Abstract
Despite the growing body of evidence supporting prolactin (PRL) actions in human breast cancer, little is known regarding PRL regulation of its own receptor in these cells. Ligand-initiated endocytosis is a key process in the regulation of receptor availability and signaling cascades that may lead to oncogenic actions. Although exposure to exogenous PRL accelerates degradation of the long isoform of the PRL receptor (lPRLR), neither the signals initiated by PRL that lead to lPRLR internalization and subsequent down-regulation, nor the relationship to downstream pathways are understood in breast cancer cells. In this study, we showed that PRL-induced down-regulation of the lPRLR was reduced by inhibition of src family kinases (SFKs), but not Janus kinase 2, in MCF-7 cells. Inhibition of SFKs also resulted in accumulation of a PRL-induced PRLR fragment containing the extracellular domain, which appeared to be generated from newly synthesized PRLR. lPRLR was constitutively associated with SFKs in lipid rafts. PRL-induced SFK activation led to recruitment of the guanosine triphosphatase, dynamin-2, to an internalization complex, resulting in endocytosis. Inhibition of endocytosis by small interfering RNA-mediated knockdown of dynamin-2 blocked PRL-induced down-regulation of lPRLR, confirming that internalization is essential for this process. Endocytosis also was required for optimal phosphorylation of ERK1/2 and Akt, but not for Janus kinase 2 or signal transducer and activator of transcription 5, indicating that internalization selectively modulates signaling cascades. Together, these data indicate that SFKs are key mediators of ligand-initiated lPRLR internalization, down-regulation, and signal transduction in breast cancer cells, and underscore the importance of target cell context in receptor trafficking and signal transduction.
Src family kinases are key mediators of ligand-initiated prolactin receptor internalization, down-regulation, and signal transduction in MCF-7 breast cancer cells.
The hormone/cytokine prolactin (PRL) is essential for mammary gland development and differentiation, in addition to roles in metabolism, osteogenesis, and immunity (reviewed in Refs. 1 and 2). PRL also contributes to pathogenesis of breast cancer, as demonstrated in both epidemiological studies, which have strongly correlated circulating PRL levels with tumors expressing the estrogen receptor (3), and transgenic mouse models (4). PRL receptor (PRLR) expression has been shown to be higher in clinical tumors than normal adjacent tissue in several studies (5,6,7). Collectively, this evidence supports a strong association between PRL exposure and development of invasive breast cancer and underscores the importance of delineating the mechanisms by which PRLR expression is regulated at this target and the relationship between PRLR trafficking and PRL-initiated signals.
PRLRs are members of the cytokine receptor superfamily and are encoded by a single gene. The long isoform (lPRLR) is preferentially expressed in many breast cancer cell lines as well as primary tumors (8). Ligand binding activates a spectrum of signaling proteins including Janus kinase (Jak)/signal transducer and activator of transcription (Stat), src family kinases (SFKs), Ras-Raf-MAPK, and phosphatidylinositol-3-kinase (PI3K; reviewed in Refs. 9 and 10). The most extensively studied PRL-initiated signaling cascade is the Jak2/Stat5 pathway, which mediates most physiological actions of PRL in mammary development and lactation. In breast cancer cells, PRL potently activates SFKs, independently of Jak2 (11). Although less is known about this pathway, PRL-induced SFKs activate ERK1/2 and PI3K, which contribute to proliferation in MCF-7 and T47D breast cancer cells (12). The relative strength of PRL-induced signals to Jak2- and SFK-dependent pathways has been shown to vary with cell context (13,14). Several emerging lines of evidence suggest that these non-Jak2–Stat5 signals may promote breast cancer progression (13,15,16).
Expression of receptors at the cell surface is a major component of target cell sensitivity. PRL treatment induces down-regulation of the PRLR by sequential proteasome-dependent proteolysis and lysosomal degradation (10,17). PRL-induced proteasomal proteolysis of the lPRLR in MCF-7 cells generates a stable fragment of the PRLR containing the extracellular domain (ECD) (17), analogous to fragments generated from some other cytokine receptors (18,19). Internalization is a key step in the response to ligand, altering receptor trafficking and modulating signal transduction (20,21,22). Therefore, the PRL-activated signals that initiate this process and the reciprocal effect of endocytosis on signaling pathways are of considerable interest. Jak2 has been shown to be critical for the increased internalization and degradation of the lPRLR upon PRL exposure in transfected 293T and γ2A fibrosarcoma cells (23). Although Jak2 plays a similarly essential role for several cytokine receptors in some experimental systems (24,25,26), some studies demonstrate independence of Jak2, even for some of the same receptors (18,27). SFKs can activate endocytic machinery, leading to internalization of multiple receptor tyrosine kinases and G protein-coupled receptors (28,29,30). These kinases are particularly important for internalization via non-clathrin-mediated, lipid raft-dependent pathways (31,32). In tumor cells, c-Src localized within these domains has been shown to facilitate signals important in oncogenesis (33,34). Together, these data suggest that SFKs may contribute to PRL-initiated lPRLR trafficking in some target cells, such as breast cancer cells, with important consequences for PRL actions in this disease.
To study how PRL regulates its own receptor in breast cancer, we examined ligand-initiated trafficking in MCF-7 cells. The study of PRL actions in breast cancer cells has been complicated by the production of PRL within the mammary epithelial cells themselves. Here we employed a well-characterized MCF-7 breast cancer subline that expresses high levels of lPRLR, but is deficient in endogenous PRL production and responds robustly to exogenous hormone (35). This model system not only allows study of endogenous lPRLR, but also permits us to test our hypotheses in a clinically relevant breast cancer cell system. Here we demonstrate that contrary to previous reports in transfected cell models, SFKs and not Jak2 are critical for ligand-initiated internalization and down-regulation of lPRLR, and internalization by this pathway selectively modulates downstream signaling cascades. These observations point to SFKs as key mediators of ligand-induced lPRLR trafficking and responsiveness in breast cancer cells and underscore the importance of cell context for receptor trafficking.
Results
Inhibition of src family kinases but not Jak2 partially reduces PRLR down-regulation in MCF-7 cells
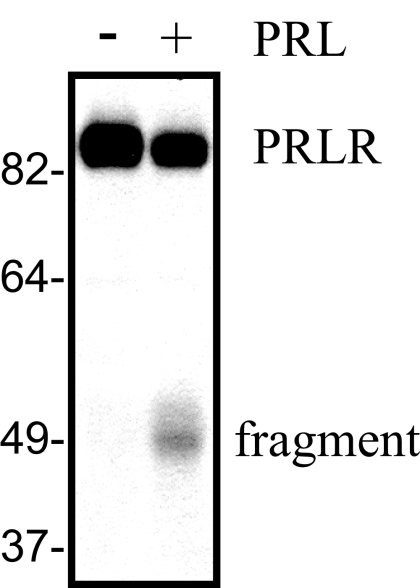
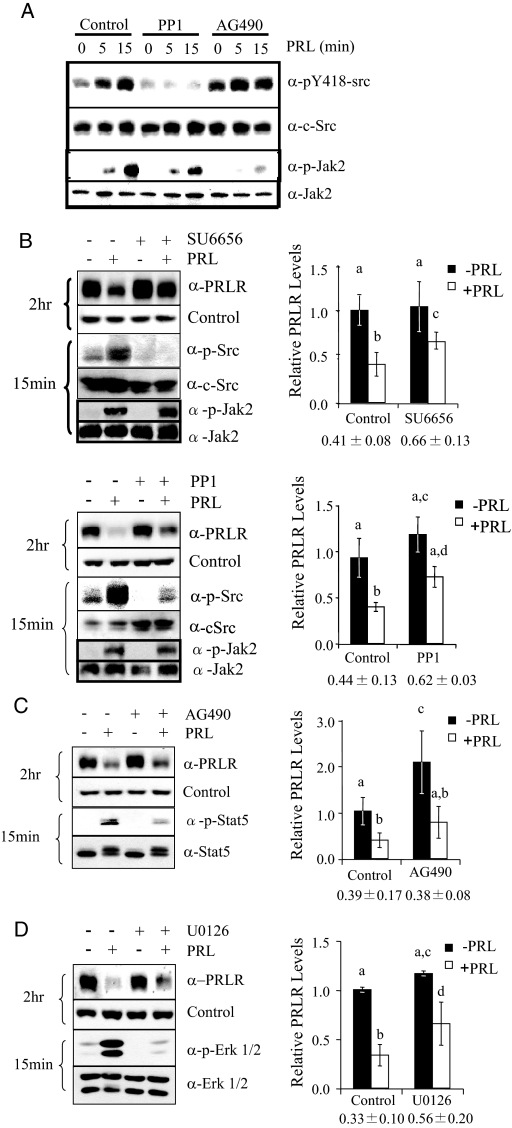
PRL-induced signaling cascades and subsequent cellular responses depend on PRLR expressed on the cell surface. PRL treatment causes rapid down-regulation of the lPRLR in MCF-7 cells, with the concomitant appearance of a fragment of about 50 kDa that is detected with an antibody to the PRLR ECD (Fig. 1), as described previously (17). However, the ligand-induced signals that initiate these events are not known. Jak2 and the SFKs are proximal kinases that are activated upon ligand engagement of the PRLR and can drive important cellular events, including proliferation (12,36). In breast cancer cells, including MCF-7 and T47D cells, PRL has been shown to activate these two pathways independently (11). To inhibit the activity of the multiple SFKs expressed in these cells [c-Src, Fyn, Yes, Blk (12)], we used chemical inhibitors for SFKs [PP1 and SU6656, as recommended (37)] and Jak2 (AG490) to confirm that these pathways were independent in our MCF-7 subline. As shown in Fig. 2, A–C, these compounds strongly reduced the expected signals, although AG490 slightly increased phosphorylation of SFKs, and both PP1 and SU6656 slightly, but not significantly, decreased pJak2, suggesting downstream cross talk to secondary modulators. We employed these inhibitors to investigate a role for Jak2 and SFKs in PRL-induced lPRLR down-regulation. As shown in Fig. 2B, both of the SFK inhibitors, SU6656 and PP1, significantly inhibited, but did not completely block, this event in MCF-7 cells. In contrast, inhibition of Jak2 with AG490 significantly increased the basal levels of PRLR, but had no effect on ligand-induced down-regulation (Fig. 2C). An alternative Jak2 inhibitor, 1,2,3,4,5,6-hexabromocyclohexane, yielded identical results (data not shown). This observed increase in unstimulated levels of PRLR after inhibition of Jak2 resembles reports that Jak2 plays an important role in the constitutive expression of other cytokine receptors, although the role of its kinase activity varies with the receptor and system (38). PRL-activated SFKs lead to the independent stimulation of ERK1/2 and PI3K, which are required for PRL-induced proliferation in MCF7 cells (12). Pretreatment of MCF-7 cells with the SFK inhibitor, SU6656, blocked both PRL-induced ERK1/2 and Akt phosphorylation, confirming their dependence on SFK kinase activity in this MCF-7 subline (supplemental Fig. 1, A and B, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). We therefore investigated the role of these downstream pathways in down-regulation of the PRLR. As shown in Fig. 2D, ligand-induced lPRLR down-regulation was significantly reduced in cells pretreated with the MAPK kinase (MEK)1/2 inhibitor, U0126, to a similar extent as observed with SFK inhibitors. In contrast, PRL-induced down-regulation was not affected by inhibition of the PI3K pathway (supplemental Fig. 1C). Together, these findings suggest an important role for SFKs, but not Jak2, in ligand-induced down-regulation of lPRLR in these cells.
Figure 1.
PRL induces lPRLR down-regulation and generation of a fragment containing the ECD, which are selectively altered by inhibition of different PRL-initiated signals. Serum-starved MCF-7 cells were treated with or without 4 nm PRL for 2 h. Cell lysates were subjected to Western analysis with an antibody recognizing the ECD of the PRLR.
Figure 2.
Inhibition of SFKs, but not Jak2, partially blocks PRLR down-regulation. A, Serum-starved MCF-7 cells were pretreated with vehicle, 10 μm PP1, or 25 μm AG490 as shown for 1 h and then treated with 4 nm PRL for 0, 5, or 15 min. Cellular lysates were subjected to immunoblotting as indicated. B–D, Serum-starved MCF-7 cells were pretreated with vehicle, 10 μm SU6656 or PP1 (B), 25 μm AG490 (C), or 10 μm U0126 (D) for 1 h, and then treated with or without 4 nm PRL for an additional 2 h at 37 C. Cellular proteins were subjected to Western analysis as indicated. lPRLR levels were quantitated from at least three independent experiments and normalized to vehicle-treated controls (mean ± sd). Treatments were compared using one-way ANOVA with Student’s-Newman-Keuls posttest. Different letters indicate significant differences among treatments (P ≤ 0.05). Numbers below charts indicate percent ligand-induced down-regulation compared with untreated samples for each inhibitor (mean ± sd).
SRKs mediate PRL-induced accelerated lPRLR turnover
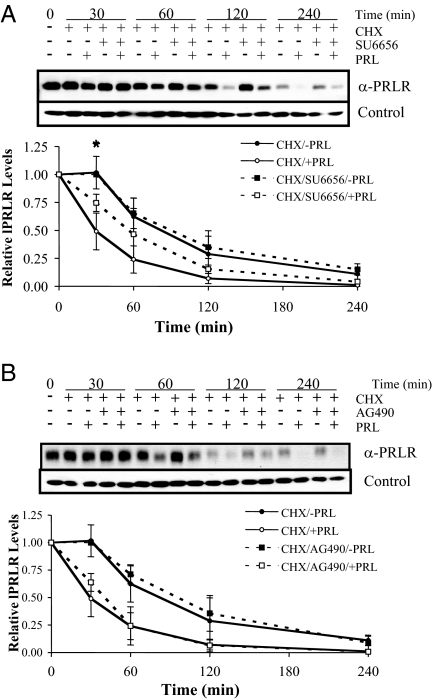
Levels of PRLR expression are determined by the balance between synthesis and degradation/proteolysis. We determined the effects of SFKs and Jak2 on lPRLR turnover using the protein synthesis inhibitor, cycloheximide, and inhibitors for these kinases. The lPRLR turns over rapidly even in the absence of PRL in many cell types (17,39,40). In agreement with our previous findings in MCF-7 cells, the lPRLR had a half-life of approximately 90 min in the absence of ligand, which fell to about 25min in the presence of PRL. Treatment with SFK inhibitors (Fig. 3A), but not those for Jak2 (Fig. 3B), retarded the early fall in PRLR. However, after 2 h exposure to cycloheximide, levels of remaining PRLR were not altered by SU6656. This experiment was also performed using alternative inhibitors for SFKs and Jak2, PP1 and 1,2,3,4,5,6-hexabromocyclohexane, respectively, and similar results were obtained (data not shown). In the absence of cycloheximide and ligand, levels of lPRLR remain constant over this time frame; treatment with PRL resulted in down-regulation over the time course previously described (Ref. 17 and data not shown). These results suggest that SFKs accelerate lPRLR degradation that is induced by ligand, but that they are not required for constitutive turnover.
Figure 3.
Inhibition of SFKs, but not Jak2, reduces PRL-induced PRLR degradation. Serum-starved MCF-7 cells were pretreated with 10 μm SU6656 (A) or 25 μm AG490 (B) for 1 h and then treated with 50 μg/ml cycloheximide (CHX) with or without 4 nm PRL for 0, 30, 60, 120, or 240 min. Cell lysates were analyzed by immunoblotting with anti-PRLR. lPRLR levels were quantitated from three independent experiments, and normalized to vehicle-treated controls at time zero (mean ± sd). The asterisk indicates a significant difference between the CHX/SU6656/+PRL and CHX/+PRL treatment groups by Student’s t test (P ≤ 0.05).
SFKs regulate the steady-state levels of PRL-induced PRLR fragment
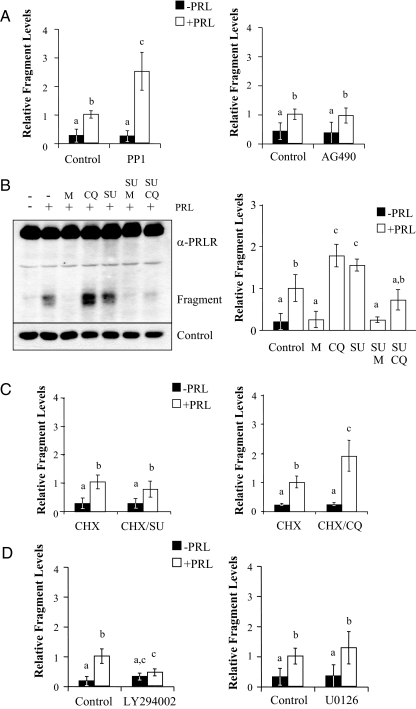
As shown in Fig. 1, treatment with PRL results in the appearance of a PRLR fragment, which temporally correlates with down-regulation of the PRLR. We have shown that this ECD fragment results from limited proteasomal cleavage of the receptor, which is then further degraded by lysosomal proteases, as revealed by proteasomal and lysosomal inhibitors, respectively (17). This PRLR fragment originates, at least in part, from surface PRLR, evidenced by biotin labeling, and in contrast to a ECD fragment of the GH receptor (GHR), is independent of metalloproteinase activity (17,41). Because our observations linked SFKs to both ligand-induced lPRLR down-regulation and internalization (see below), we hypothesized that inhibition of SFKs would also reduce steady-state levels of this fragment. However, when examined after 2 h of exposure to ligand, inhibition of SFKs with PP1 (Fig. 4A, left) or SU6656 (Fig. 4B) significantly increased PRL-induced steady-state levels of the PRLR fragment. In contrast, inhibition of Jak2 had no effect (Fig. 4A, right). Inhibition of SFKs also increased the steady-state levels of the PRL-induced PRLR fragment in T47D cells (data not shown). To test the role of proteasomes and lysosomes in this phenomenon, cells were pretreated with SFK inhibitors in combination with proteasomal and lysosomal inhibitors before stimulation with PRL. In agreement with our previous findings, pretreatment of cells with the proteasomal inhibitor, MG132, blocked the appearance of fragment, whereas inhibition of lysosomal activity with chloroquine increased the steady-state levels (Fig. 4B). Inhibition of SFKs and proteasomes in combination also blocked fragment accumulation in these cells, indicating that accumulation of the PRLR fragment induced by SFKs inhibitors also depended on proteasomal proteolysis. Interestingly, however, pretreatment of cells with SFK inhibitors in combination with the lysosomal inhibitors, chloroquine (Fig. 4B) or ammonium chloride (data not shown), blocked the increase in the PRLR fragment that had been observed with each inhibitor alone. To examine the possibility that the SFK inhibitor-induced rise in fragment level may originate from a pool of PRLR other than that in the plasma membrane, we pretreated cells with the SFK inhibitor, SU6656, or chloroquine, and then treated cells with cycloheximide in the presence or absence of PRL (Fig. 4C). Although PRL was still able to induce the appearance of the PRLR fragment, cycloheximide ablated the additional increase in steady-state levels in the presence of SFK inhibitors (compare Fig. 4, A and B with Fig. 4C, left), suggesting that the rise in PRL-induced fragment, in the presence of SFK inhibition, was generated from a less mature pool of PRLR. In contrast, chloroquine further increased the levels of PRL-induced fragment in the presence of cycloheximide, consistent with lysosomal degradation of the fragment generated from surface-associated PRLR (Fig. 4C, right). To investigate the role of signals downstream of SFKs that might modulate this event, we inhibited PI3K with LY294002, and MEK1/2 with U0126 (Fig. 4D). Inhibition of PI3K significantly decreased the steady-state levels of the PRLR fragment, suggesting that this pathway plays some role in events leading to lysosomal proteolysis, consistent with the importance of phospholipids in vesicular trafficking (42). In contrast, inhibition of MEK1/2 had no net effect on fragment levels.
Figure 4.
SFKs and PI3K modulate levels of the PRL-induced PRLR cleavage fragment. Serum-starved MCF-7 cells were pretreated with vehicle, 10 μm PP1, or 25 μm AG490 (panel A), 20 μm MG132 (M), 100 μm chloroquine (CQ), and/or 10 μm SU6656 (SU) (panel B), 20 μm LY294002 or 10 μm U0126 (panel D) for 1 h followed by treatment with or without 4 nm PRL for 2 h. C, Serum-starved MCF-7 cells were pretreated with vehicle, 10 μm SU6656 (SU, left), or 100 μm chloroquine (CQ, right) for 1 h, and then treated with 50 μg/ml cycloheximide (CHX) with or without 4 nm PRL for 2 h. Levels of the PRL-induced PRLR fragment were quantitated from at least three independent experiments and normalized to levels observed in dimethylsulfoxide/PRL-treated cells (mean ± sd). Treatment groups were compared using one-way ANOVA with Student’s-Newman-Keuls posttest. Different letters indicate significant differences among treatments (P ≤ 0.05).
The lPRLR and c-Src are located in lipid raft microdomains
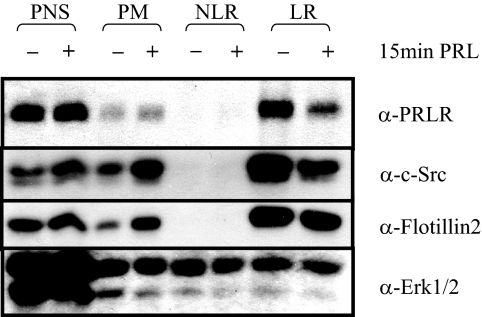
The ability of SFKs to regulate early events in the down-regulation process suggested that SFKs may play a role in lPRLR endocytosis. Recent studies addressing the role of signals in the control of internalization and trafficking have shown that SFKs activate some clathrin-independent internalization pathways (31,43). We confirmed this action of SFK in MCF-7 cells by examining the effect of various inhibitors on endocytosis of cholera toxin B (CTB) and transferrin, which internalize via clathrin-independent and -dependent pathways, respectively (31). As predicted, MCF-7 cells pretreated with inhibitors of SFKs, PP1 and SU6656, failed to internalize CTB, but continued to internalize transferrin, and inhibitors of Jak2, MEK1/2, and PI3K had no effect on endocytosis of either ligand (supplemental Fig. 2). We hypothesized that like CTB, lPRLR might be located within lipid raft microdomains in MCF-7 cells, resulting in internalization through a similar mechanism. We therefore isolated nonlipid raft and lipid raft components using the nondetergent method of membrane fractionation reported by Smart et al. (44). As shown in Fig. 5, lPRLR constitutively localized within the lipid raft fraction and was reduced by PRL treatment. Interestingly, SFKs were also selectively located in the lipid raft fraction, in contrast to ERK1/2, which was distributed throughout. Together, these data confirm that SFKs play an important role in endocytosis through lipid rafts and suggest that internalization of the lPRLR occurs primarily through this pathway in MCF-7 cells.
Figure 5.
PRLR constitutively localizes in lipid-rich fractions of the plasma membrane. Serum-starved MCF-7 cells were treated with or without 4 nm PRL for 15 min. Postnuclear supernatants (PNS), plasma membrane (PM), nonlipid-raft (NLR) and lipid raft (LR) fractions of cellular extracts were isolated as described in Materials and Methods. Total protein from the PNS fraction (30 μg) and 5 μg of total protein from the PM, NLR, and LR fractions were subjected to immunoblotting as indicated (Representative experiment). Flotillin2 was used as a marker for lipid rafts, because MCF-7 cells do not express caveolin-1 (68), and ERK1/2 was used as a positive control for protein loading.
SFKs facilitate internalization of lPRLR in COS-7 cells
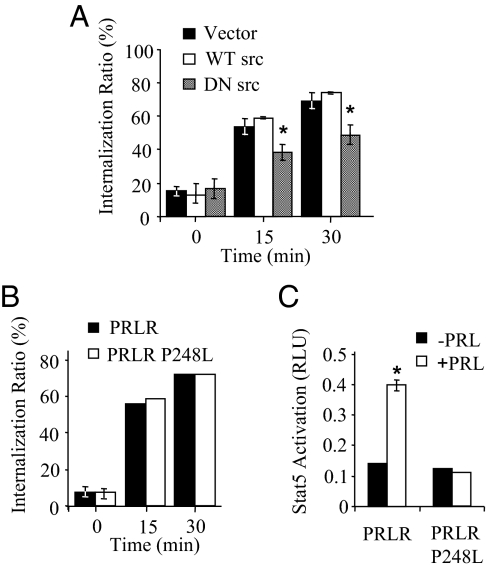
The colocalization of lPRLR and SFKs within lipid raft microdomains suggests that these kinases regulate proximal events in the PRLR down-regulation process, similar to trafficking of other receptor families, including receptor tyrosine kinases and G protein-coupled receptors (28,29,30). The relatively low specific binding of radiolabeled ligand to MCF-7 cells precluded use of this quantitative assay to examine internalization. However, the high transfection rate and resulting high expression levels of COS-7 cells are very amenable for this analysis. To assess the role of SFKs and Jak2 in ligand-induced internalization, COS-7 cells were cotransfected with wild-type PRLR and wild-type or dominant-negative Src constructs, or a PRLR construct harboring a mutation in the Box1, Jak2 binding motif (PRLRP248L). Cells expressing dominant-negative Src exhibited significantly reduced internalized ligand (Fig. 6A). In contrast, the rate of ligand internalization mediated by the mutated PRLR was not different from the wild-type PRLR (Fig. 6B). The inability of this mutated PRLR to activate the Stat5-responsive γ-interferon-activated sequence (GAS) luciferase reporter gene was confirmed (Fig. 6C). These results demonstrate that SFKs are involved in endocytosis of PRLR in COS-7 cells. In contrast, although the PRLR box1 motif is required for activation of Jak2-dependent transcription, it is not required for ligand- induced internalization.
Figure 6.
Ligand-induced lPRLR internalization is dependent on SFKs and not Jak2 activation in COS-7 cells. COS-7 cells were cotransfected with lPRLR and either vector, wild-type src (WT src), or dominant-negative c-Src (DN src) (A), or wild-type lPRLR or lPRLRP248L (B). Ligand internalization was assessed as described in Materials and Methods. Each point represents mean ± sd of triplicate measurements from at least three independent experiments. The asterisk denotes significant difference from the vector-transfected control at the same time point (P ≤ 0.05). C, COS-7 cells were cotransfected with either wild-type lPRLR or lPRLRP248L, a Stat5-responsive GAS-luciferase reporter, and a β-galactosidase construct to control for transfection efficiency. Cells were then treated with or without 4 nm PRL for 24 h, and relative light units (RLU) were determined as described in Materials and Methods. When not visible, se bars are smaller than the symbol.
PRL induces SFK/dynamin (Dnm)2 complex formation, which facilitates internalization of lPRLR in MCF-7 cells
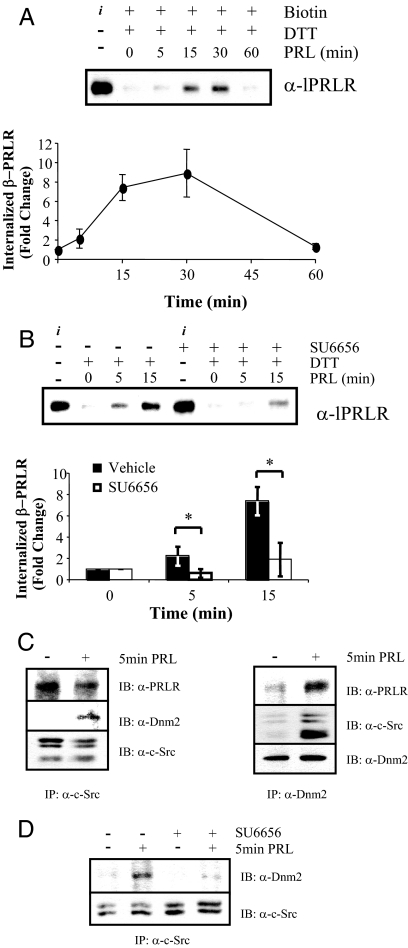
To examine internalization of endogenous lPRLR in MCF-7 cells, we employed a reversible biotinylation assay. Endocytosis of biotinylated lPRLR (β-PRLR) was initiated by treatment with PRL at 37 C, and internalized receptor was detected after cleavage of the remaining surface biotin with dithiothreitol (DTT). As shown in Fig. 7A, ligand-induced internalization was evident within 5 min, and internalized β-PRLR continued to increase up to 30 min. After 60 min, β-PRLR had fallen to baseline, indicating that the internalized β-PRLR had been degraded. To determine the role of SFK activity in this process, MCF-7 cells were biotinylated in the presence of SU6656, before treatment with PRL. As shown in Fig. 7B, inhibition of SFKs significantly reduced internalized β-PRLR, indicating that SFK activity also is critical for PRL-initiated PRLR internalization in MCF-7 cells.
Figure 7.
PRL-induced lPRLR internalization is dependent on SFK activity and Dnm2. A, Serum-starved MCF-7 cells were labeled with biotin as described in Materials and Methods, and then treated with or without 4 nm PRL at 37 C for the times indicated to allow internalization. Residual plasma membrane-associated biotin was cleaved with DTT. Total protein (1 mg) was incubated with immobilized neutravidin, and captured biotinylated proteins were immunoblotted with anti PRLR-ECD. B, Serum-starved MCF-7 cells were biotin labeled in the presence of vehicle or 10 μm SU6656 and then treated with or without 4 nm PRL at 37 C for the times indicated followed by DTT cleavage, capture, and immunoblotting as in panel A. The asterisk denotes significant differences between vehicle- and SU6656-treated groups at each time point (P ≤ 0.05) using Student’s t test. A and B, i represents the biotin-labeled PRLR, before removal of surface label with DTT. Internalized biotin-labeled PRLR (β-PRLR) was quantitated from three independent experiments, and normalized to time zero min (mean ± sd). C, Serum-starved MCF-7 cells were treated with or without 4 nm PRL for 5 min. Cellular extracts were immunoprecipitated (IP) with anti-Dnm2 (left panel) or anti-c-Src (right panel), followed by Western analysis (IB) using the antibodies indicated. Blots shown are representative of three independent experiments. D, Serum-starved MCF-7 cells were pretreated with vehicle or 10 μm SU6656 before treatment with or without 4 nm PRL for 5 min. Cellular lysates were immunoprecipitated and analyzed by immunoblotting as indicated.
SFK actions in endocytosis have been linked to the Dnm family of guanosine triphosphatases (45,46,47), and K44E-Dnm blocks PRL-induced PRLR internalization in transfected COS-7 cells (48). Dnm family members are recruited to the plasma membrane and can participate in endocytic vesicle scission in both clathrin-dependent and -independent endocytosis (32,43,49). As shown in Fig. 7C, PRLR was constitutively associated with SFKs, and PRL treatment recruited Dnm2 to this complex. This event was dependent on SFK activation; pretreatment with SU6656 greatly reduced the association of SFKs with Dnm2 (Fig. 7D).
PRL-induced PRLR endocytosis is required for PRLR down-regulation and for SFK-mediated phosphorylation of ERK1/2 and PI3K
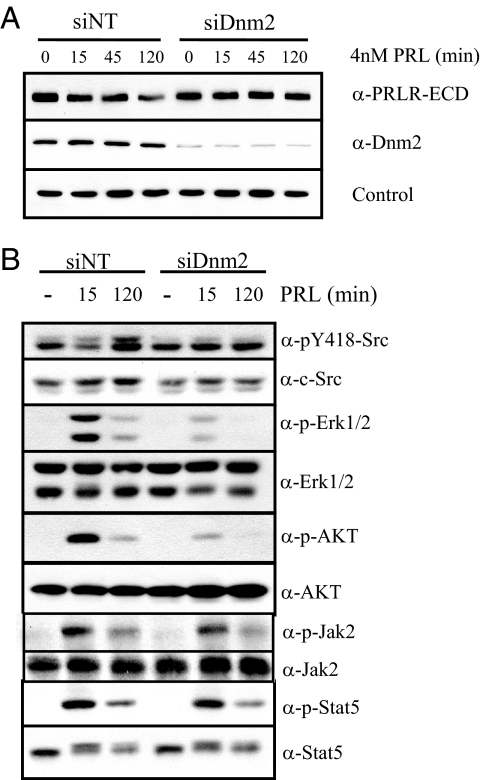
Cytokine receptors can undergo proteolysis independently of their internalization. The GHR, which is closely related to the PRLR, undergoes metalloproteolytic cleavage and ectodomain shedding at the plasma membrane (41). To confirm that internalization was required for ligand-induced PRLR down-regulation, we employed small interfering RNA (siRNA) to knock down Dnm2 [small interfering Dnm2 (siDnm2)]. Reduced Dnm2 expression blocked PRL-induced PRLR down-regulation (Fig. 8A), confirming that this event is subsequent to PRLR internalization, rather that ectodomain shedding.
Figure 8.
Dnm-2 knockdown blocks PRL-induced PRLR down-regulation and inhibits SFK signals to downstream ERK1/2 and Akt, but not Jak2 activation of Stat5. A and B, Serum-starved MCF-7 cells were transfected with either nontargeting (siNT)- or Dnm2 (siDnm2)-specific siRNA for 72 h, and then treated with or without 4 nm PRL for times indicated. Cellular proteins were subjected to Western analysis with antibodies as shown. Blots shown are representative of at least three independent experiments.
Receptor internalization can modulate downstream signaling events, which vary with receptor, host cell, and pathway. To determine whether PRL-induced PRLR endocytosis is required for activation of downstream signaling cascades, we blocked internalization with siDnm2 and assessed PRL-induced phosphorylation of signaling proteins by immunoblotting. As shown in Fig. 8B, PRL-induced activation of ERK1/2 and AKT was inhibited in cells transfected with siDnm2, compared with those transfected with nontargeting siRNA. In contrast, PRL-dependent phosphorylation of Jak2 and Stat5 were relatively unaffected by Dnm2 knockdown. These data reveal differences in the regulation of PRL-initiated signals by PRLR endocytic processes.
Discussion
The abundance of surface receptors is an important determinant of potential signal transduction and cellular responsiveness. Initiation of specific signaling cascades by ligand augments internalization and alters trafficking of membrane receptors, which are closely linked to biological effects. These processes may result in degradation of receptors by proteasomal and/or lysosomal proteases, thereby reducing levels of receptor and consequently the potential for new surface-initiated signaling events. We previously established that PRL increases the degradation of endogenous lPRLR in MCF-7 breast cancer cells by sequential action of proteasomes and lysosomes, with the associated transient appearance of a proteasome-dependent proteolytic cleavage product containing the ECD of the PRLR (17). These observations outline a mechanism by which breast cancer cells can be desensitized to PRL after ligand exposure. In the present study, we focused on identifying the proximal kinases that trigger and modulate these processes. We found that SFKs, but not Jak2, mediated ligand-induced internalization and degradation of PRLR. In these cells, PRLR was constitutively associated with SFKs in lipid rafts. PRL-induced SFK activation led to recruitment of Dnm2 to an internalization complex, resulting in endocytosis through a clathrin-independent pathway. Internalization was required for optimal activation of ERK1/2 and Akt but did not significantly modulate signals to Jak2 or Stat5. Together, these observations indicate that SFKs are key mediators of PRL-induced endocytosis and down-regulation of the lPRLR isoform, as well as modulators of downstream signaling pathways in breast cancer cells.
Lipid rafts/caveolae are membrane microdomains that have been implicated in protein scaffolding, endocytosis, and signal transduction (32,50,51). Caveolins are not required for these functions (52) or clathrin-independent internalization (53), and indeed caveolin expression is lost as cells undergo oncogenic changes (54). Using a nondetergent method of membrane fractionation, we found that the lPRLR was located within lipid raft microdomains in MCF-7 cells. SFKs were constitutively associated with lPRLR in these regions, resembling the phospho-tyrosine-independent association of the SFK, Lyn, with the βc-subunit of the IL-2, IL-3, and granulocyte macrophage colony stimulating factor receptors (55). Partitioning of receptors into lipid raft domains is dependent upon interactions with glycosylphosphatidylinositol-anchored proteins or interactions with raft resident proteins (20). A lipid raft targeting sequence has not been determined for the lPRLR; localization in these domains may result from its association with SFKs in some target cells. A recent report indicates that c-Src is preferentially localized in lipid rafts in MCF-7 cells, but not human embryonic kidney 293 cells (33), consistent with this possibility, and suggesting a basis for the difference between our findings in MCF-7 cells and findings in other cell models (see below). Recent data indicate that lipid raft-localized c-Src is a primary regulator of cell adhesion and cell cycle progression in MCF-7 cells (33), activities that are modulated by PRL (13,15,35). The relatively short half-life of the lPRLR that we previously reported in these cells (17) may result from its association with lipid rafts in this cell line. Internalization of TGF-α receptors through caveolae/lipid rafts accelerates receptor degradation, compared with endocytosis through clathrin-mediated processes (56). Together, these findings suggest that lipid rafts are attractive candidates for the temporal and spatial coordination of PRLR trafficking and signaling in MCF-7 cells. Additional analyses of PRLR-associated complexes in lipid rafts will aid in our understanding of the mechanisms that direct the lPRLR to this location and the role of these microdomains in PRL actions in tumorigenesis.
Consistent with the requirement for SFKs in clathrin-independent internalization in many cells (31,43), including MCF-7 cells as shown herein, SFK activity was required for lPRLR internalization in MCF-7 cells. Ligand treatment induced SFK-dependent recruitment of the guanosine triphosphatase, Dnm2, which was required for PRLR endocytosis and down-regulation. In other systems, SFK-induced phosphorylation of Dnm2 activates this process (45,46,47); a similar mechanism is likely to mediate PRLR internalization in MCF-7 cells. Despite the importance of SFKs in ligand-stimulated endocytosis, SFK inhibitors notably failed to completely block lPRLR down-regulation, perhaps reflecting the significant constitutive turnover of the lPRLR, similar to the reported effect on the platelet-derived growth factor receptor (28). Other signals are likely to modulate this process; our data indicate that ERK1/2 also augment lPRLR down-regulation without altering endocytosis. The target(s) of these kinases are under investigation.
Our previous report of the ligand-dependent appearance of a fragment containing the ECD of the PRLR, without a corresponding fragment containing the cytoplasmic domain, suggests that PRL induces degradation of the cytoplasmic domain of the lPRLR via proteasomal proteases (17). Steady-state levels of this PRLR fragment are increased upon inhibition of lysosomal activity, delineating a sequential pathway of lPRLR down-regulation. The ability of surface biotinylation to label this fragment indicates that at least a portion arises from plasma membrane-associated PRLR. Despite a size similar to a reported PRL-binding protein (57), the PRLR ECD fragment generated by these cells is not secreted (17), and its function is unknown. However, as shown in the current studies, it is a valuable marker for PRLR processing. In contrast to reduced degradation of the lPRLR after inhibition of SFKs, levels of the PRL-induced PRLR fragment in both MCF-7 and T47D breast cancer cells rose in the presence of these inhibitors, indicating that PRL-activated SFKs inhibit this proteolysis. Inhibition of lysosomal activity did not further increase levels of the PRLR fragment under these conditions, indicating a routing for this fragment different than that observed after PRL exposure in the absence of SFK inhibitors. Interestingly, inhibition of protein synthesis dramatically reduced this net accumulation of PRLR fragment, suggesting that it originates from a pool of newly synthesized PRLR and that PRL-activated SFKs modulate trafficking of newly synthesized receptor. Together, these data suggest that PRL-activated SFKs regulate PRLR proteolysis of two independent pools of PRLR. Further studies designed to dissect the role of these kinases in trafficking pathways are currently underway.
PRL-induced SFK activation leads to independent activation of ERK1/2 and PI3K, which contribute to proliferation in MCF-7 and T47D breast cancer cells (12). The dependence of signals downstream of SFKs upon PRL-initiated PRLR internalization is similar to reports for both the epidermal growth factor receptor (29) and RET protooncogene (58). In contrast, Dnm2 negatively regulated IL-5-dependent activation of ERK1/2 (59). In this latter report, IL-5 induced the association of Dnm2 with both Lyn and Jak2 tyrosine kinases. However, the respective roles of these kinases in endocytosis and the signals that lead to ERK1/2 activation were not examined. In some cell lines, activation of MAPKs may be independent of SFK activity. Indeed, in BaF3 cells the SFK inhibitors, PP1 and PP2, did not inhibit MAPK activation in response to IL-3 or PRL treatment, respectively (55,60). In cells lacking the SFKs, c-Src, Yes, and Fyn, IL-6 family cytokines can activate their entire repertoire of signaling pathways, including ERK1/2, and induce gene expression (61). These studies demonstrate the differences even among the cytokine receptor superfamily, and the importance of cell context in determining signaling networks and the relationship between endocytosis and downstream signaling events.
In contrast to the critical role for Jak family members in mediating ligand-induced down-regulation of multiple other cytokine receptors (18,24,25), including studies of the PRLR itself (23), Jak2 activity did not play a detectable role in ligand-induced internalization or down-regulation of the lPRLR in MCF-7 cells, nor was it required for internalization in COS-7 cells. However, inhibitors of Jak2 as well as SFKs reduced ligand-induced down-regulation of PRLR in another breast cancer cell line, T47D cells (data not shown). PRLR can be internalized via clathrin-coated pits (48) as well as lipid rafts, indicating that endocytic mechanisms vary with target cell (10). In some cells, expression of caveolins may stabilize PRLR complexes on the cell surface (32). Similar variability has been observed for related receptors, e.g. Jak2 does not play a role in GH-stimulated endocytosis of the GHR in all models (27,62,63). These differences in PRLR localization and mode of internalization have consequences for the spatial and temporal control of the signaling events that regulate internalization, desensitization, and available signaling cascades. Our studies underscore the variation in signaling pathways that are available to PRL, even among different breast cell lines (13,14). Interestingly, despite differences in the proximal mediators, reported studies of the PRLR describe a similar ultimate fate, lysosomal destruction (10,17). Further analyses of PRLR-associated signaling complexes, internalization pathways, and substrates of these kinases in different cell models will elucidate the basis of these differences.
SFKs are frequently highly activated or overexpressed in many cancers, including breast tumors, and their importance in tumor progression and metastasis is firmly established (reviewed in Refs. 64,65,66). Our current studies demonstrate that PRL can employ these kinases to regulate its own responsiveness in breast cancer cells, in addition to other reported effects on cell behavior. Understanding the mechanisms that control PRLR internalization, subsequent down-regulation and signal attenuation will aid in our understanding of how PRL contributes to human disease.
Materials and Methods
Reagents
Recombinant human PRL (lot AFP9042) was obtained through the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and Dr. Parlow. Protein A/G-Plus agarose was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies were purchased as follows: anti-PRLR-ECD (PRLR-ECD), anti-phospho-Stat5, and 34–4800 as a loading control from Zymed Laboratories, Inc. (South San Francisco, CA); anti-PRLR-cytoplasmic domain (PRLR-CYT), anti- c-Src, and anti-Stat5 from Santa Cruz Biotechnology, Inc.; anti-Jak2, anti-phospho-Jak2, and antiphosphotyrosine (4G10) from Upstate Biotechnology, Inc. (Lake Placid, NY); anti-AKT, anti-phospho-AKT (ser473), anti-ERK1/2, and anti-phospho-ERK1/2 from Cell Signaling Technologies, Inc. (Danvers, MA); anti-pY418-src from Biosource International, Inc. (Camarillo, CA); and anti-Dnm2 from Calbiochem (San Diego, CA). The kit for biotinylation was purchased from Pierce Chemical Co. (Rockford, IL), and Optiprep was purchased from Axis-Shield (Oslo, Norway). All inhibitors and culture media were purchased from Sigma (St. Louis, MO) with these exceptions: SU6656 and the JAK2 inhibitor, 1,2,3,4,5,6-hexabromocyclohexane inhibitor from Calbiochem; PP1 from Biomol International, LP (Plymouth Meeting, PA); AG490 from EMD International, Inc. (San Diego, CA); and U0126 from Promega Corp. (Madison, WI).
MCF-7 and COS-7 cell culture
PRL-deficient cells derived from the human mammary adenocarcinoma cell line, MCF-7, were grown in RPMI 1640 containing 10% horse serum and 50 μm ganciclovir, 100 U/ml penicillin G, and 100 μg/ml streptomycin as reported previously (35). Cells (106 cells/60-mm plate or 5 × 106cells/100-mm plate) were incubated in serum-free medium for 24–48 h before treatments. COS-7 cells were maintained in DMEM/Ham’s F12 (DMEM/F12) containing 5% heat-inactivated fetal bovine serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin.
RNA interference
siRNA duplexes targeting human Dnm2 were custom synthesized by Dharmacon (Lafayette, CO). The target sequence used was AGCGAAUCGUCACCACUUA, corresponding to nucleotides 1534–1554 of the Dnm2 mRNA. MCF-7-derived cells were transfected with either 10 nm Dnm2 siRNA or nontargeting siRNA using Lipofectamine 2000 from Invitrogen (Carlsbad, CA), according to the manufacturer’s protocol. Twenty four hours after transfection, cells were serum starved for an additional 48 h (72 h total) before experiments.
Radioligand internalization assay
COS-7 cells were transiently transfected using SuperFect (QIAGEN Inc., Santa Clara, CA) as described previously (48). Cells were transfected with dominant-negative and wild-type src constructs and wild-type PRLR, or PRLRP248L, and studies were initiated 24 h later. Internalized radiolabeled ligand was determined as described previously (48). Briefly, cells were incubated with radiolabeled ligand in the presence or absence of excess unlabeled ligand at 4 C, and then washed and moved to 37 C for 0, 15, or 30 min. Cells were then placed on ice and cell surface-bound ligand was harvested by acid treatment (50 mm glycine and 100 nm NaCl, pH 2.5) and radioactivity was determined. Internalized ligand was harvested by solubilization in 0.2 n NaOH/1% sodium dodecyl sulfate, and radioactivity was determined. Specific binding in each fraction was determined as the difference between 125I-labeled ligand detected in the presence and absence of a 100-fold excess of unlabeled ligand. The internalization ratio was expressed as a percentage of the specific internalized fraction with respect to total specific binding at each time point. In parallel, the ability of PRL to activate a Stat5-responsive GAS element was determined as described previously (67).
Biotin internalization assay
Cell surface proteins were biotinylated using a cell surface protein labeling kit (Pierce) with some adaptations. MCF-7 cells were plated at a density of 5 × 106 cells/100-mm dish and serum starved overnight. Cells were washed twice in ice-cold PBS and incubated at 4 C in 200 μg/ml sulfosuccinimidyl 2-(biotinamido)-ethyl-l,3 dithiopropionate in the presence or absence of inhibitors for 2 h. After removal of unbound biotin with quenching solution and two washes with ice-cold PBS, cells were stimulated with 4 nm PRL at 37 C as indicated. Residual surface-bound biotin was cleaved using two 30-min washes of 25 mm DTT on ice. Lysates were cleared by centrifugation, and 1 mg of total protein was incubated with 100 μl of neutravidin-sepharose [50% (vol/vol) in lysis buffer] overnight at 4 C. Pellets were washed three times in PBS, resuspended in sample buffer, boiled for 5 min, and analyzed by immunoblotting.
Membrane fractionation
Membrane preparations were isolated based upon buoyant density using the method of Smart et al. (44). Briefly, MCF-7 cells were homogenized in buffer A (containing 0.25 m sucrose; 1 mm EDTA; and 20 mm tricine, pH 7.8), and cellular debris was cleared by centrifugation. Postnuclear supernatants were loaded onto 30% (vol/vol) Percoll in buffer A and centrifuged for 35 min at 84,000 × g at 4 C to collect the plasma membrane fraction. The plasma membrane fraction was sonicated for 1 min and mixed with 50% (wt/vol) Optiprep in buffer B (containing 0.25 m sucrose; 6 mm EDTA; and 120 mm tricine, pH 7.8) to yield a 23% slurry. This slurry was loaded to the bottom of an ultracentrifuge tube and overlaid with 6 ml of a 10–20% (vol/vol) continuous Optiprep gradient and centrifuged for 90 min at 52,000 × g at 4 C. The resulting nonfloating fraction (bottom 3.5ml) was collected as the nonlipid raft fraction of the plasma membrane. The floating fraction was collected and adjusted to 23% (vol/vol) Optiprep in buffer B. This fraction was then transferred to the bottom of an ultracentrifuge tube and overlaid with 5% Optiprep in buffer B, and centrifuged for 90 min at 52,000 × g at 4 C. The lipid raft fraction was collected at the interface of the discontinuous gradient. Protein concentrations were determined by the bicinchoninic acid assay, and fractions were immunoblotted as indicated.
Immunoprecipitation
Protein-protein association was investigated using coimmunoprecipitation as described previously (17). Briefly, cells were harvested with immunoprecipitation assay buffer (10 mm Tris, pH 8; 150 mm NaCl; 1 mm EDTA; 1% Triton X-100; 0.5% sodium deoxycholate; 0.05% sodium dodecyl sulfate; containing 1 mm Na3VO4, 1 mm phenylmethylsulfonylfluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin). The cell extract was centrifuged at 14,000 rpm for 10 min at 4 C, and 0.5–1 mg of the supernatant was precleared by incubating with 20 μl Protein A/G-plus agarose at 4 C for 1 h. Cell lysates were centrifuged at 2500 rpm for 5 min at 4 C. The supernatant was collected and incubated with primary antibody (1 μg/mg) at 4 C for 1 h, and then 30 μl of Protein A/G-plus-agarose was added and incubated overnight. The pellet was collected by centrifugation at 2500 rpm for 5 min at 4 C, washed three times with ice-cold PBS, and then resuspended in 30 μl 2× sample buffer before immunoblotting as described below.
Western analysis
Protein from cellular lysate (30 μg) or immunoprecipitated protein was immunoblotted as described previously (48). For some experiments, signals were quantified by densitometry (ImageQuant software, version 4.2a, Amersham Biosciences, Piscataway, NJ).
Supplementary Material
Acknowledgments
We thank Norma I. Rodriguez-Malave and Debra Rugowski for experimental assistance, and Dr. Serge Fuchs for insightful discussions.
Footnotes
This work was supported by National Institutes of Health R01 Grants CA78312 and DK62783 (to L.A.S.), T32 GM08349 (to K.C.C.), and T35 ES07295 (to N.I.R-M.).
Current address for J.-C.L.: Institute of Molecular Biology, Academia Sinica, No.128, Academia Road, Section 2, Nankang, Taipei 115, Taiwan.
The authors have nothing to disclose.
First Published Online December 4, 2008
Abbreviations: CTB, Cholera toxin B; Dnm, dynamin; DTT, dithiothreitol; ECD, extracellular domain; GAS, γ-interferon-activated sequence; GHR, GH receptor; β-PRLR, biotinylated lPRLR; lPRLR, long isoform of the PRLR; MEK, MAPK kinase; PI3K, phosphatidylinositol-3-kinase; PRL, prolactin; PRLR, PRL receptor; Jak, Janus kinase; SFK, src family kinase; siDnm2, small interfering DnM2; siRNA, small interfering RNA.
References
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska S, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Hankinson SE 2006 Prolactin and breast cancer risk. Cancer Lett 243:160–169 [DOI] [PubMed] [Google Scholar]
- Arendt LM, Schuler LA 2008 Transgenic models to study actions of prolactin in mammary neoplasia. J Mammary Gland Biol Neopl 13:29–40 [DOI] [PubMed] [Google Scholar]
- Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV 1997 Expression of prolactin and its receptor in human breast carcinoma. Endocrinology 138:5555–5560 [DOI] [PubMed] [Google Scholar]
- Mertani HC, Garcia-Caballero T, Lambert A, Gérard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G 1998 Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer 79:202–211 [DOI] [PubMed] [Google Scholar]
- Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA 1998 Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab 83:667–674 [DOI] [PubMed] [Google Scholar]
- Meng JP, Tsai-Morris CH, Dufau ML 2004 Human prolactin receptor variants in breast cancer: low ratio of short forms to the long-form human prolactin receptor associated with mammary carcinoma. Cancer Res 64:5677–5682 [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA 2003 Role of prolactin in mammary carcinoma. Endocr Rev 24:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Varghese B, Fuchs SY 2008 Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia 13:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno Vara JA, Carretero MV, Geronimo H, Ballmer-Hofer K, Martin-Perez J 2000 Stimulation of c-Src by prolactin is independent of Jak2. Biochem J 345:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Lazaro-Trueba I, Martin-Perez J 2003 Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol 17:2268–2282 [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Rugowski DE, Nikolai SE, Schuler LA 2007 Stat5 activation inhibits prolactin-induced AP-1 activity: distinct prolactin initiated signals in tumorigenesis dependent on cell context. Oncogene 26:6341–6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay E, Norman M, Kilic E, Goffin V, Zachary I 1997 Prolactin stimulates the JAK2 and focal adhesion kinase pathways in human breast carcinoma T47-D cells. Biochem J 324:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H 2005 Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 24:746–760 [DOI] [PubMed] [Google Scholar]
- Arendt LM, Rose-Hellekant TA, Sandgren EP, Schuler LA 2006 Prolactin potentiates TGFα induction of mammary neoplasia in transgenic mice. Am J Pathol 168:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J-C, Piazza TM, Schuler LA 2005 Proteasomes mediate prolactin-induced receptor downregulation and fragment generation in breast cancer cells. J Biol Chem 280:33909–33916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P 2005 Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood 105:600–608 [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston DP 2001 Proteasomal regulation of βc signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization. J Clin Invest 108:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL 2005 Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 6:112–126 [DOI] [PubMed] [Google Scholar]
- Chini B, Parenti M 2004 G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol 32:325–338 [DOI] [PubMed] [Google Scholar]
- Cohen AW, Combs TP, Scherer PE, Lisanti MP 2003 Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab 285:E1151–E1160 [DOI] [PubMed] [Google Scholar]
- Swaminathan G, Varghese B, Thangavel C, Carbone CJ, Plotnikov A, Kumar KG, Jablonski EM, Clevenger CV, Goffin V, Deng L, Frank SJ, Fuchs SY 2008 Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol 196:R1–R7 [DOI] [PubMed] [Google Scholar]
- Deng L, He K, Wang XD, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ 2007 Determinants of growth hormone receptor down-regulation. Mol Endocrinol 21:1537–1551 [DOI] [PubMed] [Google Scholar]
- Marijanovic Z, Ragimbeau J, Kumar KGS, Fuchs SY, Pellegrini S 2006 TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J 397:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L, Deau B, Forejtnikova H, Dumenil D, Margottin-Goguet F, Lacombe C, Mayeux P, Verdier F 2007 β-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood 109:5215–5222 [DOI] [PubMed] [Google Scholar]
- Dos Santos GMA, Ten Broeke T, Strous GJ 2001 Growth hormone receptor ubiquitination, endocytosis, and degradation are independent of signal transduction via Janus kinase 2. J Biol Chem 276:32635–32641 [DOI] [PubMed] [Google Scholar]
- Avrov K, Kazlauskas A 2003 The role of c-Src in platelet-derived growth factor a receptor internalization. Exp Cell Res 291:426–434 [DOI] [PubMed] [Google Scholar]
- Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T 2006 Epidermal growth factor receptor exposed to oxidative stress undergoes src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem 281:14486–14493 [DOI] [PubMed] [Google Scholar]
- Huang JY, Sun YT, Huang XY 2004 Distinct roles for Src tyrosine kinase in b2-adrenergic receptor signaling to MAPK and in receptor internalization. J Biol Chem 279:21637–21642 [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M 2005 Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436:78–86 [DOI] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR 2007 Regulation of raft-dependent endocytosis. J Cell Mol Med 11:644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitosugi T, Sato M, Sasaki K, Umezawa Y 2007 Lipid raft specific knockdown of SRC family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells. Cancer Res 67:8139–8148 [DOI] [PubMed] [Google Scholar]
- Arcaro A, Aubert M, del Hierro MEE, Khanzada UK, Angelidou S, Tetley TD, Bittermann AG, Frame MC, Seckl MJ 2007 Critical role for lipid raft-associated Src kinases in activation of P13K-Akt signalling. Cell Signal 19:1081–1092 [DOI] [PubMed] [Google Scholar]
- Schroeder MD, Symowicz J, Schuler LA 2002 Prolactin modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol 16:45–57 [DOI] [PubMed] [Google Scholar]
- Xie J, LeBaron MJ, Nevalainen MT, Rui H 2002 Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J Biol Chem 277:14020–14030 [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P 2003 The specificities of protein kinase inhibitors: an update. Biochem J 371:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan C, Kreis S, Margue C, Behrmann I 2006 Jaks and cytokine receptors—an intimate relationship. Biochem Pharmacol 72:1538–1546 [DOI] [PubMed] [Google Scholar]
- Genty N, Paly J, Edery M, Kelly PA, Djiane J, Salesse R 1994 Endocytosis and degradation of prolactin and its receptor in Chinese hamster ovary cells stably transfected with prolactin receptor cDNA. Mol Cell Endocrinol 99:221–228 [DOI] [PubMed] [Google Scholar]
- Djiane J, Delouis C, Kelly PA 982 Prolactin receptor turnover in explants of pseudopregnant rabbit mammary gland. Mol Cell Endocrinol 25:163–170 [DOI] [PubMed] [Google Scholar]
- Wang XD, He K, Gerhart M, Huang Y, Jiang J, Paxton RJ, Yang SS, Lu CS, Menon RK, Black RA, Baumann G, Frank SJ 2002 Metalloprotease-mediated GH receptor proteolysis and GHBP shedding: determination of extracellular domain stem region cleavage site. J Biol Chem 277:50510–50519 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P 2006 Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657 [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE 2007 Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG 1995 A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA 92:10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Bertics PJ 2002 The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in response to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology 143:1726–1731 [DOI] [PubMed] [Google Scholar]
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y 1999 Src-mediated tyrosine phosphorylation of dynamin is required for b2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274:1185–1188 [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD 2004 Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem 279:20392–20400 [DOI] [PubMed] [Google Scholar]
- Lu J-C, Scott P, Strous GJ, Schuler LA 2002 Multiple internalization motifs differentially used by prolactin receptor isoforms mediate similar endocytic pathways. Mol Endocrinol 16:2515–2527 [DOI] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L 2007 Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol 19:417–425 [DOI] [PubMed] [Google Scholar]
- Golub T, Wacha S, Caroni P 2004 Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol 14:542–550 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR 2004 Membrane domains. Annu Rev Cell Dev Biol 20:839–866 [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH 1998 Filipin-dependent inhibition of cholera toxin: Evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 141:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J 2001 Endocytosis without clathrin coats. Trends Cell Biol 11:406–412 [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP 2005 Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 288:C494–C506 [DOI] [PubMed] [Google Scholar]
- Dahl ME, Arai K, Watanabe S 2000 Association of Lyn tyrosine kinase to the GM-CSF and IL-3 receptor common βc subunit and role of Src tyrosine kinases in DNA synthesis and anti-apoptosis. Genes Cells 5:143–153 [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL 2003 Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5:410–421 [DOI] [PubMed] [Google Scholar]
- Kline JB, Clevenger CV 2001 Identification and characterization of the prolactin-binding protein in human serum and milk. J Biol Chem 276:24760–24766 [DOI] [PubMed] [Google Scholar]
- Richardson DS, Lai AZ, Mulligan LM 2006 RET ligand-induced internalization and its consequences for downstream signaling. Oncogene 25:3206–3211 [DOI] [PubMed] [Google Scholar]
- Gorska MM, Cen O, Liang Q, Stafford SJ, Alam R 2006 Differential regulation of interleukin 5-stimulated signaling pathways by dynamin. J Biol Chem 281:14429–14439 [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Caceres MA, Silva A, Martin-Perez J 2001 Src family kinases are required for prolactin induction of cell proliferation. Mol Biol Cell 12:2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo GS, Nathanson NM 2003 Src family kinase-independent signal transduction and gene induction by leukemia inhibitory factor. J Biol Chem 278:27750–27757 [DOI] [PubMed] [Google Scholar]
- Allevato G, Billestrup N, Goujon L, Galsgaard ED, Norstedt G, Postel-Vinay M-C, Kelly PA, Nielsen JH 1995 Identification of phenylalanine 346 in the rat growth hormone receptor as being critical for ligand-mediated internalization and down-regulation. J Biol Chem 270:17210–17214 [DOI] [PubMed] [Google Scholar]
- Maamra M, Finidori J, Von Laue S, Simon S, Justice S, Webster J, Dower S, Ross R 1999 Studies with a growth hormone antagonist and dual-fluorescent confocal microscopy demonstrate that the full-length human growth hormone receptor, but not the truncated isoform, is very rapidly internalized independent of Jak2-Stat5 signaling. J Biol Chem 274:14791–14798 [DOI] [PubMed] [Google Scholar]
- Johnson FM, Gallick GE 2007 SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med Chem 7:651–659 [DOI] [PubMed] [Google Scholar]
- Finn RS 2008 Targeting Src in breast cancer. Ann Oncol 19:1379–1386 [DOI] [PubMed] [Google Scholar]
- Hiscox S, Nicholson RI 2008 Src inhibitors in breast cancer therapy. Expert Opin Ther Targets 12:757–767 [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schroeder MD, Schuler LA 2002 Prolactin activates the cyclin D1 promoter via the JAK2-STAT pathway. Mol Endocrinol 16:774–784 [DOI] [PubMed] [Google Scholar]
- Lavie Y, Fiucci G, Liscovitch M 1998 Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem 273:32380–32383 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.