Abstract
We have previously demonstrated the tumor suppressor characteristics of protein tyrosine phosphatase receptor-type O (PTPRO) in leukemia and lung cancer, including its suppression by promoter methylation. Here, we show tumor-specific methylation of the PTPRO CpG island in primary human breast cancer. PTPRO expression was significantly reduced in established breast cancer cell lines MCF-7 and MDA-MB-231 due to promoter methylation compared with its expression in normal human mammary epithelial cells (48R and 184). Further, the silenced gene could be demethylated and reactivated in MCF-7 and MDA-MB-231 cells upon treatment with 5-Azacytidine, a DNA hypomethylating agent. Because PTPRO promoter harbors estrogen-responsive elements and 17β-estradiol (E2) plays a role in breast carcinogenesis, we examined the effect of E2 and its antagonist tamoxifen on PTPRO expression in human mammary epithelial cells and PTPRO-expressing breast cancer cell line Hs578t. Treatment with E2 significantly curtailed PTPRO expression in 48R and Hs578t cells, which was facilitated by ectopic expression of estrogen receptor (ER)β but not ERα. On the contrary, treatment with tamoxifen increased PTPRO expression. Further, knockdown of ERβ by small interfering RNA abolished these effects of E2 and tamoxifen. Chromatin immunoprecipitation assay showed association of c-Fos and c-Jun with PTPRO promoter in untreated cells, which was augmented by tamoxifen-mediated recruitment of ERβ to the promoter. Estradiol treatment resulted in dissociation of c-Fos and c-Jun from the promoter. Ectopic expression of PTPRO in the nonexpressing MCF-7 cells sensitized them to growth-suppressive effects of tamoxifen. These data suggest that estrogen-mediated suppression of PTPRO is probably one of the early events in estrogen-induced tumorigenesis and that expression of PTPRO could facilitate endocrine therapy of breast cancer.
This study demonstrates methylation-mediated suppression of protein tyrosine phosphatase PTPRO gene in primary breast cancer and provides a novel mechanism for its regulation by estradiol.
Breast cancer is a heterogeneous disease with a variety of pathological entities and varied clinical behavior. Breast cancer progression is a multistep process encompassing progressive changes in genetic aberrations in normal tissue resulting in hyperplasia with or without atypia, in situ carcinomas, invasive carcinomas, and finally metastatic carcinoma (1). Molecular subtyping of the breast cancers has allowed us to better understand the clinical behavior of these tumors and the targets for better therapy (2,3). A large body of evidence confirms the role of prolonged exposure to endogenous or exogenous estrogen in the pathogenesis of breast cancer. Estrogen acts as an accelerator for growth, and this effect is primarily mediated through estrogen receptors. Estrogen receptor (ER) acts as a ligand-dependent transcription factor, and its activation results in increased tyrosine phosphorylation, cAMP response element binding protein phosphorylation, activation of ERK/MAPK cascade, phosphatidylinositol 3-kinase signaling, G protein-coupled signaling, all of which mediate cell growth, migration, and angiogenesis (4). Although the two known estrogen receptors ERα and ERβ are found in normal breast epithelial tissue (5,6), recent studies in humans indicate that ERβ expression is decreased in neoplastic breast tissue, suggesting that ERβ could be an inhibitor of tumorigenesis (7,8,9). Both ER subtypes have diverged during early evolution and differ in the N-terminal A/B domain and, to a lesser extent, in the ligand-binding domain (10,11). Although both receptors bind 17β-estradiol (E2) and activate transcription through ERE (estrogen response element), they signal in opposite ways through activator protein 1 (AP-1) sites. Thus, ERβ inhibits transcription when bound to a ligand through this site. Conversely the antiestrogen-ERβ complex works as an agonist when bound to AP-1 complex (12). Evidence suggesting the involvement of ERβ in the terminal differentiation of mammary gland epithelium in mice poses an important question as to whether ERβ plays a role in the development of breast cancer or in the response of breast tissue to endocrine therapy (13).
In recent years, there has been considerable interest in understanding the role of tyrosine phosphorylation and endocrine resistance in breast cancer (for review see Ref. 14). Tyrosine phosphorylation plays a key role in cellular processes such as cell proliferation, differentiation, metabolism, cell-to-cell communication, gene transcription, and survival (15). This rapidly reversible process is determined by a balance between the activities of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). Whereas PTKs transmit signals by a cascade of phosphorylation events, PTPs that can dephosphorylate the kinases can modulate the intensity and effectiveness of phosphorylation-mediated signaling. Significant preclinical and clinical evidence show that overexpression of epidermal growth factor receptor, a PTK, in breast cancer results in reduced survival and endocrine resistance (16,17). It is, therefore, logical to postulate that the loss of a counteracting signaling pathway involving specific PTPs could contribute to this phenomenon. Computational analysis of the human genome identified 38 classical PTP genes, 19 of which mapped to regions frequently deleted in human cancers, and 30 of these protein phosphatases have been implicated in tumorigenesis (18). Further, genetic alterations of several PTPs such as PTPRF, PTPN14, PTPRG, PTPN13, PTPN11, PTPRT, and PTPN3 in different types of cancer also strengthen their potential role as growth/tumor suppressors (19).
Our laboratory has previously demonstrated that the gene encoding membrane-bound protein tyrosine phosphatase receptor-type O (PTPRO) is methylated and suppressed in rat hepatocellular carcinomas, primary lung cancers, and in chronic lymphocytic leukemia (20,21,22). Moreover, ectopic expression of PTPRO in human lung cancer cell line A549 resulted in inhibition of anchorage-independent growth, blocked cell cycle, and increased susceptibility to apoptosis (27). We have also observed that overexpression of the truncated isoform of PTPRO (PTPROt) reduces tumor forming potential of leukemia cells upon injection into immunocompromised mice (50). These data and the location of PTPRO gene in chromosomal region 12p12.3 that is characterized by loss of heterozygosity in different types of cancers support the contention that PTPRO is a tumor suppressor. Here, we show that PTPRO is methylated in primary breast cancers relative to their normal counterpart and is densely methylated and suppressed in breast cancer cell lines. Further, we have shown that estrogen treatment suppresses PTPRO expression in primary human mammary epithelial cells whereas tamoxifen augments its expression. Finally, we have made an effort to elucidate the mechanism of hormonal regulation of this protein phosphatase gene and its probable role in enhancing tamoxifen sensitivity.
Results
PTPRO promoter is methylated in primary human breast tumor samples but not in the normal breast tissue
A 582-bp CpG island (from −168 to +415bp) that spans the PTPRO promoter and exon 1 was methylated, in a tumor- specific manner, in primary human lung cancers and in chronic lymphocytic leukemia (21,22). Based on these data and growth-suppressive property of this phosphatase (21,22) and other PTPs in general (23), we investigated the methylation status of PTPRO CpG island in primary breast tumors. Genomic DNA isolated from tumor tissue and surrounding normal tissue (n = 21) was subjected to COBRA (Combined Bisulfite Restriction Analysis). Figure 1A depicts COBRA of a representative patient sample where PTPRO CpG island amplified from bisulfite (BS) converted tumor DNA, DNA from adjacent normal tissue and that from a reduction mammoplasty sample were subjected to digestion with TaqI. DNA from tumor tissue was susceptible to TaqI digestion, indicating methylation at this site, whereas the PTPRO CpG island amplified from the normal tissues was completely resistant to TaqI digestion (Fig. 1A, lanes 3, 6, and 9). Complete digestion of the PCR products with Tsp509 I (Fig. 1A, lanes 2, 5, and 8) demonstrated efficient BS conversion of the genomic DNA. The TaqI site was methylated in 17 of 21 tumor tissues whereas their normal counterparts had no detectable methylation.
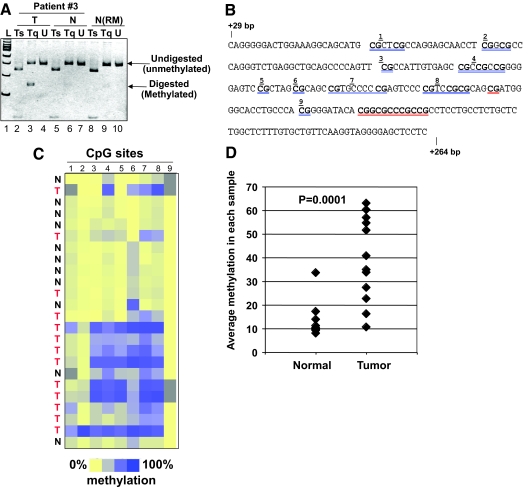
Figure 1.
PTPRO CpG island is methylated in primary breast tumors. A, Genomic DNA isolated from primary breast tumor samples and matching adjacent normal tissue (n = 21) was subjected to COBRA. PCR-amplified PTPRO promoter fragment from a representative patient [no. 3; Tumor (T); and Normal (N)] and that from a normal individual (reduction mammoplasty, N-RM) were digested with Tsp509 I (Ts) or TaqI (Tq) or mock digested (U). The digested products were separated by polyacrylamide gel electrophoresis along side 100-bp ladder (L). B, MassARRAY, Sequence of PTPRO CpG island (+29 to +264) amplified for MassARRAY. Each individual or group of CpGs underlined in blue is represented by the corresponding number in the dendrogram in panel C. Data for the CpGs underlined in red could not be obtained due to low-/high-fragment mass. C, PTPRO CpG island amplified from BS-treated DNA of primary breast cancer samples (T) and normal adjacent tissue (N) were subjected to MassARRAY (EpiTYPER). Each square represents a single CpG or a group of two or three CpGs (see panel B) and each row represents a sample. Methylation frequency ranges from 0% (yellow) to 100% (dark blue). Gray indicates unavailable data. Samples include tumor (T) and normal (N) from breast cancer patients. D, Quantification of the EpiTYPER data as percent methylation of the PTPRO CpG island in tumor and normal tissues.
The methylation status of each CpG dinucleotide spanning the PTPRO CpG island was then assessed using MassARRAY (EpiTYPER) (24). The amplicon spanning from +29 bp to +264 bp and harboring 21 CpG dinucleotides (Fig. 1B) was subjected to MassARRAY, which revealed higher methylation density (blue as opposed to yellow in the dendrogram in Fig. 1C) in all the tumor samples compared with the adjacent normal tissue. Low level of methylation was observed in few CpGs in some normal tissues. Quantification of the data obtained from the MassARRAY further confirmed the higher level of methylation of PTPRO in breast cancers compared with matching normal breast tissues (Fig. 1D, P = 0.0001).
PTPRO is silenced in breast cancer cell lines due to promoter methylation
Next, we sought to determine whether methylation of PTPRO causes its suppression in breast cancer cell lines by RT-PCR analysis of breast cancer cell lines (MCF-7, MDA-MB-231, and Hs578t) and normal human mammary epithelial cells (HMEC, 184 and 48R). HMECs derived from normal breast tissue were used in this study because these cells are the closest representation of the normal mammary cells available. These cells were developed as a tool to understand the mechanisms underlying proliferation and differentiation of normal breast epithelium, during immortalization and malignant transformation (http://lbl.gov/LBL-Programs/mrgs/review.html). The results showed expression of PTPRO in both normal mammary epithelial cells, and its suppression in two (MCF-7, MDA-MB-231) of the three breast cancer cell lines (Fig. 2A). Western blot analysis with PTPRO antibody detected an approximately 110-kDa protein in 48R cells but not in MCF-7 cells (Fig. 2B). To determine whether suppression of PTPRO in the breast cancer cell lines correlates with methylation of its CpG island, we performed BS genomic sequencing of PTPRO CpG island from one representative HMEC (48R) and one breast cancer cell line (MCF-7). The presence of cytosines in a CpG context after BS conversion indicates that it is methylated in the genomic DNA of the sample being analyzed. These data indeed demonstrated that the majority of the CpG dinucleotides within the analyzed region were heavily methylated in MCF-7 cells but were free of methylation in the HMEC 48R cells (Fig. 2C).
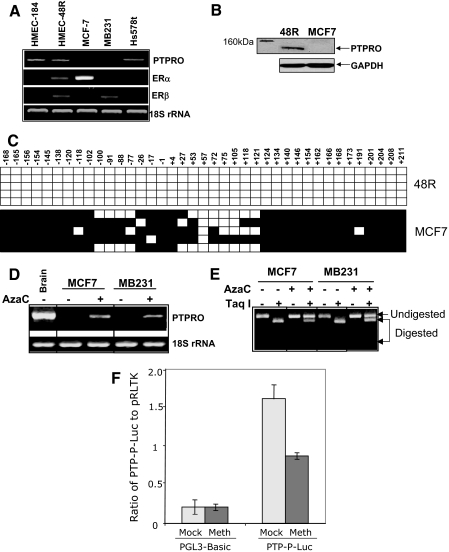
Figure 2.
PTPRO is methylated in breast cancer cell lines but not in normal breast epithelial cells. A, Total RNA isolated from normal human mammary epithelial cells (48R, 184) and breast cancer cell lines Hs578t, MCF-7, and MB-231 were subjected to RT-PCR using PTPRO-, ERα-, ERβ-, or 18S rRNA (loading control)-specific primers. B, Whole-cell extracts from 48R and MCF-7 cells were subjected to Western blot analysis with PTPRO antibody (Abcam). The same blot was probed with anti-GAPDH antibody to ensure equal protein loading. C, PTPRO CpG island from HMEC 48R and MCF-7 cells was subjected to BS genomic sequencing. Each solid square represents a methylated cytosine and an open square represents unmethylated cytosine in a CpG dinucleotide context. Each row corresponds to a single clone. D, Breast cancer cell lines MCF-7 and MB-231 were treated with 1μm 5-AzaC for 72 h and 2.5 μm 5-AzaC for 96 h, respectively. Total RNA from cells was subjected to RT-PCR to amplify PTPRO mRNA. 18S rRNA was used as a normalizer. E, Genomic DNA isolated from untreated and 5-AzaC treated (5 μm for 2 wk) MCF-7 and MB-231 cells was subjected to COBRA with TaqI. The undigested and digested products indicate unmethylated and methylated DNA (at TaqI site, TCGA), respectively. To monitor DNA demethylation, cells were treated for longer time with the drug. F, H293t cells were transfected with mock-methylated (Mock) or HhaI-methylated (Meth) PTP-P-Luc plasmid. Luciferase activity was normalized to the internal control pRLTK. HhaI-methylated and mock-methylated pGL3-Basic was used as control. Error bars represent sd of triplicate measurements.
To confirm that methylation of the PTPRO promoter in breast cancer cell lines was responsible for its suppression, the ERα(+) MCF-7 and ERα(−) MDA-MB-231 cells were treated with 5-azacytidine (5-AzaC), a DNA-hypomethylating agent. RT-PCR analysis demonstrated reexpression of PTPRO in MCF-7 cells treated with 1 μm 5-AzaC for 72 h and in MDA-MB-231 cells treated with 2.5 μm 5-AzaC for 96 h (Fig. 2D). The response of different cell lines to demethylating agents probably varies due to different drug sensitivities as well as different kinetics of association/dissociation of chromatin remodelers with specific genes. To confirm that the 5-AzaC-mediated activation of PTPRO was due to demethylation of its CpG island, we performed COBRA on the untreated and the inhibitor-treated breast cancer cells. The relative resistance of the amplicon derived from BS DNA to TaqI digestion in the 5-AzaC-treated cells compared with untreated cells (Fig. 2E) proved that the PTPRO CpG island was indeed demethylated after treatment with the methyltransferase inhibitor. These data reveal that PTPRO is silenced in breast cancer cell lines due to methylation of the CpG island within its promoter region. Additionally, we compared the activity of HhaI-methylated PTPRO promoter to that of unmethylated promoter in a transient transfection study. An approximately 950-bp minimal PTPRO promoter cloned upstream of the luciferase gene in a promoter-reporter construct harbors eight HhaI sites. In vitro HhaI methylation of the PTPRO promoter resulted in 45% inhibition of its activity compared with that of the mock-methylated promoter (Fig. 2F). These data further support the notion that methylation of the PTPRO CpG island directly inhibits its promoter activity.
E2 and tamoxifen regulate PTPRO expression in HMECs and breast cancer cell lines via ERβ
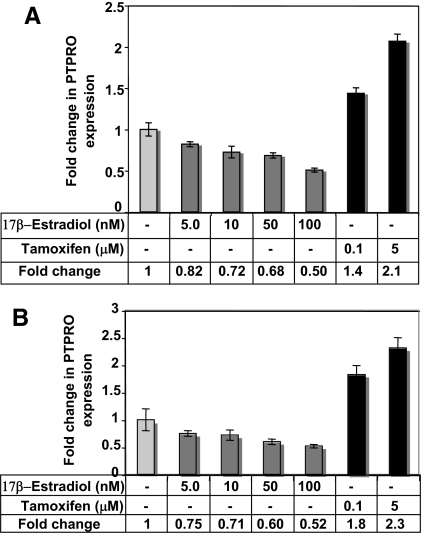
Methylation-mediated silencing of PTPRO CpG island in established breast cancer cell lines and primary breast cancer raised the question of whether an initial transcriptional repression event triggered permanent silencing of PTPRO by CpG island methylation. E2 is known to regulate gene expression in ER(+) cells by nuclear receptor signaling and via protein signaling cascades (25,26). Further, the presence of estrogen response elements in the PTPRO promoter coupled with the possibility that alteration in tyrosine phosphorylation of key signaling molecules due to loss of PTPRO could contribute to tumorigenesis in breast cancer led us to investigate whether PTPRO could be one of the target genes regulated by estrogen. For this purpose, cells were grown in charcoal-stripped serum-supplemented phenol red-free media to eliminate any estrogenic stimuli from phenol red (27) and serum components. Treatment of 48R cells with increasing concentration of E2 in this medium resulted in dose-dependent reduction in PTPRO expression with 50% decrease at 100 nm E2 (Fig. 3A). Among the three breast cancer cell lines tested, only Hs578t cells expressed PTPRO. We, therefore, investigated whether E2 had similar effect on PTPRO in this cell line. Interestingly, a similar suppression of PTPRO was observed in Hs578t cells after exposure to E2 (Fig. 3B). Conversely, treatment of 48R and Hs578t cells with the ER blocker, tamoxifen (5 μm), resulted in a 2.1- and 2.3-fold increase in the expression of PTPRO, respectively (Fig. 3, A and B). Treatment with a lower concentration of tamoxifen (0.1 μm) also resulted in 1.4- and 1.8-fold increase in PTPRO expression in 48R and Hs578t cells, respectively. This observation indicates that the estrogen-mediated suppression of PTPRO could be the primary event in PTPRO suppression in breast cancer.
Figure 3.
E2 inhibits and tamoxifen augments PTPRO expression in normal HMECs 48R and breast cancer cell line Hs578t. 48R (A) and Hs578t (B) cells were treated with indicated doses of E2 or tamoxifen for 72 h. Total RNA isolated from vehicle, E2, and tamoxifen-treated cells was subjected to real time RT-PCR with β-actin as normalizer for RNA input. The data are represented as fold change compared with control cells. Error bars represent sd of triplicate measurements.
The classical action of estrogen involves the corresponding nuclear receptor (ER) that acts as a ligand-dependent transcription factor. There are two different ERs, ERα and ERβ, encoded by different genes located on separate chromosomes in the human genome. Although these two receptors are homologous in their DNA-binding domains, they differ in their hormone-binding domains and more significantly in their N-terminal activation function-1 region (10,11). Such differences between the ER isoforms could impart differential trans-activation properties to these proteins. The ligand-bound ERα generally demonstrates trans-activation function in the presence of estrogen (12). PTPRO was, however, suppressed by E2 and activated by tamoxifen. Further these effects were observed in ERα(−) Hs578t and ERα(+) 48R cells. Therefore, we hypothesized that ERβ might be involved in the E2-mediated repression of PTPRO promoter. To test this possibility we first compared the expression of ERβ and ERα in these cells (Fig. 2A). Variable levels of ERβ was indeed detectable in Hs578t (ERα−) and 48R cells (ERα+) as well as in other cell lines. We also confirmed ERα expression in all the cells involved in this study. As expected, it was not expressed in the ERα(−) cells (MB-231 and Hs578t). To confirm the involvement of ERβ, both 48R and Hs578t cells were transiently transfected with either ERβ small interfering RNA (siRNA) or control siRNA, treated with E2 or tamoxifen, and analyzed for PTPRO expression. In both cell lines, the effect of E2 and tamoxifen was abrogated when transfected with ERβ siRNA but not with control siRNA (Fig. 4, A and B). In addition, Hs578t cells transiently transfected with ERα or ERβ expression vector were treated with 100 nm E2 for 30 h. RT-PCR analysis revealed a 50% decrease in PTPRO expression in vector-transfected cells, whereas its expression was abolished in ERβ transfected cells (Fig. 4, C and D). Ectopic expression of ERα had no further inhibitory effect compared with the vector-transfected cells (Fig. 4, C and D).
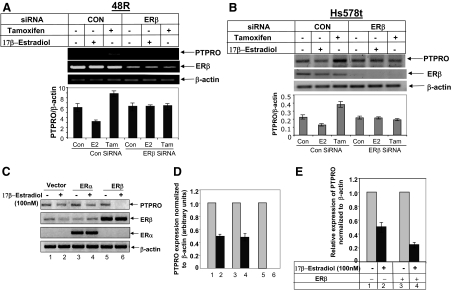
Figure 4.
ERβ mediates the effects of E2 and tamoxifen on PTPRO expression. 48R (panel A) or Hs578t cells (panel B) transiently transfected with either 100 nm control siRNA or ERβ siRNA were treated with either 100 nm E2, 5 μm tamoxifen (Tam), or left untreated. PTPRO expression analyzed by RT-PCR was normalized to β-actin. Error bars represent sd of triplicate measurements. C, Hs578t cells were transiently transfected with the vector alone, ERα expression vector, or ERβ expression vector. The transfected cells were treated with 100 nm E2 for 30 h. Total RNA isolated from these cells was subjected to RT-PCR with PTPRO-, ERα-, and ERβ-specific primers. β-actin was amplified to ensure equal input RNA. D, Quantification of the RT-PCR data. Error bars represent sd of triplicate measurements. E, 48R cells were transiently transfected with ERβ expression vector or the empty vector as indicated and treated with 100 nm E2 for 48 h. Total RNA was subjected to real-time RT-PCR for PTPRO expression. Normalized PTPRO expression in E2 untreated cells was assigned a value of 1.0. Error bars represent sd of triplicate measurements.
To determine whether estrogen-mediated down-regulation of PTPRO is a common event in breast epithelial cells, the 48R cells were transfected with ERβ expression plasmid and treated with E2 for 48 h. Real time RT-PCR analysis showed significant increase (82%) in E2-mediated suppression of PTPRO upon ectopic ERβ expression compared with the vector-transfected cells (46%) (Fig. 4E). These data suggested a key role of ERβ in estrogen-mediated suppression of PTPRO gene in breast cancer.
Hormonal regulation of PTPRO expression involves AP-1 complex
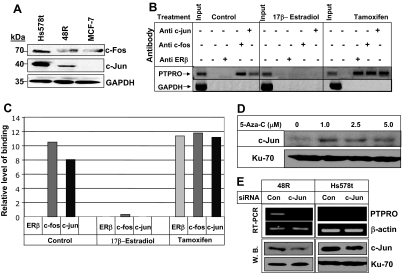
ERs can act as transcription factors after binding to the estrogen response element (ERE), which is composed of two inverted hexanucleotide repeats. ER can also mediate gene transcription from an AP-1 enhancer element. Transcriptional activation at the AP-1 site is dependent on the ligand and the AP-1 transcription factors Fos and Jun (12). ERα and ERβ exhibit similar transactivation potential at the ERE in the presence of ligand, i.e. transcriptional activation in the presence of estrogen and repression when antiestrogen is the ligand. On the contrary, these two receptors have been shown to respond differentially to the ligands at AP-1 sites. Thus, ERα acts as a transcriptional activator in the presence of E2 whereas the ligand-bound ERβ inhibits transcription at these sites. Conversely, the antiestrogens tamoxifen, raloxifene, and ICI 164384 act as potent activators at the AP-1 site when bound to ERα and ERβ (12). PTPRO promoter harbors several estrogen response elements including ERE half-site as well as several AP-1 sites. Because estrogen-bound ERβ suppressed PTPRO promoter activity, it was logical to assume that this action involved the AP-1 complex. Interestingly, because PTPRO is a TPA-responsive gene (28) AP-1 can up-regulate its transcription (29), and the AP-1 complex is likely to be involved in regulating its expression. To address this possibility, we first determined whether the expression of AP-1 components, c-Fos and c-Jun, correlated with PTPRO expression in the breast epithelial cells. Indeed, c-Fos and c-Jun expression was higher in PTPRO expressing 48R and Hs578t cells compared with nonexpressing MCF-7 cells (Fig. 5A). We then confirmed the potential involvement of ERβ and AP-1 complex in E2-mediated suppression and tamoxifen- mediated up-regulation of PTPRO promoter. For this purpose, chromatin immunoprecipitation assay was performed using antibodies specific for ERβ, c-Fos and c-Jun, and formaldehyde cross-linked chromatin prepared from untreated and E2 or tamoxifen-treated Hs578t cells followed by semiquantitative PCR for PTPRO promoter with a 32P-labeled primer. The results demonstrated that c-Fos and c-Jun were indeed associated with the PTPRO promoter in control/untreated cells, which could explain the expression of PTPRO in these cells (Fig. 5, B and C). To our surprise, instead of association of an inhibitory ERβ/E2, c-Fos, and c-Jun complex with the promoter in E2-treated cells (12), neither ERβ/E2 nor c-Fos/c-Jun was associated with the promoter under this condition. On the contrary, treatment of the cells with tamoxifen resulted in recruitment of ERβ in addition to c-Fos and c-Jun at the promoter (Fig. 5, B and C). It appears, therefore, that Fos and Jun are required for PTPRO expression. It can be speculated that in the presence of E2, the ERβ/E2 complex probably sequesters the AP-1 complex from the promoter resulting in PTPRO suppression. In contrast, tamoxifen-bound ERβ enhances recruitment of AP-1 complex and probably other transcriptional activators to the promoter and up-regulates PTPRO expression.
Figure 5.
Differential association of AP-1 components and ERβ with the PTPRO promoter in the presence of E2 and tamoxifen. A, Whole-cell extracts from Hs578t, 48R, and MCF-7 cells were subjected to Western blot analysis with anti-c-Fos and anti-c-Jun antibody. The same blot was probed with anti-GAPDH antibody to ensure equal protein loading. B, Hs578t cells were treated with either 100 nm E2 or 5 μm tamoxifen for 24 h. Formaldehyde-cross-linked chromatin prepared from untreated or treated cells was immunoprecipitated with anti-ERβ, anti-c-Fos, or anti-c-Jun antibody. PTPRO promoter was amplified and analyzed from the pulled-down chromatin by semiquantitiative PCR. Amplification of GAPDH promoter was used as negative control. One representative sample of three repeats with similar observation is shown here. C, Quantification of binding observed in Fig. 5B is calculated as a percent of input DNA. D, MCF-7 cells were treated with increasing concentration of 5-AzaC for 72 h. Western blot analysis was performed with anti-c-Jun antibody, and the blot was reprobed with Ku-70 antibody for protein normalization. The data are representative of duplicate experiments. E, 48R and Hs578t cells were transfected with 100 nm c-Jun or control siRNA. PTPRO expression was analyzed by RT-PCR and normalized to β-actin expression. For c-Jun expression, whole-cell extract from siRNA-transfected cells was subjected to Western blot analysis with c-Jun antibody and normalized to Ku-70 protein. The data are representative of triplicate experiments. Con, Control; W.B., Western blot.
Based on our data, c-Fos and c-Jun are the two key transcription factors required for PTPRO expression in breast epithelial cells. However, c-Jun protein was barely detectable in MCF-7 cells (Fig. 5A) that express PTPRO after treatment with 5-AzaC. Analysis of c-Jun expression in 5-AzaC-treated MCF-7 cells revealed an increase in c-Jun protein level compared with untreated cells (Fig. 5D). To authenticate the role of c-Jun in PTPRO expression, we depleted the protein using siRNA in both 48R and Hs578t cells. PTPRO expression in c-Jun siRNA transfected cells was reduced at least by 50% compared with cells transfected with control siRNA (Fig. 5E).
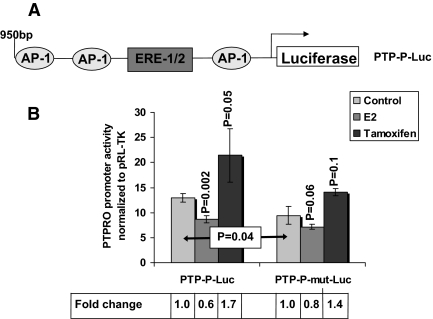
To establish further the involvement of the AP-1 site in E2-mediated suppression of PTPRO promoter, we used a promoter- reporter construct to enable mutational studies. For this purpose, an approximately 950-bp minimal promoter fragment upstream of the transcription start site harboring an ERE half-site and several AP-1 consensus was cloned in front of a luciferase reporter (PTP-P-Luc, Fig. 6A). The strongest AP-1 site (based on Transcriptional Element Search Software analysis) that is closest to the transcription start site (− 40 bp) was mutated to inhibit c-Fos/c-Jun binding (PTP-P-mut-Luc). These promoter-reporter plasmids were transfected into H293t cells along with ERβ expression vector followed by treatment with E2 or tamoxifen. E2 suppressed promoter activity of PTP-P-Luc by approximately 40% (P = 0.002), whereas tamoxifen activated the promoter nearly 2-fold (P = 0.05) compared with untreated cells (Fig. 6B, PTP-P-Luc). Mutation of the AP-1 site caused a small but significant reduction (28%, P = 0.04) in the activity of the untreated promoter, reinforcing the importance of AP-1 element(s) in the expression of PTPRO. As expected, the stimulatory effect of tamoxifen on the AP-1 mutated promoter was also reduced and was not significant (P = 0.1). Similarly, as the basal promoter activity was reduced upon AP-1 site mutation, E2 did not show a significant suppressive effect on the mutated promoter (P = 0.06). Because the cloned PTPRO promoter harbors multiple AP-1 sites (Transcriptional Element Search Software database), it is possible that further mutation of an additional AP-1 site would be necessary to abolish the effect of ER agonist (E2) or antagonist (tamoxifen). These data support our chromatin immunoprecipitation data that establish the involvement of AP-1 site(s) in the modulation of PTPRO expression.
Figure 6.
AP-1 site(s) are involved in the modulation of PTPRO expression. A, Schematic representation of the PTPRO promoter/luciferase reporter plasmid with relevant cis-regulatory elements (AP-1 and ERE) indicated. B, H293t cells were transiently transfected with PTP-P-Luc or PTP-P-mut-Luc (AP-1 site mutated to knock out c-Fos/c-Jun binding; Santa Cruz Biotechnology) and ERβ expression vector. Cells were treated with 100 nm E2, 5 μm tamoxifen, or the vehicle (EtOH) for 36 h before harvest. Luciferase activity was measured in the whole-cell lysate using the Dual Luciferase Assay System (Promega) and normalized to internal control pRL-TK. Any decrease in promoter activity due to E2 treatment or AP-1 mutation is calculated as percent reduction whereas an increase in promoter activity upon tamoxifen treatment is calculated as fold change over the untreated sample. For convenience, the reduction calculated as fold change is represented in the figure. P values were calculated using the Student’s t test.
PTPRO expression augments tamoxifen sensitivity of breast cancer cell line
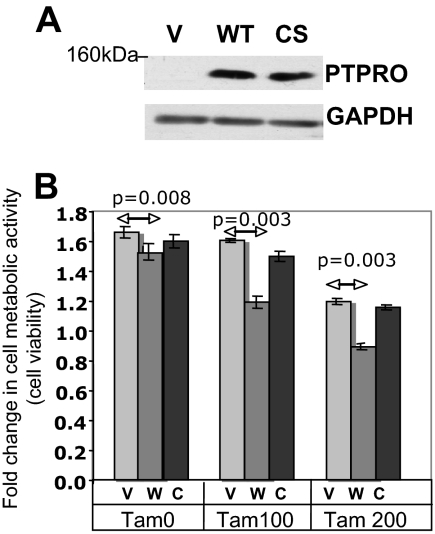
Although tamoxifen is widely used in breast cancer treatment, the development of resistance to prolonged endocrine therapy is a major problem. Earlier studies have indicated overall increase in the membrane-bound PTP in breast cancer cells treated with 4-hydroxy-tamoxifen (30). The estrogen-mediated suppression of PTPRO and its stimulation by tamoxifen raised the possibility that the increased expression of this tyrosine phosphatase might aid in antiestrogen therapy of breast cancer. Because PTPRO is silenced in MCF-7 cells, we tested the efficacy of tamoxifen in empty-vector transfected MCF-7 cells and cells transfected with the expression vector for the wild-type (WT) or catalytically inactive catalytic site (CS) mutant of PTPRO (Fig. 7A) by measuring their metabolic activity using cell viability (MTT) assay. Although expression of PTPRO-WT had a small but significant effect on metabolic activity compared with vector control and PTPRO-CS cells (Fig. 7B, TAM0), the effect of tamoxifen was enhanced by PTPRO-WT but not PTPRO-CS (Fig. 7B; compare TAM100 and TAM200 with TAM0) These data demonstrate a direct relationship between the expression of functional PTPRO and sensitivity of breast cancer cells to tamoxifen.
Figure 7.
PTPRO expression augments tamoxifen sensitivity of breast cancer cell line. A, The WT or CS mutant of FLAG-tagged PTPRO was overexpressed in MCF-7 cells. The G418 selected pool was analyzed by Western blot analysis for PTPRO overexpression using anti-FLAG M2 antibody. B, The PTPRO overexpressing pool (WT and CS mutant) cells were treated with 0, 100, and 200 nm tamoxifen. Cell viability was measured using MTT assay at 0 and 48 h. The data are represented as fold change in metabolic activity at 48 h compared with activity at 0 h. Error bars represent sd of triplicate measurements. Tam0, Tam100, Tam200, 0, 100, and 200 nm tamoxifen; V, vector; W, wild type; C, catalytic site mutant.
Discussion
We have previously presented evidence that the PTPRO is a bona fide tumor suppressor (20,21,22). The present study demonstrated hypermethylation of PTPRO promoter in approximately 81% (n = 21) of primary breast tumor samples compared with their normal adjacent tissue and lack of PTPRO promoter methylation in normal breast tissue obtained during reduction mammoplasty. Further, hypermethylation of PTPRO promoter and its reexpression after treatment with DNA methyltransferase inhibitor 5-AzaC confirm that the methylation of PTPRO promoter is indeed responsible for its silencing in breast cancer cell lines.
This study also showed significant reduction in expression of the gene encoding the tumor suppressor PTPRO after treatment of normal HMECs and PTPRO expressing breast cancer cell lines with E2. It is tempting, therefore, to speculate that the down-regulation of this gene is one of the mechanisms of estrogen-induced breast carcinogenesis. In addition, transcriptional repression of PTPRO might be an early event in silencing this tumor-suppressor gene in breast cancer.
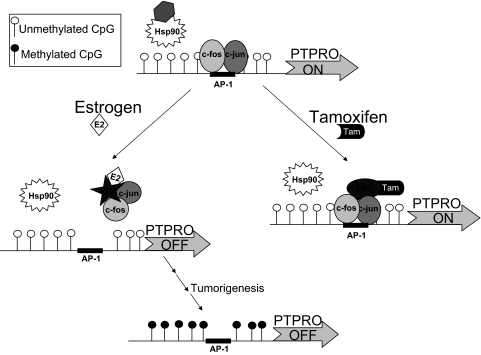
The exact mechanism for the transcriptional suppression of PTPRO merits discussion. We observed that ERβ and not ERα is involved in the estrogen-mediated suppression of PTPRO. Further, we have shown that the two positive transcription factors, c-Fos and c-Jun associated with the active PTPRO promoter, are dislodged from their cognate AP-1 binding site in the presence of E2. On the other hand, tamoxifen treatment recruits c-Fos, c-Jun, and ERβ to the promoter element. Based on these observations and existing knowledge about the interaction of ER with its agonist E2 or antiestrogen compounds like tamoxifen, we propose a model for PTPRO regulation by E2 and tamoxifen (see Fig. 8). Like other steroid receptors, ER remains associated with the chaperone protein Hsp90 in the absence of any ligand (for review see Ref. 31). Under this condition, the components of AP-1 complex, c-Fos and c-Jun, are free to bind to their cognate element in the PTPRO proximal promoter and facilitate transcription. E2 induces a conformational change of ERβ resulting in its dissociation from Hsp90. These conformational changes induced by E2 could poise the receptor molecule to sequester the activator proteins c-Fos and c-Jun, resulting in transcriptional repression of PTPRO. The antiestrogen tamoxifen inhibits estrogen action by blocking the estrogen-binding site on the receptor. A significant difference in structure between E2/ER and tamoxifen/ER has been observed by x-ray crystallographic studies (32,33). We propose that the binding of tamoxifen to ERβ could facilitate recruitment of transcriptional activators along with AP-1 components to the PTPRO promoter, resulting in up-regulation of PTPRO. A similar model for transcriptional suppression of AP-1-containing genes by E2/ERβ has been proposed based on transient transfection studies (12) and shown for progesterone receptor gene after overexpression of ERβ (34). These studies, however, suggest that recruitment of E2/ER to the promoter inhibits recruitment of the AP-1 elements, thereby suppressing the gene. On the contrary, the present study on endogenous ERβ is the first to show that the inhibitory effect of E2 results from failure of both ERβ and fos/jun complex to bind to the cognate element on the PTPRO promoter due to probable squelching of the AP-1 complex. Further detailed studies to determine the identity of these complexes with ERβ in the presence of estrogen or tamoxifen and their differential recruitment to the PTPRO promoter are indeed warranted. It is possible that the initial E2-mediated transcriptional suppression of this gene is probably followed by methylation as a means to permanently silence its expression (see Fig. 8). It is noteworthy that initial suppression of the ERα target gene encoding progesterone receptor due to loss of ER signaling eventually leads to its long-term silencing by DNA methylation. Further, reactivation of progesterone receptor required demethylation along with restoration of ER signaling (35). Similarly, the reversal of methylation-mediated suppression of PTPRO by 5-AzaC and the significance of increased PTP expression by tamoxifen provide a rationale to test epigenetic drugs in combination with tamoxifen for breast cancer therapy.
Figure 8.
Schematic model of PTPRO gene regulation by estrogen and tamoxifen in breast epithelial cells. c-Fos- and c-Jun-mediated expression of PTPRO via AP-1 site is further activated in presence of tamoxifen and inhibited by estrogen in the presence of ERβ. The transcriptional repression of PTPRO in the presence of estrogen, as observed in mammary epithelial cells, could probably lead to CpG island methylation probably during the multistep process of tumorigenesis. Hsp90, Heat shock protein 90; Tam, tamoxifen.
The growth-suppressive role of PTPRO and its increased expression by tamoxifen prompted us to explore the functional implications of its up-regulation by the widely used selective ER modulator tamoxifen. The increased tamoxifen sensitivity of the MCF-7 cells upon ectopic expression of PTPRO-WT, but not its CS mutant, indicates that proper control of protein tyrosine phosphorylation of key substrates by the functional PTPRO is important for effective endocrine therapy. It is critical to identify the key substrates of PTPRO and determine their phosphorylation states in the initiation and progression of breast cancer for developing a therapeutic strategy that involves reactivation of the silenced PTPRO.
Although few studies have addressed the expression and function of PTPs in breast cancer, most report their increased expression in breast cancer as has been demonstrated for leukocyte antigen related (36), PTPα (37), PTP1B (38,39), and Src homology phosphatase 1 (40). On the contrary, the gene encoding PTP γ (PTPG) is the only PTP gene that is down-regulated in breast tumors relative to their normal counterparts. Further, the expression of this gene was suppressed by E2 in primary normal breast cells in culture (41,42) and induced by tamoxifen in MCF-7 cells. However, the mechanism of the estrogen-mediated alteration in the expression of this gene has not been studied. To our knowledge, this is the first report on the methylation and suppression of a receptor-type tyrosine phosphatase gene in primary human breast tumors and breast cancer cell lines. Further, this is also the first detailed analysis of the mechanism of estrogen-mediated suppression and tamoxifen-mediated up-regulation of a tyrosine phosphatase gene through AP-1 site(s) present in its proximal promoter. These are significant findings given that E2 is a known tumor promoter and PTPRO exhibits tumor- suppressive properties in different cancers that now include breast cancer.
Materials and Methods
Cell culture and treatment
Human breast cancer cell lines MCF-7, MDA-MB-231 (provided by Dr. Young C. Lin, The Ohio State University, Columbus, OH), and Hs578t cells (obtained from Dr. Nancy E. Davidson, The Johns Hopkins University School of Medicine, Baltimore, MD) were maintained in DMEM supplemented with 5% fetal bovine serum and 1 mm nonessential amino acids in a 5% CO2 incubator. 48R and 184 cells (provided by Dr. Martha Stampfer, The Lawrence Berkeley National Laboratory, Berkeley, CA) were maintained in MEGM (Cambrex Corp., East Rutherford, NJ) and passage 8–11 cells were used for this study. Exponentially growing cells were treated with 5-AzaC (Sigma Chemical Co., St. Louis, MO) for different time period as indicated in the figures.
siRNA transfection
ERβ siRNA Smartpool (100 nm, Thermo Fisher Scientific, Pittsburgh, PA) and c-Jun siRNA pool (100 nm; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in exponentially growing cells for 16 h, followed by incubation of cells in phenol red free media for 8 h. The cells were then treated with 100 nm E2 or 5 μm tamoxifen for 36 h where indicated, before RNA isolation.
Breast cancer samples
Human primary breast cancer samples and matching controls were obtained from the cooperative human tissue network and from the tissue bank created through the protocol OSU 0109 (PI: Dr. Charles Shapiro) for collection and storage of breast cancer and normal tissue for research purposes.
Genomic DNA isolation, BS treatment, and COBRA
Isolation of genomic DNA and treatment with BS reagent were performed as previously described (43,44). The PTPRO CpG island between +29 and +264 bp with respect to the transcription start site was amplified using nested primers. The first round of amplification was carried out for 35 cycles with the primers hPTP-BS-F1: 5′-AGGTTGTTGTTATTTTATGG-3′ and hPTP-BS-R1: 5′-AAAACTCCCCTACCTTAAAC-3′. After initial incubation at 94 C for 3 min, each cycle involved 30 sec each at 94 C, 50 C, and 72 C. An aliquot of the reaction was then used for a second round of PCR with primers hPTP-BS-seqF: AGGAAGAGAGTAGGGGGATTGGAAA and hPTP-BS-seqT7R: CAGTAATACGACTCACTATAGGGAGA. After initial incubation at 94 C for 3 min, each of the 35 cycles involved 30 sec each at 94 C, 60 C, and 72 C. The purified PCR product was then used for digestion with Tsp 509I to check for complete BS conversion or with TaqI to study methylation status.
EpiTYPER
High-throughput analysis of DNA methylation was performed on the same amplicon generated above for COBRA using MassARRAY as described (24). Standard PTPRO amplicons (methylated at different levels) were generated by amplifying BS-treated DNA where completely methylated human lymphocyte DNA (Millipore Corp., Billerica, MA) and unmethylated DNA from peripheral blood lymphocytes were mixed at different ratios.
RNA isolation and real-time RT-PCR
H7578t cells were transfected with ERα or ERβ expression vectors (a generous gift from Dr. Leigh C. Murphy, University of Manitoba, Winnipeg, Canada). Total RNA was isolated using the guanidinium isothiocyanate-acid phenol method (45). Reverse transcription of deoxyribonuclease-treated RNA (3 μg) was carried out with random hexamers and murine Moloney leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) according to the Gene-Amp RNA PCR kit (PerkinElmer, Wellesley, MA) instructions. RT-PCR primers and details of the procedure are as described elsewhere (20,21).
Western blot analysis
MCF-7 cells transfected with PTPRO-WT-FLAG, PTPRO-CS-FLAG or vector (20,21) were selected with 500 μg/ml G418 for 10–14 d. Whole-cell extract from the G418-resistant pool was immunoblotted with M2-anti-FLAG antibody (Sigma) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Molecular Probes, Inc., Eugene, OR) antibodies following published protocol (46). Whole-cell extracts from 48R and MCF-7 cells were immunoblotted with PTPRO antibody (AbCam, Inc., Cambridge, MA). The signal was developed with ECL (GE Healthcare, Buckinghamshire, UK) after incubation with appropriate secondary antibodies.
Chromatin immunoprecipitation
Hs578t cells (70% confluent) were treated with 100 nm E2, 5 μm tamoxifen, or the vehicle (control) for 24 h and cross-linked with 1% formaldehyde. Chromatin prepared from each treatment group was immunoprecipitated with anti-ERβ (47) anti-c-Fos, or anti-c-Jun (both from Santa Cruz Biotechnology), following the protocol described by Ghoshal et al. (48).
Plasmid construction and transient transfection assay
An approximately 950-bp fragment of the PTPRO promoter (−822 to +132) was amplified from lymphocyte DNA and cloned at the SmaI/BglII sites of pGL3-Basic vector (Promega Corp., Madison, WI) to generate the PTP-P-Luc vector. In vitro methylation of the HhaI sites on the PTPRO promoter were performed following the protocol described earlier (49). To obtain the PTP-P-mutLuc reporter plasmid, the AP-1 site in the cloned PTPRO promoter was mutated by site-directed mutagenesis using the primer pairs PTP-P-F: GGAGGAGGAAGAGGGTTTGGTC/mutAP-1-R: AGGCGCTGCTGTTCTTTAACAACTAAGGAT and mutAP-1-F:ATCCTTAGTTGTTAAAGAACAGCAGCGCCT/PTP-P-R: AATAGATCTGGCGGCTCACAATGGCGAAC. The promoter reporter construct along with ERβ expression vector was transfected into H293t cells using Lipofectamine 2000 (Invitrogen). The cells were split into three dishes 24 h after transfection and treated with 100 nm E2, 5 μm tamoxifen, or the vehicle (ethanol) for an additional 36 h before harvest. Luciferase activity was measured using the Dual Luciferase assay kit (Promega Corp.).
Cell proliferation assay
Cell proliferation was monitored using Cell Proliferation Reagent Kit I (MTT) (Roche Molecular Biochemicals, Nutley, NJ). MCF-7 cells transfected with PTPRO-WT or PTPRO-CS, or the vector were allowed to grow in 96-well plates (5000 per well). Cells were serum starved for 18 h followed by tamoxifen (100 nm and 200 nm) treatment in complete media for 48 h. Cell proliferation was documented following the manufacturer’s protocol. Absorbance was measured at 550 nm in the ELISA reader (Tristar; Berthold Technology, Oak Ridge, TN) after overnight incubation. The metabolic activity is plotted as fold change at 48 h relative to activity at 0 h of tamoxifen treatment.
Statistical analysis
Statistical significance was analyzed by unpaired Student’s t test, and P ≤ 0.05 was considered to be statistically significant. Each experiment was repeated two to three times as indicated in the figure legends.
Acknowledgments
We thank Dr. Martha Stampfer (The Lawrence Berkeley National Laboratory) for providing us HMECs; Dr. Young C. Lin (The Ohio State University) for MCF-7 and MDA-MB-231 cells; and Dr. Nancy E. Davidson (The Johns Hopkins University School of Medicine) for Hs578t cells. We also thank Dr. Leigh C. Murphy (University of Manitoba) for kindly providing us the ERβ expression vector.
Footnotes
This work was supported, in part, by National Institutes of Health Grants CA101956, CA122523, and CA086978.
First Published Online December 18, 2008
Abbreviations: AP-1, Activator protein 1; 5-AzaC, 5-azacytidine; BS, bisulfite; CS, catalytic site; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HMECs, human mammary epithelial cells; MTT, 3-(4,5-dimethylthiazole-2yl)-2,5 diphenyltetrazolium bromide; PTK, protein tyrosine kinase; PTP, protein tyrosine phosphatase; PTPRO, protein tyrosine phosphatase receptor-type O; siRNA, small interfering RNA; WT, wild type.
References
- Russo J, Hu YF, Yang X, Russo IH 2000 Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr:17–37 [DOI] [PubMed] [Google Scholar]
- Peppercorn J, Perou CM, Carey LA 2008 Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest 26:1–10 [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D 2000 Molecular portraits of human breast tumours. Nature 406:747–752 [DOI] [PubMed] [Google Scholar]
- Henderson BE, Feigelson HS 2000 Hormonal carcinogenesis. Carcinogenesis 21:427–433 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Gustafsson JA 1997 The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett 410:87–90 [DOI] [PubMed] [Google Scholar]
- Dotzlaw H, Leygue E, Watson PH, Murphy LC 1999 Estrogen receptor-β messenger RNA expression in human breast tumor biopsies: relationship to steroid receptor status and regulation by progestins. Cancer Res 59:529–532 [PubMed] [Google Scholar]
- Iwao K, Miyoshi Y, Egawa C, Ikeda N, Noguchi S 2000 Quantitative analysis of estrogen receptor-β mRNA and its variants in human breast cancers. Int J Cancer 88:733–736 [DOI] [PubMed] [Google Scholar]
- Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H 2001 Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res 61:2537–2541 [PubMed] [Google Scholar]
- Katzenellenbogen BS, Frasor J 2004 Therapeutic targeting in the estrogen receptor hormonal pathway. Semin Oncol 31:28–38 [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R 1996 ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49–53 [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA 2002 Involvement of estrogen receptor β in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA 99:15578–15583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RI, Hutcheson IR, Jones HE, Hiscox SE, Giles M, Taylor KM, Gee JM 2007 Growth factor signalling in endocrine and anti-growth factor resistant breast cancer. Rev Endocr Metab Disord 8:241–253 [DOI] [PubMed] [Google Scholar]
- Hunter T 2000 Signaling–2000 and beyond. Cell 100:113–127 [DOI] [PubMed] [Google Scholar]
- Giltnane JM, Ryden L, Cregger M, Bendahl PO, Jirstrom K, Rimm DL 2007 Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J Clin Oncol 25:3007–3014 [DOI] [PubMed] [Google Scholar]
- Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD 2007 Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer 120:1874–1882 [DOI] [PubMed] [Google Scholar]
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T 2004 Protein tyrosine phosphatases in the human genome. Cell 117:699–711 [DOI] [PubMed] [Google Scholar]
- Jacob ST, Motiwala T 2005 Epigenetic regulation of protein tyrosine phosphatases: potential molecular targets for cancer therapy. Cancer Gene Ther 12:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Ghoshal K, Das A, Majumder S, Weichenhan D, Wu YZ, Holman K, James SJ, Jacob ST, Plass C 2003 Suppression of the protein tyrosine phosphatase receptor type O gene (PTPRO) by methylation in hepatocellular carcinomas. Oncogene 22:6319–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Kutay H, Ghoshal K, Bai S, Seimiya H, Tsuruo T, Suster S, Morrison C, Jacob ST 2004 Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits characteristics of a candidate tumor suppressor in human lung cancer. Proc Natl Acad Sci USA 101:13844–13849 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Motiwala T, Majumder S, Kutay H, Smith DS, Neuberg DS, Lucas DM, Byrd JC, Grever M, Jacob ST 2007 Methylation and silencing of protein tyrosine phosphatase receptor type O in chronic lymphocytic leukemia. Clin Cancer Res 13:3174–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Jacob ST 2006 Role of protein tyrosine phosphatases in cancer. Prog Nucleic Acid Res Mol Biol 81:297–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, Cantor CR, Field JK, van den Boom D 2005 Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA 102:15785–15790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis BJ, Greger JG, Nagpal S, Freedman LP 2007 Signaling by estrogens. J Cell Physiol 213:610–617 [DOI] [PubMed] [Google Scholar]
- Tang S, Zhang Z, Tan SL, Tang MH, Kumar AP, Ramadoss SK, Bajic VB 2007 KBERG: KnowledgeBase for estrogen responsive genes. Nucleic Acids Res 35:D732–D736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS 1986 Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83:2496–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimiya H, Sawabe T, Inazawa J, Tsuruo T 1995 Cloning, expression and chromosomal localization of a novel gene for protein tyrosine phosphatase (PTP-U2) induced by various differentiation-inducing agents. Oncogene 10:1731–1738 [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M 2004 AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973 [DOI] [PubMed] [Google Scholar]
- Freiss G, Vignon F 1994 Antiestrogens increase protein tyrosine phosphatase activity in human breast cancer cells. Mol Endocrinol 8:1389–1396 [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M 1997 Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389: 753–758 [DOI] [PubMed] [Google Scholar]
- Levenson AS, Catherino WH, Jordan VC 1997 Estrogenic activity is increased for an antiestrogen by a natural mutation of the estrogen receptor. J Steroid Biochem Mol Biol 60:261–268 [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA 2006 Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- Leu YW, Yan PS, Fan M, Jin VX, Liu JC, Curran EM, Welshons WV, Wei SH, Davuluri RV, Plass C, Nephew KP, Huang TH 2004 Loss of estrogen receptor signaling triggers epigenetic silencing of downstream targets in breast cancer. Cancer Res 64:8184–8192 [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang JS, Massa SM, Han X, Longo FM 1999 Leukocyte common antigen-related tyrosine phosphatase receptor: increased expression and neuronal-type splicing in breast cancer cells and tissue. Mol Carcinog 25:139–149 [PubMed] [Google Scholar]
- Ardini E, Agresti R, Tagliabue E, Greco M, Aiello P, Yang LT, Menard S, Sap J 2000 Expression of protein tyrosine phosphatase α (RPTPα) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene 19:4979–4987 [DOI] [PubMed] [Google Scholar]
- Wiener JR, Kerns BJ, Harvey EL, Conaway MR, Iglehart JD, Berchuck A, Bast Jr RC 1994 Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J Natl Cancer Inst 86:372–378 [DOI] [PubMed] [Google Scholar]
- Zhai YF, Beittenmiller H, Wang B, Gould MN, Oakley C, Esselman WJ, Welsch CW 1993 Increased expression of specific protein tyrosine phosphatases in human breast epithelial cells neoplastically transformed by the neu oncogene. Cancer Res 53:2272–2278 [PubMed] [Google Scholar]
- Yip SS, Crew AJ, Gee JM, Hui R, Blamey RW, Robertson JF, Nicholson RI, Sutherland RL, Daly RJ 2000 Up-regulation of the protein tyrosine phosphatase SHP-1 in human breast cancer and correlation with GRB2 expression. Int J Cancer 88:363–368 [PubMed] [Google Scholar]
- Liu S, Sugimoto Y, Sorio C, Tecchio C, Lin YC 2004 Function analysis of estrogenically regulated protein tyrosine phosphatase γ (PTPγ) in human breast cancer cell line MCF-7. Oncogene 23:1256–1262 [DOI] [PubMed] [Google Scholar]
- Zheng J, Kulp SK, Zhang Y, Sugimoto Y, Dayton MA, Govindan MV, Brueggemeier RW, Lin YC 2000 17β-Estradiol-regulated expression of protein tyrosine phosphatase γ gene in cultured human normal breast and breast cancer cells. Anticancer Res 20:11–19 [PubMed] [Google Scholar]
- Ghoshal K, Majumder S, Li Z, Dong X, Jacob ST 2000 Suppression of metallothionein gene expression in a rat hepatoma because of promoter-specific DNA methylation. J Biol Chem 275:539–547 [DOI] [PubMed] [Google Scholar]
- Majumder S, Ghoshal K, Li Z, Jacob ST 1999 Hypermethylation of metallothionein-I promoter and suppression of its induction in cell lines overexpressing the large subunit of Ku protein. J Biol Chem 274:28584–28589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, Cha HC, MacDougald OA, Jacob ST, Ghoshal K 2007 Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein α by phosphatidylinositol 3-kinase signaling cascade. Cancer Res 67:2736–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, Murphy LC 2006 Assessment of multiple different estrogen receptor-β antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat 100:23–31 [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Datta J, Majumder S, Bai S, Dong X, Parthun M, Jacob ST 2002 Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol Cell Biol 22:8302–8319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal K, Majumder S, Datta J, Motiwala T, Bai S, Sharma SM, Frankel W, Jacob ST 2004 Role of human ribosomal RNA (rRNA) promoter methylation and of methyl-CpG-binding protein MBD2 in the suppression of rRNA gene expression. J Biol Chem 279:6783–6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Majumder S, Ghoshal K, Kutay H, Datta J, Roy S, Lucas DM, Jacob ST 2008 PTPROt inactivates the oncogenic fusion protein BCR/ABL and suppresses transformation of K562 cells. J Biol Chem 284:455–464 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]