Abstract
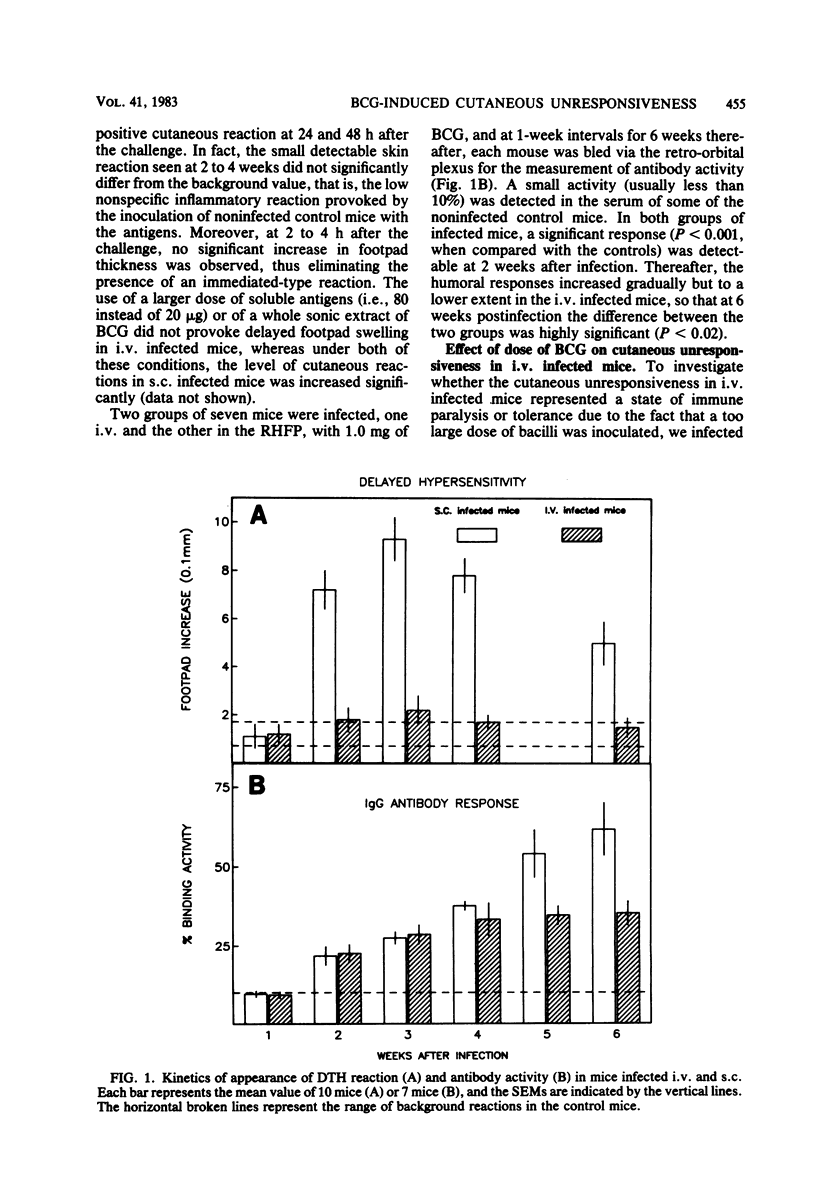
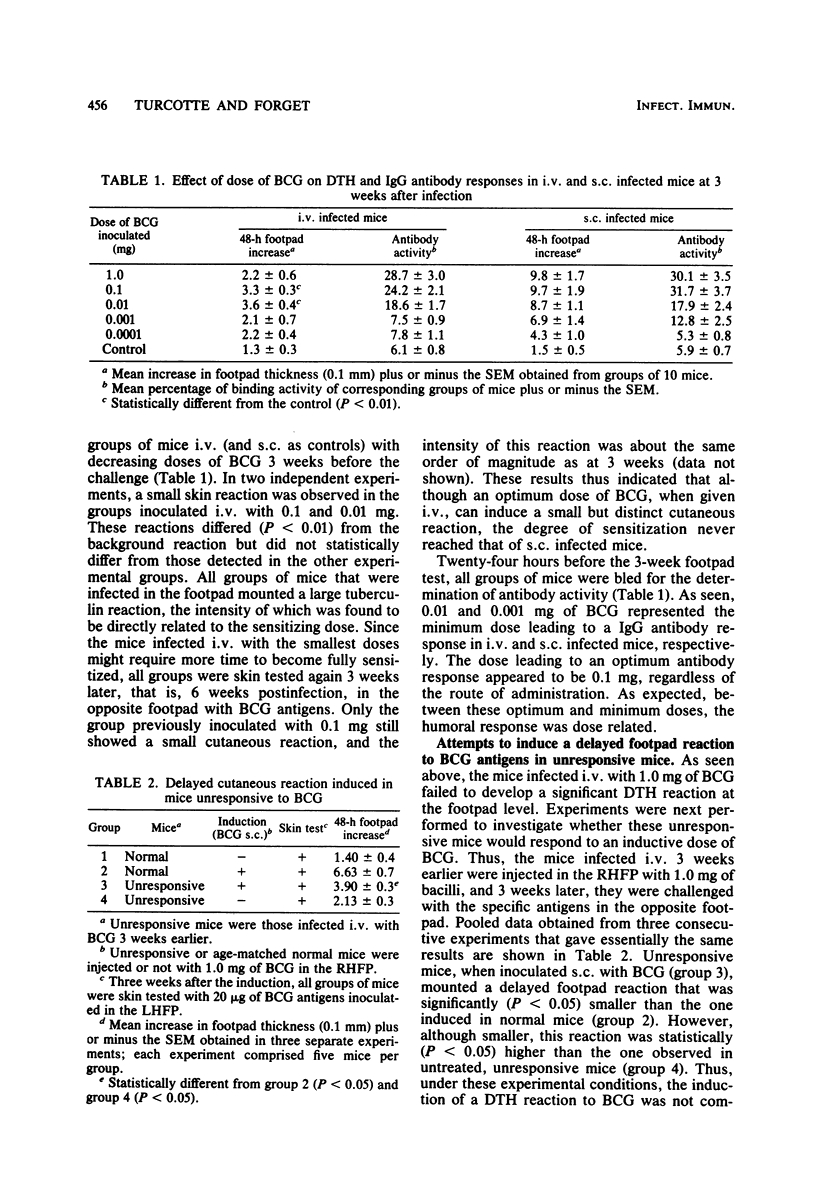
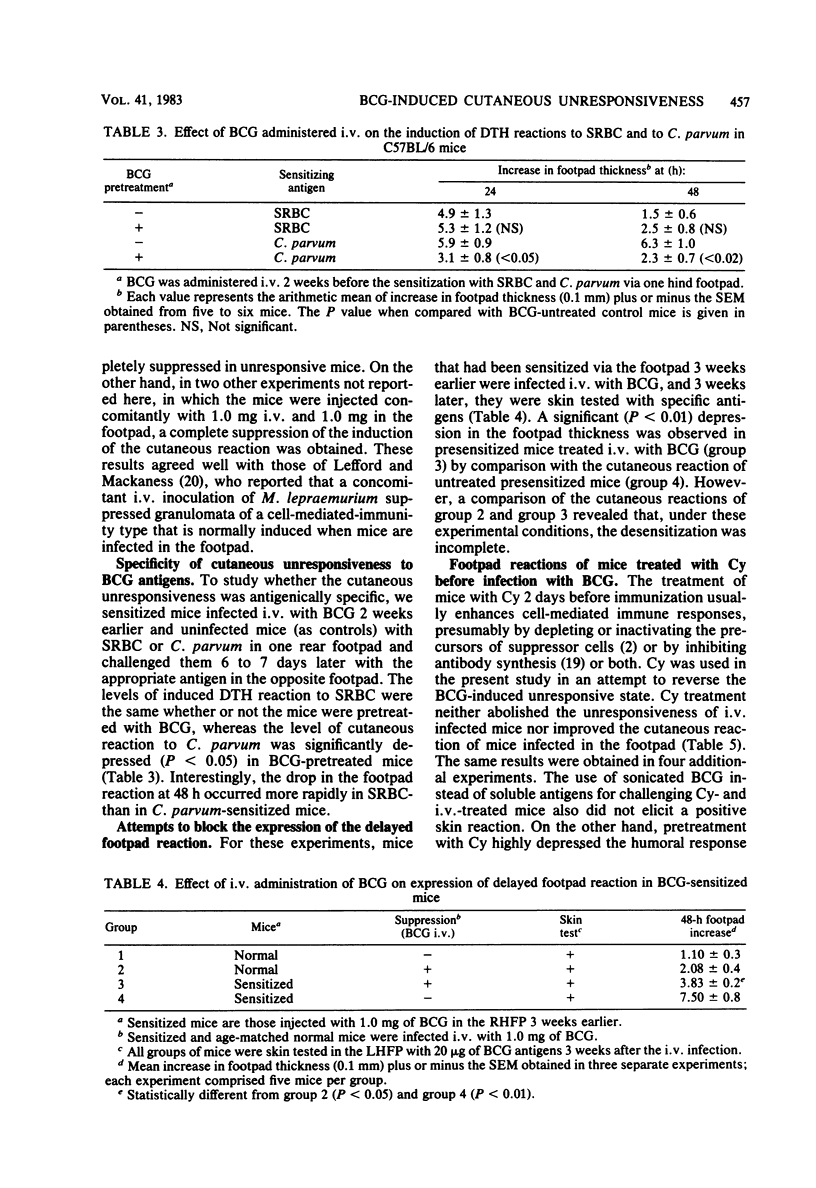
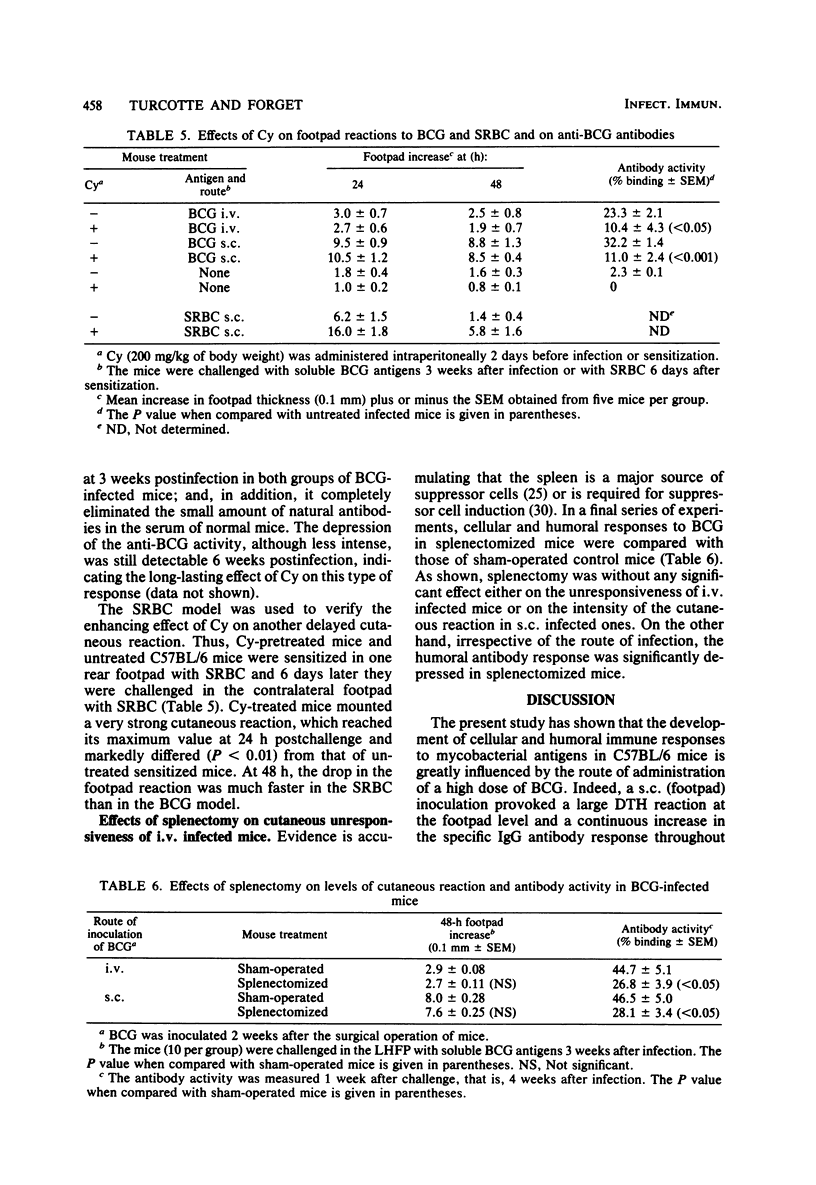
Mice injected with 1 mg (about 1 X 10(7) CFU) of Mycobacterium bovis BCG in the footpad showed high levels of delayed-type hypersensitivity (DTH) to BCG antigens and a continuous increase in circulating specific anti-immunoglobulin G (IgG) antibodies throughout a 6-week observation period. In contrast, mice injected intravenously with a 1-mg dose failed to mount a DTH and showed a depression in antibody production at week 5 postinfection. A dose-response study revealed that an optimum dose of BCG, when injected intravenously, can induce a small but significant DTH response. The delayed cutaneous unresponsiveness in intravenously infected mice lasted at least 6 weeks and was not antigenically specific, in that it depressed the DTH response to Corynebacterium parvum. No simple relationship existed between levels of DTH and the amount of circulating anti-IgG antibodies. Splenectomy and treatment with a high dose of cyclophosphamide before infection, although greatly reducing the humoral response, did not reverse the BCG-induced unresponsiveness nor enhance levels of DTH in mice infected in the footpad. It is concluded that the intravenous infection of mice with BCG exerts a nonspecific inhibitory effect on delayed-type immune reactions by the induction of some type of suppressor mechanisms that are resistant to cyclophosphamide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L. Antigen-mediated depression of delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):24–29. doi: 10.1093/oxfordjournals.bmb.a070510. [DOI] [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. A., Rao V. S., Mitchell M. S. Systemic bacillus Calmette-Guérin (BCG) activates natural suppressor cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5142–5144. doi: 10.1073/pnas.75.10.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Suppressed or enhanced antibody responses in vitro after BCG treatment of mice: importance of BCG viability. Immunology. 1979 Nov;38(3):481–488. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- CROWLE A. J., HU C. C. INHIBITION OF THE DEVELOPMENT OF DELAYED HYPERSENSITIVITY BY TREATMENT WITH ANTISERUM. J Immunol. 1965 Apr;94:555–562. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Parker D., Turk J. L. Suppression of delayed hypersensitivity to tuberculin by antigenic competition. A positive immunoregulatory mechanism sensitive to cyclophosphamide. Immunology. 1981 Apr;42(4):549–559. [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The sequence of enhanced cellular activity and protective humoral factors in murine pertussis immunity. Immunology. 1969 Dec;17(6):889–896. [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjune G., Kronvall G., Axelsen N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay. Scand J Immunol. 1978;7(2):111–120. doi: 10.1111/j.1365-3083.1978.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Ito M., Ralph P., Moore M. A. Suppression of spleen natural killing activity induced by BCG. Clin Immunol Immunopathol. 1980 May;16(1):30–38. doi: 10.1016/0090-1229(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Mackaness G. B. Suppression of immunity to Mycobacterium lepraemurium infection. Infect Immun. 1977 Nov;18(2):363–369. doi: 10.1128/iai.18.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. Specific depression of delayed hypersensitivity to purified proteins, with relation to production of circulating antibody. Cell Immunol. 1973 Nov;9(2):242–250. doi: 10.1016/0008-8749(73)90075-0. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A. Regulation of immune responses by suppressor T cells. Tohoku J Exp Med. 1976;5:91–143. [PubMed] [Google Scholar]
- Rook G. A. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin Exp Immunol. 1975 Jan;19(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Kowach H. B., Claman H. N. A splenic requirement for the generation of suppressor T cells. J Immunol. 1977 Dec;119(6):2095–2099. [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Yield of non-dialyzable mycobacterial constituents during the growth cycle. Can J Microbiol. 1969 Jan;15(1):35–41. doi: 10.1139/m69-006. [DOI] [PubMed] [Google Scholar]



