Abstract
Chlordecone (CD) is one of many banned organochlorine (OC) insecticides that are widespread persistent organic pollutants. OC insecticides alter lipid homeostasis in rodents at doses that are not neurotoxic or carcinogenic. Pretreatment of mice or rats with CD altered tissue distribution of a subsequent dose of [14C]CD or [14C]cholesterol (CH). Nuclear receptors regulate expression of genes important in the homeostasis of CH and other lipids. In this study, we report that CD suppresses in vitro reporter systems for human liver X receptors (LXRs) and activates those for human farnesoid X receptor (FXR), pregnane X receptor (PXR) and estrogen receptor α (ERα) in a concentration-dependent manner (0–50 μM). Consistent with human PXR activation in vitro, three days after a single dose of CD (15 mg/kg) hepatic microsomal CYP3A11 protein increases in C57BL/6 mice. CD decreases hepatic CH ester content without altering total CH concentration. Apolipoprotein A-I (apoA-I) contents of hepatic lipoprotein-rich and microsomal fractions of CD-treated mice are higher than controls. There is a significant reduction in non-high density lipoprotein CH but not apolipoprotein B-48/100 (apoB-48/100) in plasma from CD-treated mice after a 4 h fast. At 14 days after 15 mg CD/kg apoA-I and apoB-100 proteins but not CYP3A11 protein in hepatic microsomes are similar to controls. This work indicates that altered CH homeostasis is a mode of OC insecticide action of relevance after a single dose. This at least partially explains altered CH tissue distribution in CD-pretreated mice.
Keywords: Apolipoproteins, Cholesterol, Pregnane X receptor, Estrogen receptor α, Liver X receptors, Organochlorine insecticides, Chlordecone
Introduction
Banned organochlorine (OC) insecticides are widespread persistent organic pollutants that bioaccumulate in wildlife and humans. While OC insecticides are structurally diverse, they share a number of modes of toxic action. They are neurotoxic through their interactions with ion channels (Narahashi et al., 1998). Some OC insecticides, chlordecone (CD) for example, are carcinogenic in rodent bioassays (Reuber 1978). Others, such as dieldrin, are tumor promoters in rodent models (Kolaja et al., 1998). Alterations in lipid homeostasis occur in rodents at OC insecticide doses that are not overtly neurotoxic or carcinogenic. Single doses of CD and dieldrin decrease plasma triglycerides and total cholesterol (CH) in rats 21 and 60 days after administration, respectively (Ishikawa et al., 1978). Carpenter et al. (1996) report a dose-dependent loss of lipid droplets in hepatocytes, but not Ito cells, of mice after 5–40 mg CD/kg. A single dose of 5 mg CD/kg also alters tissue distribution of exogenous [14C]CH (Carpenter and Curtis 1991; Lee et al., 2008). The mechanisms that underlie OC insecticide alterations in lipid homeostasis are unclear. The work below employs CD since its toxicities are representative of the OC insecticide class. It is novel in that it is refractory to metabolism in rodents (Guzelian 1982); therefore contributions of metabolites of this OC to altering CH regulatory pathways are highly unlikely.
Liver X receptors (LXRs), farnesoid X receptor (FXR), peroxisome proliferator-activated receptors (PPARs), and pregnane X receptor (PXR) are nuclear receptors which function as ligand activated transcription factors (Makishima et al., 1999; Parks et al., 1999; Peet et al., 1998). These receptors bind specific response elements in 5′ upstream promoter regions of genes after heterodimerization with retinoid X receptor (RXR). LXRs respond to elevated oxysterol concentration and increase the transcription of the cyp7a1 gene that governs oxidation of CH to bile acids. Physiological concentrations of bile acids activate FXR and thereby repress the transcription of the cyp7a1 gene (Makishima et al., 1999). Therefore, LXR and FXR coordinate CH homeostasis by regulating feed forward and feed back pathways, respectively. PPARs also regulate lipid metabolism: PPARα activation increases hepatic CYP8B1, the sterol 12α-hydroxylase, that oxidizes CH leading to biosynthesis of bile acids (Hunt et al., 2000). Recent work suggests a potential role of PXR in CH metabolism. Bile acids activate PXR, which reduces their production through repression of the cyp7a1 gene (Staudinger et al., 2001a,b; Xie et al., 2001).
Most banned OC insecticides bind and activate PXR (Goodwin et al., 2002) but exhibit negligible affinity for the aryl hydrocarbon receptor (Poland and Knutson,1982). Some, including p,o-DDT, CD, and dieldrin are also estrogenic in whole animals or cell based reporter gene assays (Charles et al., 2002; Donohoe and Curtis 1996). CD competes for high affinity estrogen binding sites in rainbow trout liver and increases plasma concentration of the estrogen receptor α (ERα)-dependent lipoprotein, vitellogenin (Donohoe and Curtis 1996). Estrogen increases apolipoprotein A-I (apoA-I) secretion in Hep G2 cells, but ERα probably does not directly mediate this effect (Lamon-Fava et al., 1999). Modulating transcription of the apoA-I gene promoter appears more likely. PXR agonists but not a selective constitutive androstane receptor (CAR) agonist increase expression of the apoA-I gene in mice (Bachmann et al., 2004). ApoA-I protein is a principal component of high density lipoprotein (HDL). HDL is the lipoprotein central to CH transport from peripheral tissues to the liver (reverse CH transport) (Lee and Parks 2005). This research addresses the hypothesis that modulation of lipoprotein metabolism at least partially explains CD alterations in CH homeostasis in male C57BL/6 mice.
Materials and methods
Chemicals
CD (99% purity) was purchased from Chem Service (West Chester, PA) and purity was verified by GC-EI/MS. LG268 and T0901317 were acquired from Ligand Pharmaceuticals (San Diego, CA) and Cayman Chemical (Chicago, IL), respectively. [1-14C] Lauric acid (55 mCi/mmol) was obtained from American Radiolabeled Chemicals. Inc. (St. Louis, MO). Other chemicals including 17β-estradiol (E2) were obtained from Sigma (St. Louis, MO).
Animals
Male C57BL/6 mice, 6 to 7-week-old (20–25 g in weight), were obtained from Simeonson Laboratory (Gilroy, CA). The animals were housed in a temperature controlled room (22±1 °C) with a daily cycle of 12 h of light and 12 h darkness and fed ad libitum with AIN93 diet (Dyets, Inc., Bethlehem, PA) with free access to water. Treatments were initiated after seven days of acclimatization. CD was dissolved in corn oil. Mice received CD (5 or 15 mg/kg body weight) or corn oil alone by intraperitoneal (ip) injection (5 ml/kg body weight). After 3 or 14 days, animals were fasted for 4 h and killed by CO2 anesthesia and exsanguinations. Blood, liver, and gallbladder were collected. The procedures for animal use were approved by the Oregon State University Institutional Animal Care and Use Committee.
Cell culture and reporter gene assay
The human embryonic kidney cell line, HEK293, was maintained at 37 °C, 95% O2 and 5% CO2 in DMEM containing 10% fetal bovine serum. In some experiments HEK 293 cells were cotransfected with a plasmid encoding a fusion protein of the Gal4 DNA binding domain and a nuclear receptor ligand-binding domain along with a luciferase reporter plasmid-containing response element. In other experiments HEK293 cells were contransfected with plasmids encoding full length RXRα with LXRα, LXRβ, or FXR along with a luciferase reporter containing the LXR response element (LXRE) or FXR response element (FXRE). CMX-β-gal was contransfected as an internal control. Luciferase cotransfection assays were performed as described (Makishima et al., 2002). Nuclear receptor ligand-binding domains were from human LXRα, LXRβ, FXR, RXR, ERα and ERβ, or murine PPARα, PPARδ, PPARγ. Cells were treated with vehicle (ethanol) alone or 1 to 50 μM CD and/or 1 nM T0901317 (LXR agonist), 100 nM LG268 (RXR agonist), and 1 nM E2 (ER agonist). Cell morphology by light microscopy and β-galactosidase activity assessed potential cytotoxicity. Activation of nuclear receptors was assessed by measuring luciferase activity. Individual assays were repeated at least three times.
Tissue preparation
Blood was collected by cardiac puncture in heparinized syringes and kept on ice until plasma was isolated by centrifugation at 3000 g for 25 min at 4 °C. Plasma was stored at −80 °C. Liver was homogenized in buffer (0.01 M potassium phosphate, pH 7.5; 0.15 M KCl; 1.0 mM ethylenediaminetetraacetic acid (EDTA); 0.1 mM butylated hydroxytoluene (BHT); 0.1 mM phenylmethylsulfonyl fluoride (PMSF)) with a polytron PT 3000 (Brinkmann, Westbury, NY). Liver homogenate was centrifuged at 12,000 g for 30 min. The supernatant was again centrifuged at 100,000 g for 90 min at 4 °C with Ti 70 rotor (Beckman, Fullerton, CA). The floating fatty layer in the supernatant was regarded as lipoprotein-rich fraction. The remaining supernatant and resulting pellet were cytosolic and microsomal fractions, respectively. Microsomes were resuspended in microsome resuspension buffer (0.1 M potassium phosphate, pH 7.25; 1.0 mM EDTA; 30% glycerol; 0.1 mM PMSF, 1.0 mM DTT (dithiothreitol), and 20 μM BHT). Protein concentration was determined by the BCA assay (Pierce Biotechnology, Inc. Rockford, IL).
Western blot analysis
Mouse liver subcellular fractions (lipoprotein-rich, cytosol and microsomes) were prepared from control, 5 or 15 mg CD/kg treated animals as above. Proteins from each preparation were separated on Tris/glycine gels (Bio-Rad, Richmond, CA) under reducing conditions. Following gel electrophoresis at 100 V for 2 h, proteins were electrophoretically transferred onto PVDF membranes utilizing a Mini trans-blot transfer cell (Bio-Rad, Richmond, CA). Membranes were then reacted with antibodies. Rabbit anti-CYP7A antibody was a generous gift from Professor John Y. L. Chiang (Northwestern Ohio University) or purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-CYP3A antibody was a generous gift from Professor Donald Buhler (Oregon State University). Goat anti-CYP4A antibody was purchased from BD Gentest (Woburn, MA). ApoAl and apoB antibodies were purchased from Biodesign (Saco, ME). The blots were then probed with horseradish peroxidase conjugated goat anti-rabbit IgG or rabbit anti-goat IgG as the secondary antibody (Bio-Rad, Richmond, CA). The proteins were detected after development with enhanced chemiluminescence (ECL) detection (Amersham, Piscataway, NJ). Quantification of the intensity of the protein bands was performed with NIH-image software.
Enzyme activities
Hepatic microsomal enzyme activities were analyzed with a HPLC (Waters 2690) and a reverse phase C18 HPLC column (4.6 mm×25 cm, 5 μm, ultrasphere, Beckman). In the assay of CH 7α-hydroxylase, reaction products after the enzymatic conversion of 7α-hydroxylase-CH to 7α-hydroxy-4-cholesten-3-one (7α-HCO) by CH oxidase (Sigma, St. Louis, MO) were detected by a UV spectrophotometer (Chiang 1991). [1-14C] Laurate hydroxylation was measured using slight modifications of the procedure described by Williams et al. (1984). Samples were analyzed using 62% methanol containing 0.2% acetic acid to elute ω- and ω-1 hydroxylaurate at a flow rate of 1 ml/min and the mobile phase was switched to 100% methanol to elute parent compound and detected by Flo-One TR505 radioactivity flow monitor. 6β-Testosterone hydroxylase activity was determined as described by Purdon and Lehman-McKeeman (1997).
Plasma lipid analyses
Total plasma CH concentrations were determined after a 4 h fast using enzymatic kits (Sigma Diagnostic, St. Louis, MO). Plasma HDL-CH concentrations were measured after precipitation of apoB containing particles with dextran sulfate and magnesium (Rachem, San Diego, CA). Plasma contents of apoA-I and apoB-48/100 were quantified by densitometric scanning following electrophoresis on 4–15% Tris/glycine gels (Bio-Rad, Richmond, CA) and transfer onto PVDF membranes (Bio-Rad, Richmond, CA). The membranes were incubated with rabbit anti-apoA-I or rabbit anti-apoB-48/100 polyclonal antibodies (Biodesign, Saco, ME) and probed with horseradish peroxidase conjugated goat anti-rabbit IgG as the secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The proteins were visualized by enhanced chemiluminescence (ECL) detection (Amersham, Piscataway, NJ).
Hepatic cholesterol content
For extraction of lipids, ~100 mg homogenized liver was incubated in 1.5 N KOH in ethanol/water (2:1) at 70 °C for 30 min. Following incubation, 2 ml hexane was added and the solution was inverted three times and centrifuged for 3 min at 3000 g. The lipid layer (upper phase) of each sample was collected and dried under nitrogen. Dried lipids were dissolved in 2-propanol containing 10% Triton-X-100 as assay samples. The CH concentration was determined using a Cholesterol/Cholesteryl ester Quantitation KitR (BioVision, Mt. View, CA). Hepatic esterified CH concentration was taken as the difference between the total CH and free CH.
Statistical methods
Statistical analyses were conducted with StatGraphics software (StatPoint, Herndon, VA). Normality of data sets was assessed with standardized kurtosis, standardized skewness, and homogeneity of variance. Data that failed one or more tests for normality were log base 10 transformed. Transformed data were assessed for normality with the criteria specified above. Data were expressed as means±standard error (SE). Comparisons among groups validated for normality were submitted to a one-way analysis of variance (ANOVA). For comparison between two groups, Student’s t-test with equal-variance was applied. In all analyses, a 95% confidence level was used as the criterion for significance. For outlier identification, Grubbs’ test (assumes normality) was applied.
Results
Cell morphology and β-galactosidase activity were not significantly altered by CD (data not shown). A series of reporter gene cotransfection assays in HEK 293 cells characterized potential interactions of CD with nuclear receptors involved with lipid homeostasis. CD activated or inhibited transactivation by several nuclear receptors (Fig. 1). At 10 μM CD there was mild but statistically significant suppression of reporter construct alone (Gal4) and RXRα (about 30%) (Fig. 1A). CD substantially inhibited LXRα and strongly suppressed activation for LXRβ. CD modestly but significantly activated (about 1.3-fold) FXR and PPARα and strongly activated PXR about 3.5-fold over background (Figs. 1A, B). PPARδ and PPARγ were not affected by CD treatment (Fig. 1B).
Fig.1.
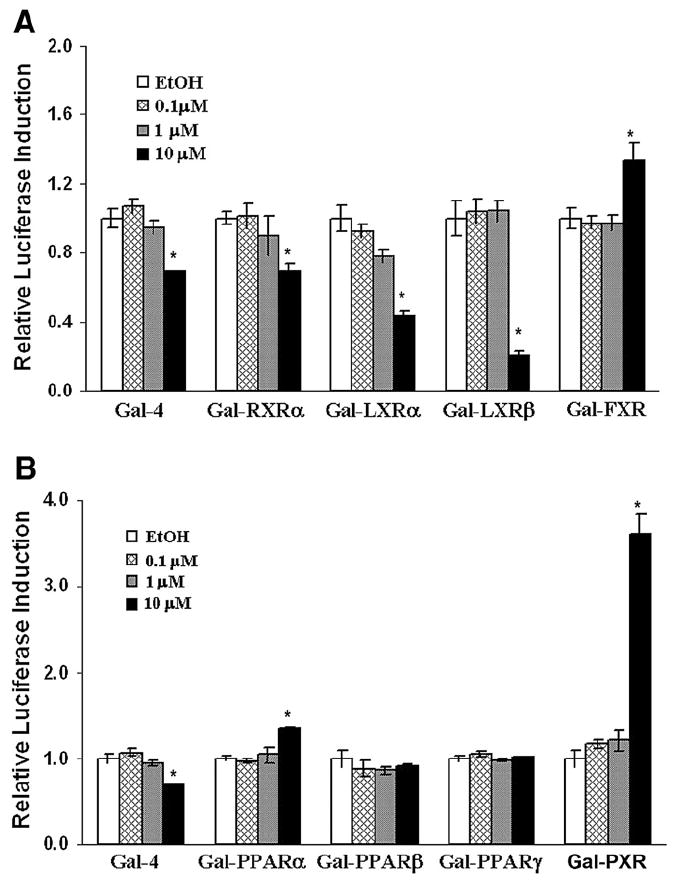
Chlordecone activates pregnane X receptor (human homolog). HEK 293 cells were cotransfected with the Gal4 luciferase reporter and a series of chimeras in which Gal4 DNA binding domain was fused to the indicated nuclear receptor ligand-binding domain. Cells were treated with ethanol or CD (0.1, 1, or 10 μM). Activation of nuclear receptors was assessed by measuring luciferase activity. Values were presented as the mean relative luciferase induction from triplicate assays±SE. Significant differences from ethanol controls detected as described in Materials and methods were indicated by an asterisk.
Activation of human ERs by CD was also assessed. CD significantly activated a chimera consisting of the DNA binding domain of Gal4 and the ligand-binding domain of ERα slightly but significantly at 1 μM and almost 10-fold at 10 μM and 50 μM (Fig. 2A). There was no CD significant effect on human ERβ activity (Fig. 2A) but CD repressed E2-induced ERβ activation (40%) (Fig. 2B). There was no significant difference in activation of ERα by E2 in the presence or absence of 10 μM CD (Fig. 2B). To further characterize CD (Fig. 3A) activation of nuclear receptors involved in lipid homeostasis not previously assessed with regard to OC insecticides, a transient transfection assay using a synthetic LXR or FXR responsive reporter plasmid was assessed. CD alone strongly suppressed activities of the murine LXRα-RXR and LXRβ-RXR heterodimers in a concentration-dependent manner (Figs. 3A and C). CD (10 μM) strongly inhibited LXRα-RXR activation by LG268 (RXR agonist) (Fig. 3B), but insignificantly inhibited the receptor activation by T0901317 (LXR agonist) (data not shown). 50 μM CD moderately but significantly repressed activation of LXRβ-RXR activated by T0901317 (Fig. 3C). CD (50 μM) activated FXR approximately 50% as effectively as the specific RXR agonist LG268 (Fig. 3D). In the presence of LG268, CD increased FXR transactivation cooperatively (Fig. 3D).
Fig. 2.
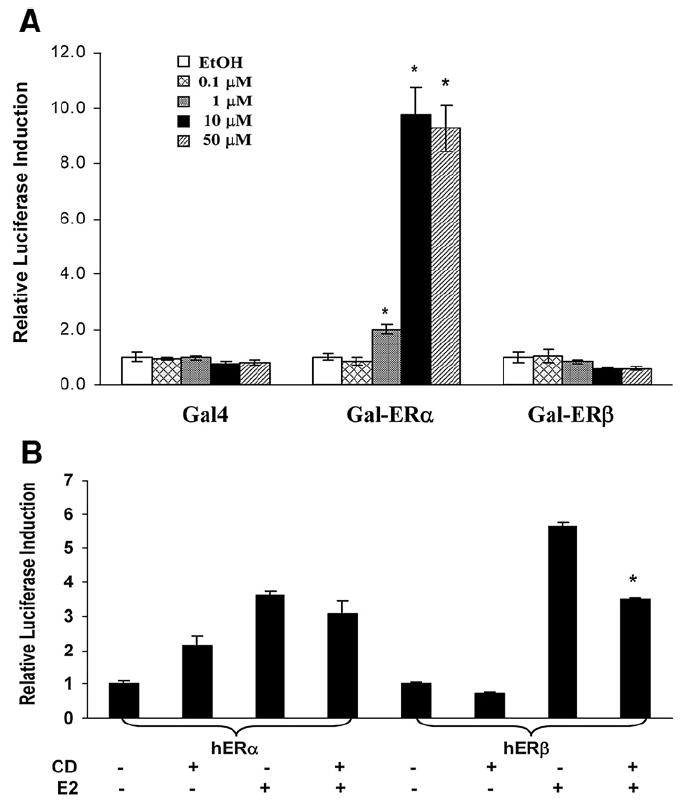
Chlordecone activates ERα. (A) HEK 293 cells were cotransfected with the Gal4 luciferase reporter and chimeras in which Gal4 DNA binding domain was fused to the indicated nuclear receptor ligand-binding domain. (B) Expression vectors for human ERα or ERβ were cotransfected with luciferase report construct containing ER response element [ERE(EFP)Tkluc] plasmid along with the CMX-β-gal internal control. Cells were treated with ethanol as vehicle, 10 μM CD or 1 nM E2 (ER agonist). Values were presented as the mean relative luciferase induction from triplicate assays±SE. Significant differences from appropriate controls detected as described in Materials and methods were indicated by an asterisk.
Fig. 3.
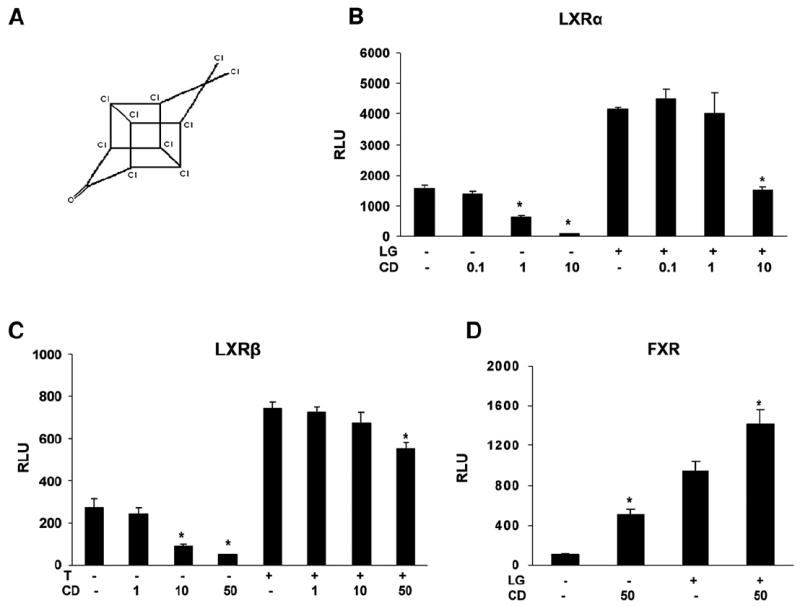
Chlordecone inhibits LXRs and activates FXR. (A) Structure of chlordecone (CD). (B) HEK 293 cells were cotransfected with a luciferase reporter plasmid [(LXRE)3 Tklc] containing three copies of the LXR response element upstream of the thymidine kinase (TK) promoter and expression vector for murine LXRα and RXR along with the CMX-β-gal internal control. (C) HEK 293 cells were cotransfected as in B for LXRβ and RXR. Cells were treated with vehicle (ethanol) alone or 0.1 to 50 μM CD and/or 1 μM T0901317 (LXR agonist) or 100 nM LG268 (RXR agonist) as indicated. (D) HEK 293 cells were cotransfected with a luciferase reporter plasmid [(FXRE) Tklc] construct containing an FXR response element upstream of the TK promoter and expression vector for FXR and RXR. Cells were treated with vehicle (ethanol) alone or 50 μM CD and/or 100 nM LG268 (RXR agonist) as indicated. Values were presented as the mean relative luciferase units (RLU) from triplicate assays±SE. Significant differences from appropriate controls detected as described in Materials and methods were indicated by an asterisk.
The relevance of CD activation of nuclear receptors in vitro was assessed with in vivo assays in C57BL/6 mice. Interactions with LXRα and FXR were assessed by hepatic microsomal CYP7A1 protein immunoquantitation and CH 7α-hydroxylase activity. Neither CYP7A1 protein content nor its enzyme activity were statistically significantly altered in CD-treated mice (Figs. 4A, B). Interaction with PPARα was assessed by hepatic microsomal CYP4A1 protein immunoquantitation and lauric acid ω-1 hydroxylase activity. There was no evidence for activation of this receptor by the CD doses administered to mice (Figs. 4A, B). Finally, interaction with PXR was assessed by hepatic microsomal CYP3A11 protein immunoquantitation and 6β-testosterone hydroxylase activity. CYP3A11 protein content was highly increased (4-fold) in livers from CD-treated animals while 6β-testosterone hydroxylase activity was unchanged. (Fig. 4A). Increased hepatic microsomal CYP3A11 protein after CD treatment indicated PXR activation.
Fig. 4.
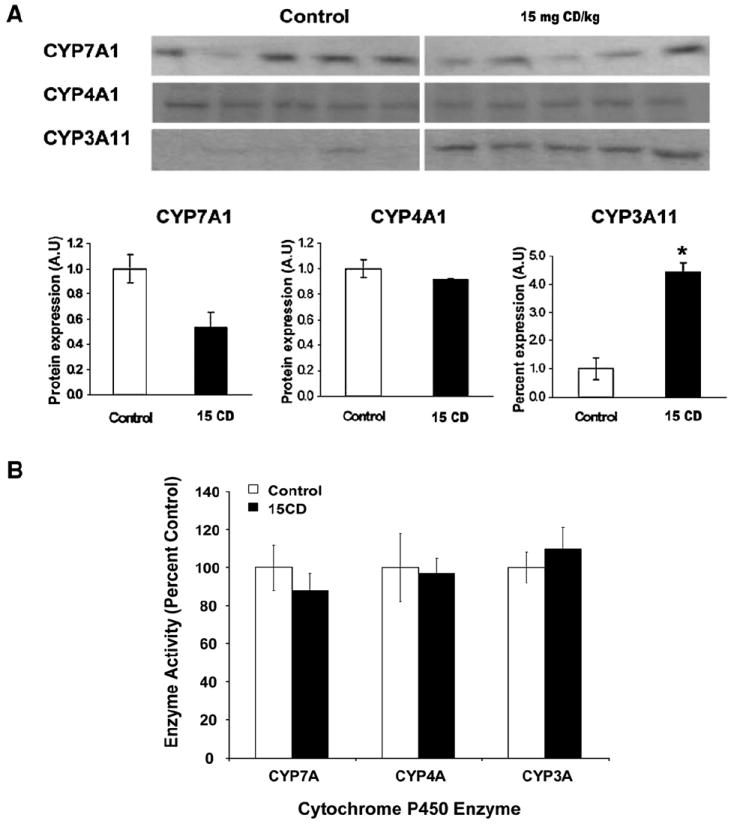
In vivo evidence for chlordecone activation of PXR. Animals were treated with corn oil, or 15 mg CD/kg (15 CD) body weight by ip injection. Hepatic microsomes were prepared from individual animals (5–6 mice in each group) after 3 days as described under Materials and methods. (A) Immunoblot analyses of CYP7A1, CYP4A1, and CYP3A11 proteins in liver microsomes. Total 20 μg protein samples were separated by SDS-PAGE and blotted with antibodies against the specific CYP isoforms as described under Materials and methods. Values were expressed as the mean relative protein expression (A.U.)±SE compared with the controls. (B) Enzyme activity analyses of CH 7 α-hydroxylase for CYP7A1, lauric acid ω-1 hydroxylase for CYP4A1, and 6β-testosterone hydroxylase for CYP3A. Values were expressed as the mean relative enzyme activity (%)±SE compared with the controls. A significant difference from vehicle injected controls was detected as described in Materials and methods and indicated by an asterisk.
The effects of CD on total plasma CH and HDL-CH concentrations were measured using enzymatic analyses. Plasma non-HDL-CH concentration was significantly decreased by CD treatment (Fig. 5A). Plasma HDL-CH was unchanged but the ratio of HDL-CH to total plasma CH was mildly but significantly increased (Figs. 5A, B).
Fig. 5.
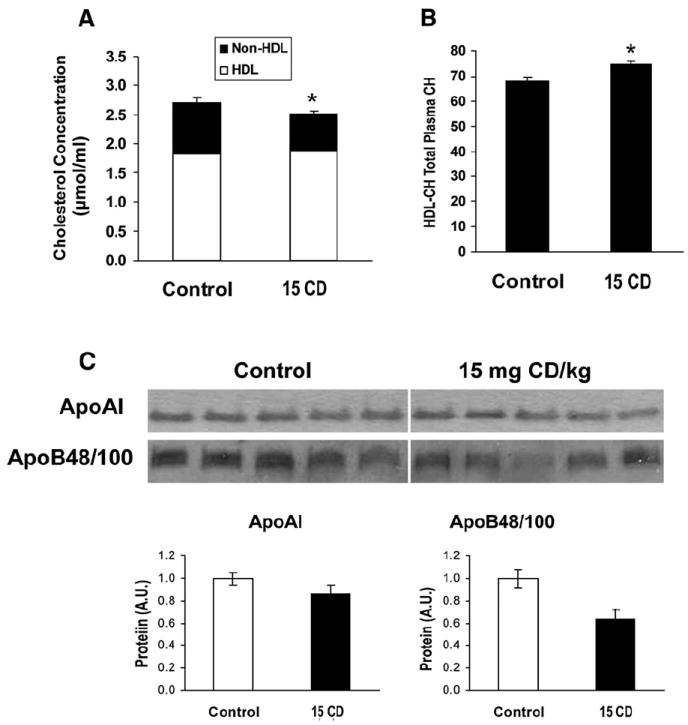
Chlordecone reduces non-HDL plasma cholesterol after a 4 h fast. Individual plasma was collected from animals (5–6 mice in each group) treated with either corn oil or 15 mg/kg (15 CD) body weight by ip injection. (A) Plasma total CH and HDL-CH was determined enzymatically as described in Materials and methods. Non-HDL-CH content was taken as the difference between the total plasma total CH and HDL-CH. (B) Ratio of HDL-CH to plasma CH. (C) Western blot analyses of plasma apoA-I and apoB48/100 protein. Plasma apolipoprotein content was measured by western blotting with either apoA-I or apoB-100 antibodies. Values were expressed as mean±SE. Statistically significant differences were indicated by an asterisk.
Immunoblot analysis revealed no significant difference in plasma apoB-48/100 or apoA-I in CD-treated mice compared to controls (Fig. 5C). The effect of CD on apolipoprotein content was further characterized in liver subcellular fractions: lipoprotein-rich, cytosol and microsomes. Interestingly apoA-I significantly increased in the hepatic lipoprotein-rich and microsomal fractions from CD-treated mice (1.5-fold,1.8-fold, respectively) (Fig. 6A). There was no significant difference in hepatic microsomal apoB-100 between CD-treated and control mice (Fig. 6B).
Fig. 6.

Effect of Chlordecone on hepatic apolipoprotein content. Animals were treated with corn oil, 5 mg CD/kg (5 CD) or 15 mg CD/kg (15 CD) body weight by ip injection. Hepatic subcellular fractions were prepared from individual animals (5–6 mice in each group) after 3 days as described under Materials and methods. (A) Hepatic apolipoprotein A-I or (B) apolipoprotein B-100 was measured by immunoblotting with either apoA-I or apoB-100 antibodies. Values were expressed as the mean relative protein expression (A.U.)±SE compared with the controls. ** Plasma CH concentration was 55.3 mg/dl and Grubbs’ test indicated this as an outlier. Statistically significant differences were indicated by an asterisk.
Hepatic total, free and esterified CH content were measured enzymatically. About 90% of hepatic CH was in the unesterified form (Fig. 7). Hepatic total and free CH contents from CD-treated mice were not different from control mice (Fig. 7). However, CD significantly decreased hepatic esterified CH concentration (about 40% of control) (Fig. 7).
Fig. 7.
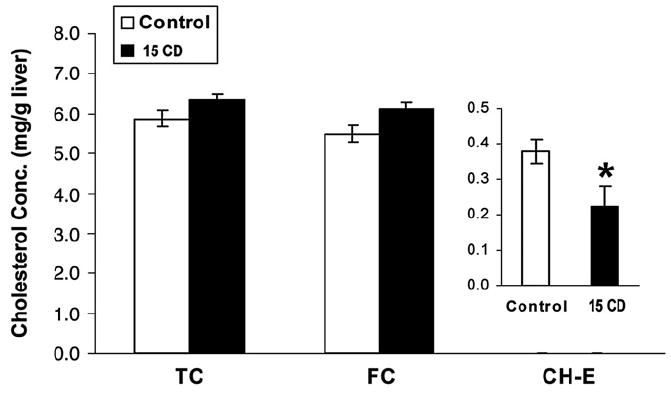
Chlordecone reduces hepatic cholesteryl ester content. Animals were treated with corn oil, or 15 mg CD/kg (15 CD) body weight by ip injection. Liver was collected from individual animals (5–6 mice in each group) after 3 days. After lipid extraction with hexane, liver total (TC) and free CH (FC) content were determined using an enzymatic method as in Materials and methods. Hepatic esterified CH (CH-E) content was taken as the difference between the total CH and free CH. Values were expressed as mean CH level±SE. A statistically significant difference from the control was indicated by an asterisk.
An ancillary experiment examined the persistence of CD-induced protein changes in liver. Hepatic microsomal CYP3A11 was significantly higher in 15 mg CD/kg treated mice than controls 14 days after injection (Fig. 8). Hepatic microsomal CYP7A1 and CYP 4A1 were not different from controls in the same mice (Fig. 8). Plasma and hepatic microsomal apoA-I were similar to controls 14 days after 15 mg CD/kg (Fig. 8). Hepatic microsomal apoB-100 was not different from controls in the same mice (Fig. 8).
Fig. 8.
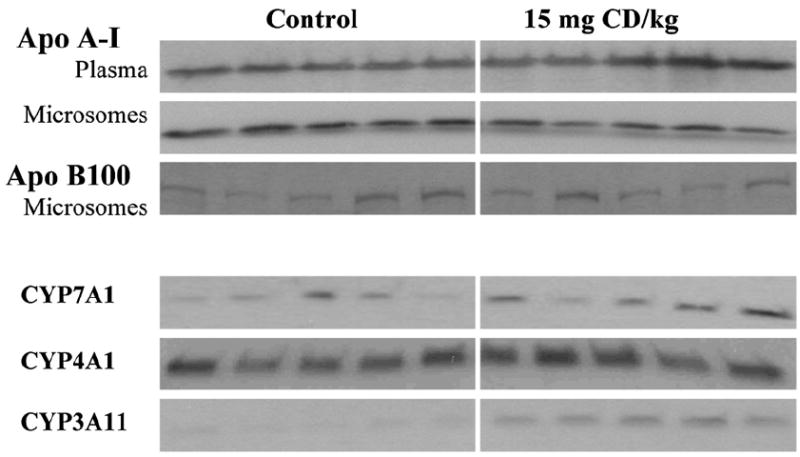
Apolipoproteins and cytochrome P450 contents at 14 days after 15 mg CD/kg treatment. Animals were treated with corn oil or 15 mg CD/kg body weight by ip injection. Plasma and hepatic microsomal fractions were prepared from individual animals (5–6 mice in each group) after 14 days as described under Materials and methods. Immunoblotting was performed with apoA-I, apoB-100, CYP7A, CYP4A or CYP3A antibodies. There was a statistically significant difference (p<0.05) in the mean intensity of CYP3A11 bands of hepatic microsomes from CD-treated compared to control mice by densitometry.
Discussion
Humans and wildlife are exposed to complex mixtures of xenobiotics including pesticides and other environmental contaminants. In the body, xenobiotics, dietary nutrients, and endogenous substances may interact to modulate regulatory pathways for intermediary metabolism. Inhibition or activation of nutrient metabolism or transport pathways may yield beneficial or adverse effects. Prior reports indicate that a single dose of CD, an OC insecticide, alters tissue distribution of exogenous CH in mice and rats (Carpenter and Curtis 1991; Gilroy et al., 1994; Lee et al., 2008). Subsequent experiments and other work suggests that CD also modulates other aspects of lipid homeostasis (Carpenter et al., 1996; Chetty et al., 1993). However, molecular mechanisms underlying these observations are unclear.
The liver is the major organ that controls CH homeostasis in mammals. The role of the liver in CH homeostasis involves coordinate regulation of biosynthesis of CH, its uptake from plasma and catabolism to bile acids (Brown and Goldstein 1999). A large body of literature shows that nuclear receptors play important roles in the regulation of metabolism of CH and other lipids through a coordinated network of transcriptional programs (Chawla et al., 2001; Lobaccaro et al., 2001; Makishima 2003). To increase understanding of how low doses of CD alter CH homeostasis, we assessed CD interactions with several nuclear receptors in vitro and in vivo. Whole livers of C57BL/6 mice contained 84 μM CD 16 h after 5 mg/kg (Carpenter and Curtis 1989). Hepatic CD residues were persistent in livers with less than a 50% reduction from 3 to 14 days after exposure. Therefore, in vitro CD concentrations used herein were relevant to those detected in livers of mice. CD significantly affected transactivation by chimeras consisting of the DNA binding domain of Gal4 and the ligand-binding domains of LXRs or FXR (Fig. 1A). CD (10 μM) minimally increased FXR (Fig. 1A) activation and inhibited LXRs to different extents (Figs. 1A, 2B, 2C). Inhibition of LXRβ was about twice as marked as that of LXRα. Additional in vitro experiments and in vivo work further assessed CD interaction with LXRs and FXR (detailed below).
PPARα was slightly but significantly activated by 10 μM CD (Fig. 1B). PPARδ and PPARγ were not activated or inhibited by CD treatment (Fig. 1B). The CYP4A proteins were generally recognized as increased by PPARα agonists (Hunt et. al., 2000). CD altered neither the CYP4A protein content nor the lauric acid ω-1 hydroxylase activity characteristic of it (Fig. 4). Therefore, physiological relevance of PPAR contributions to CD interaction with lipid homeostasis in mice seemed unlikely.
PXR was identified as a key regulator of CYP3A expression since it was activated by diverse compounds that induced CYP3A expression and bound xenobiotic response elements in cyp3a promoters (Xie et al., 2000). CD (10 μM) activated human PXR 6-fold in a stable HepG2 cell culture system (Lemaire et al., 2004). Because of evolutionary divergence of the PXR ligand-binding domain, species-specific differences were evident between humans and mice (Kliewer 2003; Xie et al., 2000). Rifampicin was a potent inducer of human CYP3A which was only weakly effective in mice; while pregnenolone 16 α-carbonitrate potently induced CYP3A in mice with low efficacy in human hepatocytes (Bachmann et al., 2004). Here CD not only transactivated human PXR in an in vitro assay (Fig. 1B) but also increased hepatic microsomal CYP3A11 protein in hepatic microsomes from mice (Fig. 4A). The 6β-testosterone hydroxylase activity characteristic of CYP3A was not significantly increased 3 days after CD however. Increased CYP3A protein without increased enzymatic activity was not unprecedented. Rifampicin treatment of mice increased CYP3A protein in immunoblots 4-fold but not an associated enzyme activity in hepatic microsomes (Schuetz et al., 1996). Presence of catalytically inactive apoenzyme was one possible explanation for our results. CD inhibition of CYP3A-mediated 6β-testosterone hydroxylation was perhaps a more likely explanation. Taken together, the data indicated that CD activated PXR in both humans and mice. Recent results suggested that PXR activation strongly induced the cyp3a4 gene by inhibiting small heterodimer partner (SHP) gene transcription in human livers (reviewed in Kalaany and Mangelsdorf 2006).
CD was an ERα but not an ERβ agonist in the micromolar range (Fig. 2A). CD activated human ERα cooperatively with E2, although the activity was 10,000 times lower than E2 (Fig. 2B); This difference in potency was similar to that reported for competition assays for estrogen binding sites in rainbow trout liver (Donohoe and Curtis 1996). CD inhibited E2-induced ERβ activation effectively (Fig. 2B). A role for ERα in CD-altered CH homeostasis was of potential importance since estrogens increased apoB expression (Srivastava et al., 1993, 1997). Work with mice indicated lipid availability interacted with apoB availability in influencing plasma non-HDL-CH. (1) Plasma non-HDL-CH was elevated in CD-treated compared to control mice that received 5 ml corn oil plus 10 mg CH/kg 4 h before sampling (Lee et al., 2008). (2) The reverse was observed in CD-treated mice fasted 4 h before sampling (Fig. 5A). (3) CD depleted lipid droplets and increased putative VLDL particles in mouse liver (Carpenter et al., 1996). Perhaps low hepatic lipid masked CD-stimulated non-HDL-CH secretory capacity in fasted mice and this was reversed when exogenous lipid was provided by ip corn oil. Increased clearance of apoB containing lipoproteins and/or reduced VLDL/LDL-CH secretion potentially contributed to decreased plasma non-HDL-CH in CD-treated fasted mice. E2 increased LDL-receptor protein in livers of rats but not mice (Srivastava et al., 1993). Activation of LXRs induced hepatic LDL-receptor expression in mice (Masson et al., 2004). Since LXRα activation was inhibited by CD in vitro (Figs. 1A and 3B) and LDL receptors were not up-regulated by E2 in mice, reduced VLDL/LDL-CH production was the most plausible explanation for decreased plasma non-HDL-CH concentration in CD-treated fasted mice.
ApoB is synthesized constitutively and regulated primarily by co- and post-transitional mechanisms in the secretory pathway (Avramoglu and Adeli 2004). Ubiquitin-mediated proteasomal degradation or non-proteasomal degradation pathways are important regulators of apoB secretion. There is a positive correlation between hepatic availability of neutral lipids proximal to the site of apoB synthesis and the amount of hepatic apoB secretion (Avramoglu and Adeli 2004). Reduced hepatic CH ester not free CH inhibits VLDL apoB production (Telford et al., 2005). Previous work demonstrates that CD decreases cytosolic lipid droplets in hepatocytes of C57BL/6 mice (Carpenter et al., 1996). CD decreases hepatic CH ester, not total or free CH (Fig. 8) but there is no difference in apoB content in the liver microsomal fraction from CD-treated animals. Thus, insufficient lipid perhaps increases apoB degradation by ubiquitin-mediated proteasomal degradation and reduces VLDL secretion in fasted CD-treated mice.
Co-expression of FXR with exogenous RXR and its ligand significantly enhanced activation (about 5-fold over background) (Fig. 3D). This indicated the requirement of RXR (heterodimer partner) for CD-induced FXR activation. CH 7α-hydroxylase (CYP7A) was the first and a rate limiting enzyme in bile acid synthesis, the major CH elimination pathway (Russell, 1999). LXRα and FXR regulated the expression of CH 7α-hydroxylase in opposite ways (Makishima, 2003). Therefore, bile acid synthesis was regulated by feed forward and feedback mechanisms. Although CD activated FXR and inhibited LXRα activation in vitro, there was no statistically significant reduction of hepatic CYP7A1 protein and its enzyme activity by CD treatment in C57BL/6 mice (Figs. 4A, B). LXRα induced CYP7A1 by binding to the 5′-flanking region and increased expression of mouse and rat cyp7al gene. FXR repressed the cyp7a1 gene indirectly through the induction of the transcriptional repressor SHP (Kalaany and Mangelsdorf, 2006). Hepatic content of other proteins in CD-treated mice indicated little roles for LXRs or FXR in CD-altered CH homeostasis. ATP binding cassette (ABC) G8 and scavenger receptor B1 (SRBI) genes were positively regulated by LXRs (Kalaany and Mangelsdorf, 2006), but hepatic contents of these proteins were not altered by CD (Lee et al., 2008). Activation of FXR negatively regulated expression of apoA1 and apoB genes (Kalaany and Mangelsdorf, 2006). Hepatic microsomal apoB 100 was unchanged while apoA-I significantly increased in that fraction for CD-treated mice (Fig. 6).
LXRβ knockout mice eliminated excess CH as effectively as wild type (Alberti et al., 2001). However, recent studies demonstrated that the LXRβ isoform also contributed to CH homeostasis through activation of ABCAI in macrophages, liver, and intestine (Quinet et al., 2006; Repa et al., 2000). ABCAI was classified as a member of a large family of ABC transporters (Langmann et al., 1999). The ABCAI transporter increased efflux of intracellular CH to lipid-poor apoA-I to form preβ-HDL or nascent HDL (Wang et al., 2000). While results of the reporter construct assays (Fig. 2A) suggested potential for CD inhibition of LXRβ signaling and that potentially disrupted ABCAI activation, there was no reduction in plasma HDL-CH in CD-treated mice (Fig. 5A).
PXR activation increased HDL-CH and expression of apoA-I (the major apolipoprotein in HDL) in vitro and in vivo (Bachmann et al., 2004; Sporstol et al., 2005). Estrogens were suggested as antiatherogenic since they reduced LDL-CH and elevated HDL-CH (Hargrove et al., 1999). While CD was a mixed agonist for human PXR and ERα, HDL-CH and plasma apoA-I contents were not changed in fasted mice (Figs. 5A, C). Instead, we observed that CD increased apoA-I content in hepatic lipoprotein-rich and microsomal fractions (Fig. 6A). Gilroy et al. (1994) reported circumstantial evidence that CD stimulated hepatic HDL secretion. CD was strongly associated with HDL rather than other lipoproteins (Guzelian 1982). In situ rat livers were perfused with HDL and albumin complexed [14C]CD for 1 h, which was then replaced with fresh solution. Efflux of [14C]CD into the perfusate from livers of CD-pretreated rats about doubled over 1 h compared to controls. Plasma HDL-to-total [14C]CH ratio increased in CD-treated compared to control mice that received 5 ml corn oil/kg 4 h prior to sampling (Lee et al., 2008). Therefore, lipid availability was likely a key factor in secretion of apoAl-I containing lipoprotein by liver. Since HDL-CH was widely considered as the good CH that provided some protection from heart disease, the potential role of PXR agonists in raising HDL-CH was an important area of current interest. However not all HDL-subclasses were equally protective. Large HDL2b was believed more important for protection from coronary heart disease in clinical observational studies (Pascot et al., 2001).
Hepatic CD concentrations declined slowly in C57BL/6 mice (Carpenter and Curtis 1989). Liver residues declined less than 50% from 3 to 14 days after ip doses similar to those used in this study. Elevated CYP3A11content in hepatic microsomes 14 days after injection was consistent with persistent PXR signaling (Fig. 8). Hepatic content of apoA-I and apoB were not elevated at that time (Fig. 8). Perhaps differential adaptation of signaling pathways contributed to this phenomenon.
Even though the United States bans most uses of persistent OC pesticides, they continue to occur in tissues of humans and wildlife. Trophic transfer through food webs that provide dietary fish, meat and dairy products is the principal route of environmental exposure to these agents. Residues of OC insecticides occur in tissues of humans and wildlife as complex mixtures. In human fat from the United States, the sum of OC insecticides averages in the nM range (Lordo et al., 1996). Concentrations for wildlife that are top predators are higher. For example, fat of grizzly bears that feed heavily on Pacific salmon contain 25-fold higher maximum concentrations (Christensen et al., 2005) than humans. Since many OC insecticides or their metabolites are PXR (Goodwin et al, 2002) and ERα agonists (Charles et al., 2002; Donohoe and Curtis, 1996) their potential interactions with lipid regulatory pathways deserves attention. Perturbation of CH homeostasis by a low dose of CD may represent an environmentally relevant mode of endocrine disruption. Therefore, it is necessary to continue studies to explain altered CH and lipid homeostasis by banned OC insecticides. In summary: (1) CD was a mixed agonist for human PXR and ERα and an effective antagonist for LXRβ. (2) CD probably modulated lipoprotein metabolism by multiple interactions with nuclear receptors. (3) This work indicated altered CH homeostasis and lipoproteins as modes of OC insecticide action of potential environmental relevance.
Acknowledgments
This work was supported by the Oregon Agricultural Experiment Station (ORE00871) and grant number T32 ES007060 from the National Institutes of Health.
References
- Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest. 2001;107:565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramoglu RK, Adeli K. Hepatic regulation of apolipoprotein B. Rev Endoc Metab Disord. 2004;5:293–301. doi: 10.1023/B:REMD.0000045100.66675.92. [DOI] [PubMed] [Google Scholar]
- Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter HM, Curtis LR. A characterization of chlordecone pretreatment-altered pharmacokinetics in mice. Drug Metab Dispos. 1989;17:131–138. [PubMed] [Google Scholar]
- Carpenter HM, Curtis LR. Low dose chlordecone pretreatment altered cholesterol disposition without induction of cytochrome P-450. Drug Metab Dispos. 1991;19:673–678. [PubMed] [Google Scholar]
- Carpenter HM, Hedstrom OR, Siddens LK, Duimstra JR, Cai ZW, Fisher KA, Curtis LR. Ultrastructural, protein, and lipid changes in liver associated with chlordecone treatment of mice. Fundam Appl Toxicol. 1996;34:157–164. doi: 10.1006/faat.1996.0186. [DOI] [PubMed] [Google Scholar]
- Charles GD, Gennings C, Zacharewski TR, Gollapudi BB, Carney EW. Assessment of interactions of diverse ternary mixtures in an estrogen receptor-alpha reporter assay. Toxicol Appl Pharmacol. 2002;180:11–21. doi: 10.1006/taap.2001.9346. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chetty KN, Walker J, Brown K, Ivie GW. The effects of dietary calcium and chlordecone on cholinesterase, triglycerides, low density lipoproteins, and cholesterol in serum of rat. Arch Environ Contam Toxicol. 1993;24:365–367. doi: 10.1007/BF01128735. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Reversed-phase high-performance liquid chromatography assay of cholesterol 7 alpha-hydroxylase. Methods Enzymol. 1991;206:483–491. doi: 10.1016/0076-6879(91)06117-l. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Mac Duffee M, Mac Donald RW, Whiticar M, Ross PS. Persistent organic pollutants in British Columbia grizzley bears: consequence of divergent diets. Environ Sci Technol. 2005;39:6952–6960. doi: 10.1021/es050749f. [DOI] [PubMed] [Google Scholar]
- Donohoe R, Curtis L. Estrogenic activity of chlordecone, o,p’-DDT, and o,p’-DDE in juvenile rainbow trout: induction of vitellogenesis and interaction with hepatic estrogen binding sites. Aquat Toxicol. 1996;36:31–52. [Google Scholar]
- Gilroy DJ, Carpenter HM, Curtis LR. Chlordecone pretreatment alters [14C] chlordecone and [14C]cholesterol transport kinetics in the perfused rat liver. Fundam Appl Toxicol. 1994;22:286–292. doi: 10.1006/faat.1994.1032. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane X receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- Guzelian PS. Comparative toxicology of chlordecone (Kepone) in humans and experimental animals. Annu Rev Pharmacol Toxicol. 1982;22:89–113. doi: 10.1146/annurev.pa.22.040182.000513. [DOI] [PubMed] [Google Scholar]
- Hargrove GM, Junco A, Wong NC. Hormonal regulation of apolipoprotein AI. J Mol Endocrinol. 1999;22:103–111. doi: 10.1677/jme.0.0220103. [DOI] [PubMed] [Google Scholar]
- Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gafvels M, Einarsson C, Alexson SE. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem. 2000;275:28947–28953. doi: 10.1074/jbc.M002782200. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, McNeeley S, Steiner PM, Glueck CJ, Mellies M, Gartside PS, McMillin C. Effects of chlorinated hydrocarbon on plasma alpha-lipoprotein cholesterol in rats. Metabolism. 1978;27:89–96. doi: 10.1016/0026-0495(78)90127-0. [DOI] [PubMed] [Google Scholar]
- Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr. 2003;133:2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRs and FXR: The yin and yang of cholesterol and fat metabolism. Ann Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Xu Y, Walborg EF, Jr, Stevenson DE, Klaunig JE. Vitamin E modulation of dieldrin-induced hepatic focal lesion growth in mice. J Toxicol Environ Health A. 1998;53:479–492. doi: 10.1080/009841098159196. [DOI] [PubMed] [Google Scholar]
- Lamon-Fava S, Ordovas JM, Schaefer EJ. Estrogen increases apolipoprotein (apo) A-I secretion in hep G2 cells by modulating transcription of the apo A-I gene promoter. Arterioscler Thromb Vasc Biol. 1999;19:2960–2965. doi: 10.1161/01.atv.19.12.2960. [DOI] [PubMed] [Google Scholar]
- Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Lee J, Scheri RC, Curtis LR. Chlordecone altered hepatic disposition of [14C] cholesterol and plasma cholesterol distribution but not SR-B1 or ABCG8 proteins in livers of C57BL/6 mice. Toxicol Appl Pharmacol. 2008;229:265–272. doi: 10.1016/j.taap.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G, de Sousa G, Rahmani R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 2004;68:2347–2358. doi: 10.1016/j.bcp.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Lobaccaro JM, Repa JJ, Lu TT, Caira F, Henry-Berger J, Volle DH, Mangelsdorf DJ. Regulation of lipid metabolism by the orphan nuclear receptors. Ann Endocrinol (Paris) 2001;62:239–247. [PubMed] [Google Scholar]
- Lordo RA, Dinh KT, Schwemberger JG. Semivolatile organic compounds in adipose tissue: estimated averages for the U.S. population and selected subpopulations. Amer J Pub Hlth. 1996;86:1253–1259. doi: 10.2105/ajph.86.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M. Lipid metabolism and nuclear receptors. Seikagaku. 2003;75:391–395. [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- Masson D, Staels B, Gautier T, Desrumaux C, Athias A, Le Guern N, Schneider M, Zak Z, Dumont L, Deckert V, Tall A, Jiang XC, Lagrost L. Cholesteryl ester transfer protein modulates the effect of liver X receptor agonists on cholesterol transport and excretion in the mouse. J Lipid Res. 2004;45:543–550. doi: 10.1194/jlr.M300432-JLR200. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Ginsburg KS, Nagata K, Song JH, Tatebayashi H. Ion channels as targets for insecticides. Neurotoxicology. 1998;19:581–590. [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Pascot A, Lemieux I, Prud’homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Despres JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res. 2001;42:2007–2014. [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlordibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Ann Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Purdon MP, Lehman-Mckeeman LD. Improved high-performance liquid chromatographic procedure for the separation and quantification of hydroxytestosterone metabolites. Pharmacol Toxicol Meth. 1997;37:67–73. doi: 10.1016/s1056-8719(97)00013-0. [DOI] [PubMed] [Google Scholar]
- Quinet EM, Savio DA, Halpern AR, Chen L, Schuster GU, Gustafsson JA, Basso MD, Nambi P. Liver X receptor (LXR)-beta regulation in LXRalpha-deficient mice: implications for therapeutic targeting. Mol Pharmacol. 2006;70:1340–1349. doi: 10.1124/mol.106.022608. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber MD. Carcinogenicity of kepone. J Toxicol Environ Health. 1978;4:895–911. doi: 10.1080/15287397809529710. [DOI] [PubMed] [Google Scholar]
- Russell DW. Nuclear orphan receptors control cholesterol catabolism. Cell. 1999;97:539–542. doi: 10.1016/s0092-8674(00)80763-1. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Schinkkel AH, Relling MV, Schuets JD. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome 4503A in mice and humans. Proc Nat Acad Sci U S A. 1996;93:4001–4005. doi: 10.1073/pnas.93.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporstol M, Tapia G, Malerod L, Mousavi SA, Berg T. Pregnane X receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun. 2005;331:1533–1541. doi: 10.1016/j.bbrc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Baumann D, Schonfeld G. In vivo regulation of low-density lipoprotein receptors by estrogen differs at the post-transcriptional level in rat and mouse. Eur J Biochem. 1993;216:527–538. doi: 10.1111/j.1432-1033.1993.tb18171.x. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Srivastava N, Averna M, Lin RC, Korach KS, Lubahn DB, Schonfeld G. Estrogen up-regulates apolipoprotein E (ApoE) gene expression by increasing ApoE mRNA in the translating pool via the estrogen receptor alpha-mediated pathway. J Biol Chem. 1997;272:33360–33366. doi: 10.1074/jbc.272.52.33360. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001a;29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001b;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford DE, Lipson SM, Barrett PH, Sutherland BG, Edwards JY, Aebi JD, Dehmlow H, Morand OH, Huff MW. A novel inhibitor of oxidosqualene: lanosterol cyclase inhibits very low-density lipoprotein apolipoprotein B100 (apoB100) production and enhances low-density lipoprotein apoB100 catabolism through marked reduction in hepatic cholesterol content. Arterioscler Thromb Vasc Biol. 2005;25:2608–2614. doi: 10.1161/01.ATV.0000189158.28455.94. [DOI] [PubMed] [Google Scholar]
- Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- Williams DE, Okita RT, Buhler DR, Masters BS. Regiospecific hydroxylation of lauric acid at the (omega-1) position by hepatic and kidney microsomal cytochromes P-450 from rainbow trout. Arch Biochem Biophys. 1984;231:503–510. doi: 10.1016/0003-9861(84)90414-4. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]


