminescence as described previously (Lin et al., 2005). Chemiluminescence was measured every minute using a Wallac Multilabel Counter plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA). Unstimulated controls were recorded simultaneously. Alternatively, some samples were preincubated with lipid or inhibitors before stimulation.
Endothelial Cell Culture and Transendothelial Electrical Resistance Measurement. Human pulmonary microvessel endothelial cells (HPMECs) were purchased from Sciencell (Carlsbad, CA) and were cultured in endothelial basal medium supplemented with EGM-2MV from Lonza (Walkersville, MD). HPMECs were plated on gelatin-coated electric cell-substrate impedance-sensing 8-well 10-electrode arrays from Applied Biophysics (Piscataway, NJ) until confluence before experiment. Isolated human neutrophils (4 × 104/well) were preincubated with vehicle, lysoPAF (1 μM), or PAF (1 μM) for 10 min before addition to endothelial cell monolayers. The electrode was then connected, and the resistance was monitored as described previously (Tiruppathi et al., 1992). Then, fMLF (1 μM) was added to indicated samples, and the recording continued for a further 5 h.
Calcium Mobilization. Increase in intracellular calcium was detected using Indo-1/AM-labeling of human PMNs kept in a 0.5% bovine serum albumin/HBSS buffer as described previously (He et al., 2003).
cAMP Assay. cAMP was measured using a competitive enzyme-linked immunosorbent assay (BIOMOL Research Laboratories, Plymouth Meeting, PA) as described previously (Lin et al., 2005).
Platelet Aggregation. Platelet aggregation was measured as described previously (Li et al., 2006). In brief, washed human platelets, at a concentration of 3 × 108/ml, were pretreated with lysoPAF (10 nM to 1 μM) or control for 2 min, then stimulated with α-thrombin from Enzyme Research Laboratories (South Bend, IN).
Statistical Analysis. Data were analyzed by unpaired Student's t test using Prism (version 4.0) software from GraphPad Software Inc., (San Diego, CA).
Results
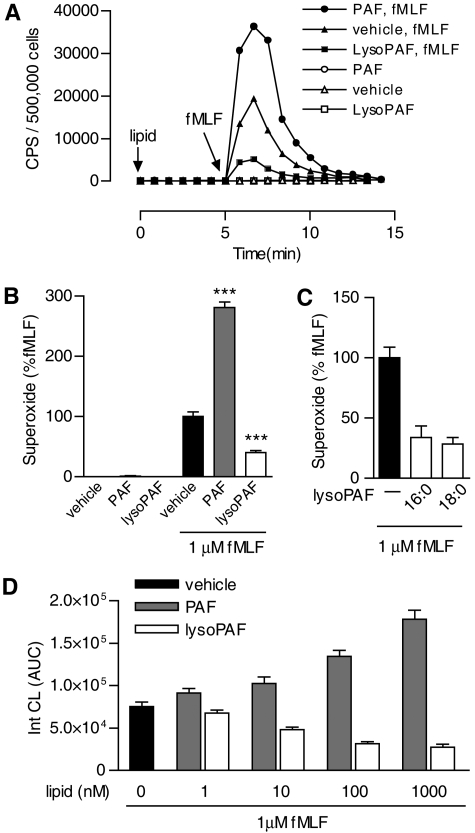
LysoPAF Inhibits Neutrophil NADPH Oxidase Activation and Neutrophil-Induced Vascular Damage. Treatment of human neutrophils with PAF does not lead to significant superoxide production. Instead, the cells are sensitized and respond more potently to subsequent stimulation with another agonist (Dewald and Baggiolini, 1985; Vercellotti et al., 1988). This “priming” effect is evident in human neutrophils first incubated with PAF and then stimulated with fMLF, a full agonist of neutrophils (Fig. 1A). In PAF-treated neutrophils, fMLF (1 μM)-induced superoxide production was 2.85-fold higher than the level in buffer-treated cells (p < 0.01), based on isoluminol-enhanced chemiluminescence (Fig. 1, A and B). LysoPAF, widely considered a nonactive PAF metabolite and precursor, is often used as a negative control in studies of the bioactivity of PAF. When superoxide production in lysoPAF-treated neutrophils was set as baseline (Fig. 1B, open bar), the “priming” effect of PAF became more evident (a 6.92-fold increase) after fMLF stimulation (p < 0.01). LysoPAF (1 μM) caused a 57% reduction of the fMLF-induced superoxide production compared with buffer-treated neutrophils under the same experimental conditions (Fig. 1, A and B). These results suggest that lysoPAF is bioactive and produces an inhibitory effect on fMLF-induced neutrophil superoxide production.
Fig. 1.
PAF and lysoPAF differentially regulate fMLF-induced neutrophil NADPH oxidase activation. A, detection of fMLF-induced superoxide generation as a function of time. CPS, counts per second of chemiluminescent light emitted. Isolated human neutrophils (5 × 105/sample) were preincubated with indicated lipid (1 μM) or vehicle for 5 min before stimulation with 1 μM fMLF. Shown is a set of representative tracings from one of the three experiments that produced similar results. B, data from all three experiments were quantified based on integrated areas under curve (AUC) and then expressed as a percentage of fMLF-induced response using the condition without lipid pretreatment as 100%. C, comparison of the effects of lysoPAF pretreatment using two lysoPAF species with different chain lengths (16:0, 18:0) on fMLF-induced superoxide production in neutrophils from different donors (n = 3). D, dose-response of lysoPAF and PAF in fMLF-induced superoxide generation. Shown is a representative set of data, as mean ± S.E.M., from one of the three experiments that produced similar results.
The alkyl group in PAF and lysoPAF is connected through an ether linkage at the C1 position to a carbon chain of variable lengths. In the above study, 16:0 PAF and lysoPAF was used. To determine whether the length of the carbon chain affects the bioactivity of lysoPAF, we examined 18:0 lysoPAF from the same source and found it to be equally effective in the inhibition of fMLF-induced superoxide production (Fig. 1C). Next, we determined the potency of PAF and lysoPAF in superoxide production assays. Both PAF and lysoPAF exhibited bioactivity at concentrations as low as 10 nM. The opposing effects of PAF and lysoPAF on superoxide production continue to increase up to the concentration of 1 μM (Fig. 1D).
Neutrophil activation can lead to microendothelial injury, manifested as increased endothelial permeability and loss of barrier function. These changes are seen during Gram-negative bacterial infection and in numerous inflammatory disorders such as adult respiratory distress syndrome (Lee and Downey, 2001). In addition, pulmonary edema is one of the hallmarks of anaphylaxis. We determined the effects of PAF and lysoPAF on neutrophil-dependent changes of endothelial permeability by measuring transendothelial electrical resistance (TER), a well established in vitro assay of endothelial barrier function (Furie et al., 1984; Tiruppathi et al., 1992). In this assay, changes in TER reflect the integrity of the endothelial monolayer, which is compromised in the presence of reactive oxygen species from activated neutrophils. As shown in Fig. 2A, fMLF stimulation of neutrophils caused a decrease in TER over time, and this change was further enhanced by pretreatment of neutrophils with PAF (1 μM). In contrast, lysoPAF used at the same concentration significantly reduced the fMLF-stimulated decrease in TER but had no significant effect on TER when applied alone (Fig. 2B). These results suggest a potential function of lysoPAF in preventing neutrophil-mediated endothelial injury.
Fig. 2.
PAF- and lysoPAF-treated neutrophils affect endothelial barrier function. A, HPMECs were grown to confluence on a gold electrode. Human neutrophils (4 × 104) were preincubated with 1 μM concentration of the indicated lipid or vehicle control for 10 min, added to the electrode well, and stimulated with 1 μM fMLF. The changes in TER were recorded over time. Data shown are from one representative experiment chosen from a total of four similar experiments. No significant changes in TER were observed with PAF or lysoPAF in the absence of PMNs (data not shown). B, the peak values at the 4-h time point of the above experiments are shown as mean ± S.E.M. based on four separate experiments; ns, not significant, *, p < 0.05; ***, p < 0.001.
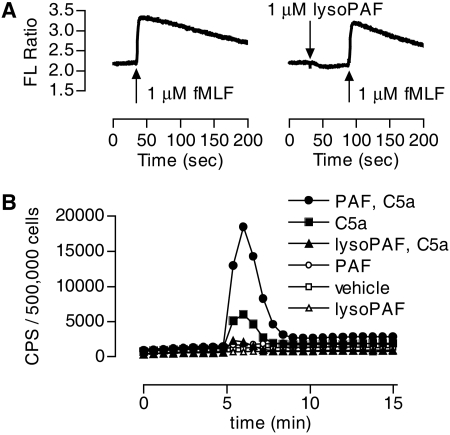
LysoPAF Does Not Block the fMLF Receptor. Inhibition of fMLF-stimulated superoxide generation could occur at multiple steps. To identify the related mechanisms, we first examined whether lysoPAF could block fMLF interaction with the formyl peptide receptor (FPR). One of the proximal signaling events downstream of the activated FPR is mobilization of intracellular Ca2+. Neutrophils were loaded with Indo-1/AM, then treated with either lysoPAF or vehicle control before stimulation with fMLF (Fig. 3A). The results indicate that lysoPAF did not induce Ca2+ mobilization in neutrophils, and treatment of neutrophils with lysoPAF had no effect on the fMLF-induced Ca2+ mobilization. These results preclude that lysoPAF blocks the fMLF interaction to FPR.
Fig. 3.
The effect of lysoPAF is not mediated through inhibition of the fMLF receptor or confined to fMLF stimulation. A, real-time measurement of Ca2+ mobilization in human neutrophils stimulated with 1 μM fMLF in the presence or absence of 1 μM lysoPAF. B, detection of the effects of lysoPAF and PAF on C5a-induced superoxide generation. CPS, counts per second of chemiluminescent light emitted. Isolated human neutrophils (5 × 105/sample) were preincubated with the indicated lipid (1 μM) or vehicle [same concentration of ethanol, 0.025% (v/v)] for 5 min before stimulation with 100 nM C5a. Shown is a representative set of tracings from one of the three independent experiments, and each produced similar results.
To rule out the possibility that the inhibitory effect of lysoPAF is specifically targeted at FPR signaling pathways, we investigated whether lysoPAF affects C5a signaling. Stimulation of neutrophils with C5a caused superoxide production (Fig. 3B, ▪). In cells treated with PAF, the C5a-induced superoxide production was markedly increased (•). In contrast, lysoPAF treatment markedly decreased the C5a-induced superoxide production (▴). Therefore, the inhibitory effect of lysoPAF is not confined to one chemoattractant receptor.
The Inhibitory Effect of lysoPAF Is Independent of the PAF Receptor. Because of the structural similarity between PAF and lysoPAF, we investigated whether the inhibitory effects of lysoPAF are mediated through the PAF receptor. When neutrophils were exposed to both PAF and lysoPAF at equal molar concentrations, the effect of the individual lipids was negated (Fig. 4A). To determine whether lysoPAF directly affects PAFR-mediated signaling, we conducted a Ca2+ mobilization assay in which neutrophils were treated with lysoPAF before measurement of PAF-induced increase in intracellular Ca2+ concentration. As shown in Fig. 4B, lysoPAF did not induce Ca2+ mobilization, and it produced no effect on PAF-induced Ca2+ mobilization. We also used a PAF receptor antagonist, SR27417 (Herbert et al., 1991), to determine whether blocking PAFR could alter the effects of PAF and lysoPAF on fMLF-induced superoxide production. Neutrophils were incubated in the presence or absence of SR27417 (10 nM) and either PAF or lysoPAF and then stimulated with fMLF. As shown in Fig. 3C, SR27417 abrogated PAF priming in the neutrophils but did not change the inhibitory effect of lysoPAF. This result argues against the notion that lysoPAF competes with PAF in using PAFR, although it does not rule out the possibility that lysoPAF may interact with the receptor at a site different from the PAF binding site. This latter possibility was tested using neutrophils derived from PAFR knockout mice (Ishii et al., 1998). As shown in Fig. 4D, genetic deletion of the PAFR gene abolished the priming effect of PAF but did not alter the inhibitory effect of lysoPAF on fMLF-induced superoxide production. Taken together, results from these experiments indicate that inhibition by lysoPAF is independent of PAFR.
Fig. 4.
The inhibitory effect of lysoPAF is not mediated through the PAF receptor. A, human neutrophils (5 × 105/sample) were incubated with the indicated lipid(s) (1 μM each) for 5 min before stimulation with 1 μM fMLF. The changes in superoxide generation, expressed as integrated AUC, are shown, with fMLF-induced response in the absence of lipid set as 100%. Data shown are mean ± S.E.M. from three independent experiments. B, real-time measurement of calcium mobilization in human neutrophils loaded with Indo-1/AM and stimulated with 1 μM PAF or with 1 μM lysoPAF and then PAF. A representative set of tracings, from a total of three experiments, is shown. C, superoxide generation was assessed in neutrophils treated for 5 min with the PAFR antagonist SR27417 (10 nM) before the addition of PAF or lysoPAF (1 μM) or vehicle. Cells were then stimulated with fMLF (1 μM) and production of superoxide was measured over time. Data procession and presentation are similar to that in A. D, mouse neutrophils (1 × 106/sample) isolated from PAFR+/+ or PAFR-/- mice were incubated with indicated lipid for 5 min before stimulation with fMLF (1 μM). Integrated AUC was calculated for each mouse and data (mean ± S.E.M.) expressed as a percentage of fMLF stimulation for each experiment (n = 3).
LysoPAF Activates Adenylyl Cyclase and Increases Intracellular cAMP Concentration in Neutrophils. Molecular characterization of PAFR has led to the identification of its signaling pathways that include functional coupling to the Gq class of G proteins, activation of phospholipase Cβ, and induction of second messengers inositol (1,4,5)-trisphosphate and diacyl glycerol (Honda et al., 2002). PAFR also couples to the Gi class of G proteins that mediate the chemotactic and cross-regulatory signals and also contribute to the activation of phospholipase Cβ and exocytosis in transfected RBL-2H3 cells with the G proteins fused to the PAFR (Brown et al., 2006). As a result, PAF stimulation leads to Ca2+ mobilization in neutrophils. We have shown that stimulation of neutrophils with lysoPAF does not cause an increase in intracellular Ca2+ concentration (Fig. 4B), suggesting differences between PAF and lysoPAF in the activation mechanism and the use of downstream effectors. Because several anti-inflammatory molecules stimulate the adenylyl cyclase-cAMP signaling pathway (Rivkin and Becker, 1976; Fantone et al., 1983, 1984), we tested whether lysoPAF could activate this pathway and increase cAMP concentration in human neutrophils. As shown in Fig. 5A, stimulation of neutrophils with lysoPAF resulted in dose-dependent increases in intracellular cAMP concentrations that reached up to 2-fold above baseline. It is possible that lysoPAF activates a Gαs-coupled receptor, leading to cAMP production. This is suggested by the observation that cholera toxin, which ADP ribosylates and activates Gαs, increased cAMP level to the same extent as in lysoPAF-stimulated cells (Fig. 5B). Likewise, cholera toxin dose-dependently inhibited fMLF-stimulated superoxide production (Fig. 5C). Pertussis toxin, which ADP ribosylates the Gαi proteins and interferes with Gαi-receptor interaction, had no effect on lysoPAF-induced elevation of cAMP concentration.
Fig. 5.
LysoPAF activates the adenylyl cyclase-cAMP-PKA pathway. A, human neutrophils were incubated with lysoPAF at different concentrations or with PAF at 1 μM for 5 min in the presence of the phosphodiesterase inhibitor IBMX. The cells were lysed, and cAMP was assayed by competitive immunoassay. Shown is a representative experiment of three that produced similar results. B, cAMP measurement of human neutrophils in the presence or absence of lysoPAF (1 μM, 5 min), CTx (1 μg/ml, 1 h with or without lysoPAF), or pertussis toxin (0.5 μg/ml) for 1 h with or without lysoPAF (1 μM) in the presence of IBMX. cAMP was measured as described above. Shown are data from two experiments, normalized based on nonstimulated controls. C, superoxide generation in the presence or absence of increasing concentrations of CTx, in the presence or absence of 1 μM lysoPAF. Human neutrophils were incubated with CTx for 1 h before the addition of 1 μM lysoPAF for 5 min and then stimulated with 1 μM fMLF. Data are shown as the percentage relative to fMLF-induced superoxide generation without lysoPAF and are based on two different donors. D, superoxide generation in the presence or absence of increasing concentrations of the adenylyl cyclase inhibitor SQ22536. Human neutrophils were incubated with the indicated dose of SQ22536 or vehicle for 10 min before the addition of lysoPAF (1 μM) for 5 min. Data are shown as a percentage relative to fMLF-induced superoxide generation without lysoPAF or SQ22536, using neutrophils from three different donors. E, superoxide generation was assessed in neutrophils treated with or without the PKA inhibitors H-89 (5 μM) or KT5720 (5 μM) before the addition of lysoPAF (1 μM) or vehicle. The fMLF (1 μM)-induced superoxide generation was measured and expressed as the percentage of change relative to superoxide generation in the absence of inhibitor and lysoPAF. Data shown are mean ± S.E.M. based on three independent experiments.
The involvement of an adenylyl cyclase is suggested by the results from experiments using the adenylyl cyclase inhibitor SQ22536 (Graber and Hawiger, 1982), which dose-dependently reversed the inhibitory effect of lysoPAF on the production of syperoxide (Fig. 5D). PKA, a key kinase activated by cAMP, also plays a role in the inhibition by lysoPAF. Using the PKA inhibitor H-89, we observed a partial restoration (p < 0.05) of superoxide production in fMLF-stimulated neutrophils that were pretreated with lysoPAF (Fig. 5E). This result is confirmed in a parallel experiment using another PKA inhibitor, KT5720, which produced a similar effect in reversal of the effect of lysoPAF. These results indicate that lysoPAF signaling involves adenylyl cyclase-mediated generation of cAMP, and that PKA contributes to the inhibitory effect of lysoPAF.
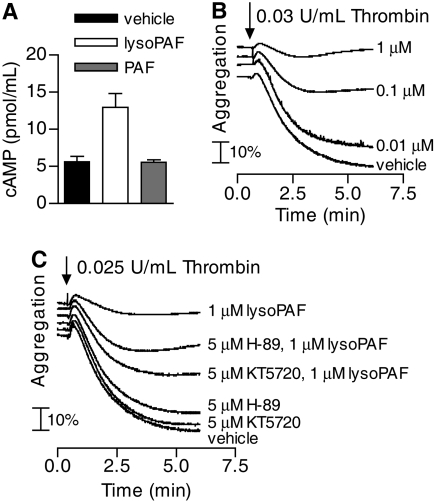
LysoPAF Induces cAMP Elevation in Platelets and Inhibits Platelet Aggregation. In addition to neutrophils, other blood cells, including platelets, are involved in inflammatory responses. PAF was originally named for its activating effect on platelets (Benveniste et al., 1972). However, a bioactivity of lysoPAF on platelets has not been reported. We conducted experiments to determine whether lysoPAF could affect the intracellular cAMP levels in platelets and whether treatment of platelets with lysoPAF would affect its activation. In washed human platelets, lysoPAF induced a significant increase in cAMP levels (p < 0.01) (Fig. 6A), whereas PAF does not have an effect on the intracellular cAMP levels in platelets under the same experimental conditions. Similar to neutrophils, cAMP inhibits many activation processes in platelets, including platelet aggregation (Salzman et al., 1972). We therefore tested whether lysoPAF-treated platelets display changes in agonist-induced platelet aggregation. When washed, platelets were preincubated with lysoPAF and then stimulated with α-thrombin (0.03 U/ml), a lysoPAF dose-dependent inhibition in platelet aggregation was observed (Fig. 6B). Also similar to neutrophils, PKA is known to be involved in downstream signaling of cAMP. The inhibitory effect of lysoPAF on thrombin-induced platelet aggregation is partially reversed by the PKA inhibitors H-89 and KT5720 (Fig. 6C).
Fig. 6.
LysoPAF induces cAMP elevation in platelets and suppresses thrombin-induces platelet aggregation. A, human platelets (3 × 108/ml) were incubated with the indicated lipid (1 μM) for 2 min. Cells were lysed in IBMX-containing buffer, and cAMP was assayed by competitive immunoassay. Data shown are mean ± S.E.M. and are representative of three experiments with similar results. B, thrombin-induced platelet aggregation in the absence or presence of lysoPAF. Human platelets were incubated with the indicated concentrations of lysoPAF for 2 min before stimulation with α-thrombin (0.03 U/ml) at 37°C under stirring conditions. Platelet aggregation, measured by light transmission, was determined over time. C, thrombin-induced platelet aggregation in the absence or presence of the PKA inhibitors H-89 (5 μM) or KT5720 (5 μM) before stimulation with vehicle or lysoPAF (1 μM). Platelet aggregation was measured as in B. Data shown are representative of two independent experiments with similar results.
Discussion
Results from this study demonstrate that lysoPAF is bioactive and produces an effect opposite that of PAF in neutrophil superoxide production. Whereas PAF potentiates NADPH oxidase activation and superoxide production, lysoPAF inhibits this function. The inhibitory effect of lysoPAF does not depend on PAFR and is not limited to a particular agonist such as fMLF or one cell type such as neutrophils. In our experiments, the C5a-stimulated neutrophil superoxide production and thrombin-induced platelet aggregation are also inhibited by lysoPAF. These observations are novel and provide evidence for a possible function of lysoPAF in balancing the proinflammatory activities of PAF. Although PAF plays important roles in regulating cellular functions, its proinflammatory activities require temporal regulatory mechanisms, including induced synthesis and efficient degradation. Therefore, enzymes responsible for PAF synthesis and degeneration, mainly the PAF-ATs and PAF-AHs, are potential targets of therapeutic intervention. Results from the current study suggest that PAF-AHs, in addition to removing PAF from circulation and tissues, may at the same time create a bioactive molecule with opposing functions, thereby effectively negating the bioactivity of PAF. It is likely that the inhibitory effects of lysoPAF on neutrophils and platelets contribute to the anti-inflammatory functions of PAF-AHs (Henderson et al., 2000).
Previous studies of the bioactivity of PAF often included lysoPAF as a negative control. Other reports showed that lysoPAF displayed PAF-like activities in stimulating DNA synthesis in smooth muscle cells (Chai et al., 2000) and microvascular leakage when administered to guinea pigs by inhalation (Sakamoto et al., 1993). These PAF-like activities, including the ability to induce Ca2+ mobilization, were blocked by PAFR antagonists, indicating that they are mediated by the identified PAFR (Sakamoto et al., 1993). Marathe and colleagues (2001) have shown that treatment of the bioactive lysoPAF and lysoPC with PAF-AH or with saponification abolishes the activities of these lysophospholipids, indicating that these activities come from contaminating phospholipids. Before chemically synthesized PAF became widely available, many studies were conducted using PAF isolated from crude methanol extracts of cells or egg yolk, and those studies were prone to contamination with PAF-like phospholipids. The same methods apparently contributed to the identification of lipids migrating with a similar Rf values as PAF on thin-layer chromatography and containing both platelet-activating and neutrophil-inhibiting properties (O'Donnell et al., 1981). To eliminate contaminations with other phospholipids, in this study, we used synthetic lysoPAF and PAF from the same source and prepared stock solutions for both lipids using exactly the same method. Experiments that compared lysoPAF and PAF were strictly carried out in parallel. Our study has shown dose-dependent inhibition of neutrophil NADPH oxidase activation by lysoPAF, with minimal active concentrations as low as 10 nM. Under the same experimental conditions, the potentiation effect of PAF was detectable.
The exact mechanism by which lysoPAF exerts its bioactivity is still unknown. As the first step toward an answer to this question, we determined whether elevation of intracellular cAMP level contributes to the inhibitory effects of lysoPAF. Our results confirmed that lysoPAF, but not PAF, was able to stimulate an increase in cAMP levels in both neutrophils and platelets. Moreover, pharmacological inhibition of PKA, an effector of cAMP, partially reversed the inhibitory effect of lysoPAF, indicating that the cAMP-PKA pathway is involved, but PKA-independent mechanism may also exist. We also found that treating neutrophils with SQ22536, an inhibitor of adenylyl cyclase, dose-dependently reversed the inhibitory effect of lysoPAF on superoxide generation. These results confirm that adenylyl cyclase-mediated production of cAMP is key to the inhibitory activity of lysoPAF. These effects are not likely to be the result of nonspecific actions of the inhibitors on other kinases, most of which are important for neutrophil NADPH oxidase activation and platelet aggregation.
We hypothesize that lysoPAF activates adenylyl cyclase through either one or both of the following pathways. First, lysoPAF may bind to a receptor that couples to the Gs class of G proteins. Because Gαs activates adenylyl cyclase, lysoPAF binding to this receptor leads to increased cAMP production. This is likely given the emergence of an increasing number of GPCRs for bioactive lipids (Im, 2004). Our study using cholera toxin, which activates Gαs, produced a similar effect in the elevation of cAMP levels and inhibition of superoxide generation as seen in lysoPAF-stimulated cells. To determine which receptor(s) is activated by lysoPAF, we have conducted an exhaustive search of the existing GPCR database and identified 28 receptors that are related in sequence to known lipid receptors. These receptors were individually analyzed in transfected cells for their abilities to mediate lysoPAF-induced increase in intracellular cAMP, cAMP response element-binding protein-driven luciferase reporter expression, and calcium mobilization. G2A has been shown to mediate the bioactivity of lysoPC (Lin et al., 2005; Wang et al., 2005), although it lacks certain pharmacological properties of a receptor such as direct binding of lysoPC (Kabarowski et al., 2001; Witte et al., 2005). When tested in our assays, G2A and structurally similar receptors such as GPR4, TDAG8, and OGR1, did not respond significantly to lysoPAF stimulation (Supplemental Table 1). Other GPCRs tested also failed to respond to lysoPAF in these functional assays. Therefore, screening of additional GPCRs is necessary to further test the hypothesis. We noticed that fMLF, which activates a Gi-coupled receptor FPR, is reported to enhance intracellular cAMP levels through a pertussis toxin-sensitive mechanism (Ali et al., 1998). However, fMLF also stimulates Ca2+ flux, whereas lysoPAF does not, indicating that lysoPAF does not activate the same signaling pathway that is induced by fMLF. Nevertheless, expanding the search to other GPCRs may be beneficial to understanding how lysoPAF induces cAMP elevation. The second possible mechanism for the inhibitory effect of lysoPAF involves direct activation of adenylyl cyclase. To date, 10 isoforms of transmembrane adenylyl cyclase have been identified (Hurley, 1999). Adenylyl cyclase isoform 1 expression is restricted to the brain. Isoforms 6 and 7 are ubiquitously expressed in cells and tissues. We have tested several other cell types, including HPMECs, which were unable to respond to lysoPAF with increased cAMP levels (data not shown). Therefore, it is unlikely that the candidate is a ubiquitously expressed isoform of adenylyl cyclase. Store-operated calcium channels are known to activate adenylyl cyclase isoforms 3 and 8; however, we observed no changes in calcium levels in cells treated with lysoPAF. A further analysis is required to more carefully examine these cyclase isoforms.
In summary, results from the current study identify lysoPAF as a bioactive lipid with inhibitory functions in neutrophil NADPH oxidase activation and platelet aggregation. This novel finding may create an opportunity to investigate the biological functions of PAF-AHs in regulating the activities of PAF. Because lysoPAF is a precursor and a metabolite of PAF, its tissue concentration is regulated by the presence of enzymes in the PAF remodeling pathway. Recent cloning and characterization of acetyl-CoA:lyso-PAF acetyltransferase (Shindou et al., 2007) and clinical correlation between lowered PAF-AH concentration and severity of anaphylaxis (Vadas et al., 2008) are expected to promote continued exploration of PAF-AH and related pathways. The discovery of lysoPAF as having opposite activities of PAF in the functional assays performed in this study may contribute to a better understanding of these phospholipids for their functions in human physiology.
Supplementary Material
Acknowledgments
We thank Dr. Jean-Marc Herbert for the gift of SR27417, Dr. Guy Le Breton and Dr. Tohru Kozasa for suggestions on cAMP assays, Dr. Feng Qian for assistance with blood cell preparation, Fumie Hamano for technical assistance in receptor screening assays, and members of the Ye Laboratory for helpful discussions.
This work was supported by the National Institutes of Health [Grants AI033503, HL077806] and by an American Heart Association Predoctoral Fellowship [Grant 0615496Z].
ABBREVIATIONS: PAF, platelet-activating factor (1-O-hexadecyl-2-acetoyl-sn-glycero-3-phosphocholine); lysoPAF, 1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine; GPCR, G-protein coupled receptor; PKA, protein kinase A; PAFR, platelet-activating factor receptor; fMLF, N-formyl-l-methionyl-l-leucyl-l-phenylalanine; HPMEC, human pulmonary microvessel endothelial cell; PAF-AH, platelet-activating factor acetylhydrolase; PAF-AT, platelet-activating factor acetyltransferase; TER, transendothelial electrical resistance; SQ22536, 9-(tetrahydro-2-furyl)-adenine; KT5720, (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]-benzodiazocine-10-carboxylic acid hexyl ester; H-89, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride CTx, cholera toxin; Rf, retardation factor; IBMX, 3-isobutyl-1-methylxanthine; HBSS, Hanks' balanced salt solution; PMN, polymorphonuclear neutrophil; AM, acetoxymethyl ester; FPR, formyl peptide receptor; AUC, area under the curve; SR27417, N-(2-dimethylaminoethyl)-N-(3-pyridinylmethyl)(4-[2,4,6-triisopropylphenyl]thoiazol-2-yl)amine.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Ali H, Sozzani S, Fisher I, Barr AJ, Richardson RM, Haribabu B, and Snyderman R (1998) Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cβ3 phosphorylation by protein kinase A. J Biol Chem 273 11012-11016. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Qian JY, Baatar D, Karasawa K, Asada Y, Sasaguri Y, Miller ER, Witztum JL, and Ueno H (2005) Local expression of platelet-activating factor-acetylhydrolase reduces accumulation of oxidized lipoproteins and inhibits inflammation, shear stress-induced thrombosis, and neointima formation in balloon-injured carotid arteries in nonhyperlipidemic rabbits. Circulation 111 3302-3309. [DOI] [PubMed] [Google Scholar]
- Benveniste J, Henson PM, and Cochrane CG (1972) Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med 136 1356-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Jala VR, Raghuwanshi SK, Nasser MW, Haribabu B, and Richardson RM (2006) Activation and regulation of platelet-activating factor receptor: role of Gi and Gq in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J Immunol 177 3242-3249. [DOI] [PubMed] [Google Scholar]
- Chai YC, Binion DG, and Chisolm GM (2000) Relationship of molecular structure to the mechanism of lysophospholipid-induced smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol 279 H1830-H1838. [DOI] [PubMed] [Google Scholar]
- Dewald B and Baggiolini M (1985) Activation of NADPH oxidase in human neutrophils. Synergism between fMLP and the neutrophil products PAF and LTB4. Biochem Biophys Res Commun 128 297-304. [DOI] [PubMed] [Google Scholar]
- Fantone JC, Marasco WA, Elgas LJ, and Ward PA (1983) Anti-inflammatory effects of prostaglandin E1: in vivo modulation of the formyl peptide chemotactic receptor on the rat neutrophil. J Immunol 130 1495-1497. [PubMed] [Google Scholar]
- Fantone JC, Marasco WA, Elgas LJ, and Ward PA (1984) Stimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion production. Am J Pathol 115 9-16. [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Kawashima H, Saito K, Inomata N, Matsui M, and Nakanishi T (2000) Effect of human plasma-type platelet-activating factor acetylhydrolase in two anaphylactic shock models. Eur J Pharmacol 390 203-207. [DOI] [PubMed] [Google Scholar]
- Furie MB, Cramer EB, Naprstek BL, and Silverstein SC (1984) Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol 98 1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RN, Bozza FA, Amâncio RT, Japiassú AM, Vianna RC, Larangeira AP, Gouvêa JM, Bastos MS, Zimmerman GA, Stafforini DM, et al. (2006) Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock 26 41-49. [DOI] [PubMed] [Google Scholar]
- Graber SE and Hawiger J (1982) Evidence that changes in platelet cyclic AMP levels regulate the fibrinogen receptor on human platelets. J Biol Chem 257 14606-14609. [PubMed] [Google Scholar]
- He R, Sang H, and Ye RD (2003) Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101 1572-1581. [DOI] [PubMed] [Google Scholar]
- Henderson WR Jr, Lu J, Poole KM, Dietsch GN, and Chi EY (2000) Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J Immunol 164 3360-3367. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Bernat A, Valette G, Gigo V, Lale A, LaPlace MC, Lespy L, Savi P, Maffrand JP, and Le Fur G (1991) Biochemical and pharmacological activities of SR 27417, a highly potent, long-acting platelet-activating factor receptor antagonist. J Pharmacol Exp Ther 259 44-51. [PubMed] [Google Scholar]
- Honda Z, Ishii S, and Shimizu T (2002) Platelet-activating factor receptor. J Biochem 131 773-779. [DOI] [PubMed] [Google Scholar]
- Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, and Miyamoto T (1991) Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature 349 342-346. [DOI] [PubMed] [Google Scholar]
- Hurley JH (1999) Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem 274 7599-7602. [DOI] [PubMed] [Google Scholar]
- Hwang SB, Lee CS, Cheah MJ, and Shen TY (1983) Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry 22 4756-4763. [DOI] [PubMed] [Google Scholar]
- Im DS (2004) Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res 45 410-418. [DOI] [PubMed] [Google Scholar]
- IIshii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, et al. (1998) Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med 187 1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarowski JH, Zhu K, Le LQ, Witte ON, and Xu Y (2001) Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293 702-705. [DOI] [PubMed] [Google Scholar]
- Karasawa K, Harada A, Satoh N, Inoue K, and Setaka M (2003) Plasma platelet activating factor-acetylhydrolase (PAF-AH). Prog Lipid Res 42 93-114. [DOI] [PubMed] [Google Scholar]
- Lee WL and Downey GP (2001) Neutrophil activation and acute lung injury. Cur Opin Crit Care 7 1-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang G, Feil R, Han J, and Du X (2006) Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin αIIbβ3. Blood 107 965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Welch EJ, Gao XP, Malik AB, and Ye RD (2005) Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol 174 2981-2989. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Silva AR, de Castro Faria Neto HC, Tjoelker LW, Prescott SM, Zimmerman GA, and McIntyre TM (2001) Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J Lipid Res 42 1430-1437. [PubMed] [Google Scholar]
- O'Donnell MC, Siegel JN, and Fiedel BA (1981) Platelet activating factor: an inhibitor of neutrophil activation? Clin Exp Immunol 43 135-142. [PMC free article] [PubMed] [Google Scholar]
- Quarck R, De Geest B, Stengel D, Mertens A, Lox M, Theilmeier G, Michiels C, Raes M, Bult H, Collen D, et al. (2001) Adenovirus-mediated gene transfer of human platelet-activating factor-acetylhydrolase prevents injury-induced neointima formation and reduces spontaneous atherosclerosis in apolipoprotein E-deficient mice. Circulation 103 2495-2500. [DOI] [PubMed] [Google Scholar]
- Reinhold SL, Zimmerman GA, Prescott SM, and McIntyre TM (1989) Phospholipid remodeling in human neutrophils. Parallel activation of a deacylation/reacylation cycle and platelet-activating factor synthesis. J Biol Chem 264 21652-21659. [PubMed] [Google Scholar]
- Rivkin I and Becker EL (1976) Effect of exogenous cyclic AMP and other adenine nucleotides on neutrophil chemotaxis and motility. Int Arch Allergy Appl Immunol 50 95-102. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Elwood W, Barnes PJ, and Chung KF (1993) Effect of inhaled lyso-platelet-activating factor on airway microvascular leakage in the guinea pig. J Appl Physiol 74 1117-1122. [DOI] [PubMed] [Google Scholar]
- Salzman EW, Kensler PC, and Levine L (1972) Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann N Y Acad Sci 201 61-71. [DOI] [PubMed] [Google Scholar]
- Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taquchi R, and Shimizu T (2007) A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem 282 6532-6539. [DOI] [PubMed] [Google Scholar]
- Szabó C, Wu CC, Mitchell JA, Gross SS, Thiemermann C, and Vane JR (1993) Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res 73 991-999. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, and Giaever I (1992) Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A 89 7919-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, and Yeung J (2008) Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 358 28-35. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, Yin HQ, Gustafson KS, Nelson RD, and Jacob HS (1988) Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood 71 1100-1107. [PubMed] [Google Scholar]
- Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, and Witte ON (2005) Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell 16 2234-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte ON, Kabarowski JH, Xu Y, Le LQ, and Zhu K (2005) Retraction. Science 307 206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.