Abstract
IGF-binding proteins (IGFBPs) have multiple cellular effects, which occur by both IGF-dependent and -independent mechanisms. IGFBP-2 is involved in the regulation of both normal and carcinogenic cell growth. To further understand the actions of IGFBP-2, we carried out a yeast two-hybrid screen to search for intracellular partner proteins using a human prostate cDNA library. We isolated Pim-1-associated protein-1 (PAP-1)-associated protein-1 (PAPA-1) as an IGFBP-2-binding protein, whose expression and subcellular localization is regulated by both IGFBP-2 and androgens. Coimmunoprecipitation and glutathione S-transferase pull-down assay confirmed the interaction in vitro, and confocal microscopy showed the colocalization of IGFBP-2 and PAPA-1 in the nucleus. Suppression of PAPA-1 by small interfering RNA treatment enhanced the growth-promoting effect of IGFBP-2. Conversely, IGFBP-2-promoted bromodeoxyuridine incorporation into LNCaP cells was abrogated by the simultaneous overexpression of myc-hPAPA-1. Mouse embryonic fibroblasts from IGFBP-2 knockout mouse showed diminished growth activity compared with wild type, and expression of FLAG-mPAPA-1 decreased cell proliferation in IGFBP-2 knockout, but not control mouse embryonic fibroblasts. These studies suggest that the growth-promoting role of IGFBP-2 in prostate cancer is inhibited by its intracellular interaction with PAPA-1.
The growth inhibitory transcription factor PAPA-1 is shown to be a binding partner for IGFBP-2, which acts as a prostate cancer growth promoter.
The IGF family is composed of the ligands (IGF-I and -II), their receptors [IGF type I receptor (IGF-IR) and IGF-IIR], and a family of six high-affinity IGF-binding proteins (IGFBPs) and plays an important role in the regulation of cell growth (1). The IGFBPs were originally believed to regulate cell proliferation by sequestering IGFs. However, in addition to the modulation of IGF action, diverse IGF-independent effects on cellular function have been identified for many of the IGFBPs that can be both growth promoting and inhibitory (2).
IGFBP-2 is the second most abundant IGFBP in the circulation. High levels of IGFBP-2 in serum correlates with several types of cancer including prostate (3,4), ovarian (5), colorectal (6), and central nervous system (7). Overexpression of IGFBP-2 is proposed to play a role in carcinogenesis and tumor progression (8,9). Moreover, the increased IGFBP-2 induced by castration plays a role in the proliferation of androgen-independent prostate LNCaP xenograft tumors (10). Because IGFBP-2 has an Arg-Gly-Asp (RGD) integrin-binding motif, one of the possible molecular mechanisms of carcinogenesis promotion by IGFBP-2 is through integrin binding. Indeed, IGFBP-2 can interact with many different integrins to elicit a variety of cellular responses. For example, it can interact with α5β1-integrin in A673 Ewing’s sarcoma cells (11), α5B1 to activate cell motility in SNB19 cells (12), and αvβ3 to suppress IGF-I-mediated breast tumor migration and growth (13).
The regulation of cell growth by IGFBP-2 is highly cell specific. We have previously demonstrated an IGF-independent proliferative function of IGFBP-2, which is specific to prostate cancer cells and not normal prostate epithelial cells (14). Interestingly, IGFBP-2 has been proposed as a marker for PTEN-negative (invasive) prostate cancer (15) as well as a regulator for PTEN activity (16). A role for IGFBP-2 as a local growth factor for mononuclear cells (17), adrenal carcinoma cells (18), and DU145 human prostate cancer cells (19) has also been reported. Recently, the role of IGFBP-2 as a glioblastoma promoter has also been highlighted (20,21). In contrast, an IGF-independent proapoptotic effect of IGFBP-2 was demonstrated in the human breast cancer cell line Hs578T, which has no functional IGF-I receptor (22), and IGFBP-2 has been proposed a mediator of p53 actions in lung cancer (18).
Recent data suggest that in addition to IGFBP-3 and -5, IGFBP-2 can also be isolated in the nucleus (24,25). However, unlike the specific interactions of IGFBP-3 with nuclear receptors such as retinoid X receptor (26), any role of intranuclear IGFBP-2 in cell growth regulation remains uncharacterized. To elucidate potential nuclear roles of IGFBP-2, we performed a yeast two-hybrid screen using a human prostate cDNA library to specifically identify binding partner proteins of IGFBP-2.
Results
In vitro binding of IGFBP-2 to Pim-1-associated protein-1 (PAP-1)-associated protein-1 (PAPA-1)
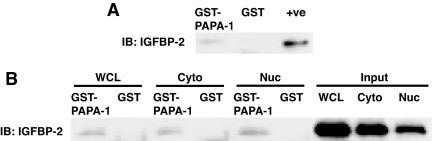
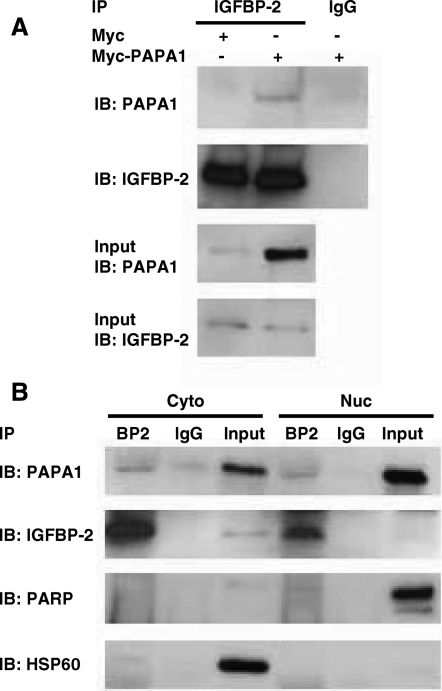
In a yeast two-hybrid screen, we isolated a 788-bp fragment corresponding to positions 377–1164 of PAPA-1 cDNA (GenBank accession no. AB054538) as an IGFBP-2-binding protein. To confirm the interaction between IGFBP-2 and PAPA-1 in vitro, we carried out both glutathione S-transferase (GST) pull-down (Fig. 1) and coimmunoprecipitation (Fig. 2) assays. Recombinant human IGFBP-2 protein was incubated with GST-PAPA-1, and a GST pull-down assay was performed. GST-PAPA-1, but not control GST, was able to interact with IGFBP-2 (Fig. 1A). To determine whether PAPA-1 can interact with endogenous intracellular IGFBP-2, we incubated GST-hPAPA-1 with LNCaP whole-cell extract, cytoplasmic and nuclear fractions, and a GST pull-down assay was performed. GST-hPAPA-1 bound to endogenous IGFBP-2 in all cell fractions was detected (Fig. 1B). The specificity of subcellular fractionation was confirmed by immunoblotting with anti-heat-shock protein 60 (anti-HSP60) or anti-poly (ADP-ribose) polymerase (anti-PARP) antibody. For further verification of the interaction, we carried out coimmunoprecipitation experiments. LNCaP cells were transfected with myc-hPAPA-1 or empty vector control. Endogenous IGFBP-2 was immunoprecipitated using rabbit antiserum anti-IGFBP-2 followed by SDS-PAGE. Interaction with PAPA-1 was then detected by immunoblotting with anti-PAPA-1 antibody (Fig. 2A). IGFBP-2 was also detected after immunoprecipitation with anti-myc tag antibody from LNCaP whole-cell lysate cotransfected with myc-hPAPA-1 and hIGFBP-2-FLAG (data not shown). We confirmed the coimmunoprecipitation in subcellular fractions of LNCaP cells transfected with myc-hPAPA-1. IGFBP-2 and PAPA-1 were coimmunoprecipitated from both the cytoplasmic and the nuclear fractions of transfected LNCaP cells, suggesting that the interaction of these proteins is not specific to the nucleus (Fig. 2B).
Figure 1.
Confirmation of IGFBP-2 and PAPA-1 interaction by GST pull down. Fifty micrograms of GST-hPAPA-1 or GST were incubated with either 25 ng recombinant human IGFBP-2 (A) or 500 μg LNCaP whole-cell extract or subcellular fraction (+ve, positive control) 4 ng rhIGFBP-2 (B). GST-bound proteins were captured by glutathione Sepharose and separated by SDS-PAGE. IGFBP-2 was identified by immunoblotting (IB) with anti-IGFBP-2. Cyto, Cytoplasmic; Nuc, nuclear; WCL, whole-cell lysate.
Figure 2.
Coimmunoprecipitation of IGFBP-2 and PAPA-1 in LNCaP cells. LNCaP cells were transfected with myc-hPAPA-1 or myc vector control. IGFBP-2 bound to PAPA-1 was coimmunoprecipitated from 500 μg whole-cell extract (A) or subcellular fractions (B) by immunoprecipitation (IP) with IGFBP-2 or IgG control antibodies followed by immunoblotting (IB) for PAPA-1. Validity of fractionation was confirmed by immunoblot for HSP60 (cytoplasmic fraction, Cyto) and PARP (nuclear fraction, Nuc). Input refers to 20 μg whole-cell lysate or subcellular fraction used for coimmunoprecipitations, as described.
Colocalization of IGFBP-2 and PAPA-1
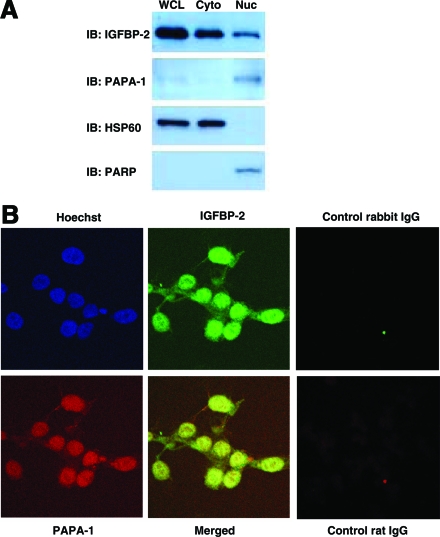
Once the physical interaction between PAPA-1 and IGFBP-2 had been confirmed, we investigated whether the two proteins colocalize within the cell. First, we analyzed the subcellular localization of IGFBP-2 and PAPA-1 by immunoblot after subcellular fractionation into nuclear and cytoplasmic fractions (Fig. 3A). IGFBP-2 was detected in both the cytoplasmic and the nuclear fractions. In contrast, PAPA-1 was detected mainly in the nuclear fraction. We also analyzed the intracellular localization of endogenous IGFBP-2 and PAPA-1 by confocal microscopy (Fig. 3B). IGFBP-2 was detected throughout the cell. In contrast, the localization of PAPA-1 was predominantly nuclear. When the images were merged, clear colocalization of IGFBP-2 and PAPA-1 was shown in the nuclei.
Figure 3.
Nuclear colocalization of IGFBP-2 and PAPA-1 in LNCaP cells. A, The subcellular localization of endogenous IGFBP-2 and PAPA-1 was detected by immunoblotting (IB) in 20 μg LNCaP whole-cell extract and cytoplasmic (Cyto) and nuclear (Nuc) fractions. The validity of subcellular fractionation was confirmed by immunoblotting for Hsp60 (cytoplasmic) and PARP (nuclear). WCL, Whole-cell lysate. B, The localization of endogenous IGFBP-2 and PAPA-1 was observed by confocal microscopy.
Suppression of PAPA-1 enhances the growth-promoting effect of IGFBP-2
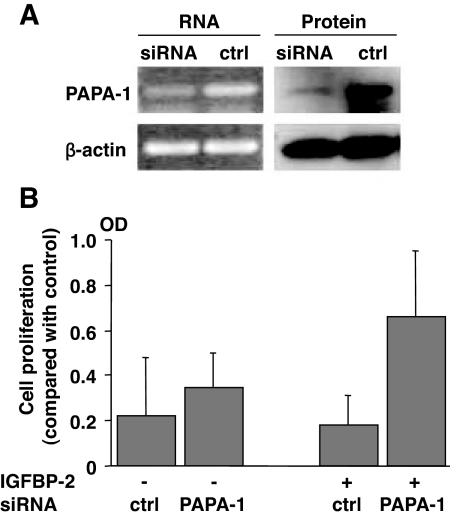
To investigate the functional significance of the interaction, we used small interfering RNA (siRNA) against PAPA-1. SiRNA against hPAPA-1 specifically suppressed PAPA-1 expression at both the mRNA and protein levels (Fig. 4A). Next, we evaluated the effect of PAPA-1 knockdown on IGFBP-2-induced cell proliferation. Although PAPA-1 gene knockdown did not lead to a significant increase in cell viability/proliferation, reducing PAPA-1 expression with siRNA potentiated IGFBP-2-induced cell proliferation relative to control oligonucleotide (Fig. 4B).
Figure 4.
Knockdown of PAPA-1 expression enhances IGFBP-2-induced proliferation. Endogenous PAPA-1 levels were decreased in LNCaP cells by transfection with PAPA-1 siRNA. A, Analysis of mRNA and protein by RT-PCR and Western blot, respectively. β-Actin was simultaneously evaluated as a loading control (ctrl). B, Cell viability/proliferation was assessed by the CellTiter 96 AQueous One Cell Proliferation ELISA after 72 h in serum-containing media (n = 3).
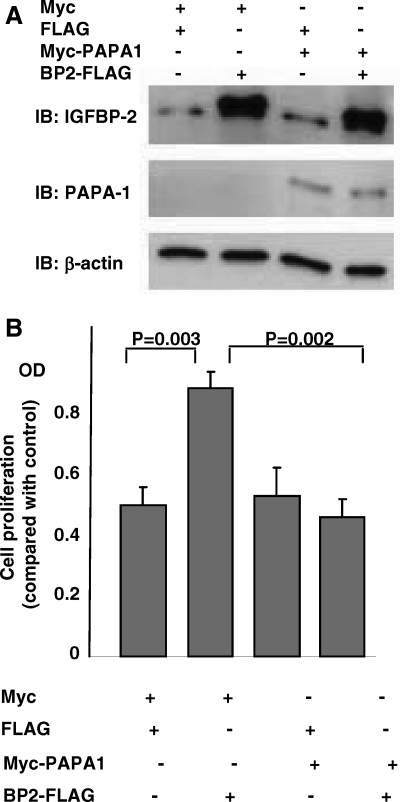
To further investigate the relationship between IGFBP-2 and PAPA-1, we transfected LNCaP cells with myc-hPAPA-1 and/or hIGFBP-2-FLAG and evaluated cell proliferation by bromodeoxyuridine (BrdU) incorporation. Myc-hPAPA-1 and hIGFBP-2-FLAG were both successfully overexpressed after transfection (Fig. 5A). Overexpression of hIGFBP-2-FLAG promoted BrdU incorporation into LNCaP cells (P = 0.003) (Fig. 5B). However, this proliferative effect was completely abrogated by the simultaneous expression of myc-hPAPA-1.
Figure 5.
Overexpression of PAPA-1 inhibits IGFBP-2-induced cell proliferation. LNCaP cells were transiently transfected with combinations (as indicated) of control vector, myc-PAPA-1, or FLAG-IGFBP-2. A, Success of transfection was confirmed by immunoblotting (IB) for PAPA-1, IGFBP-2, and β-actin (loading control). Endogenous PAPA-1 is not seen in this image. β-Actin was used as a loading control. B, Cell proliferation was assessed by BrdU incorporation after 24 h in SF media (n = 4).
IGFBP-2 abrogates the growth-inhibitory effect of PAPA-1
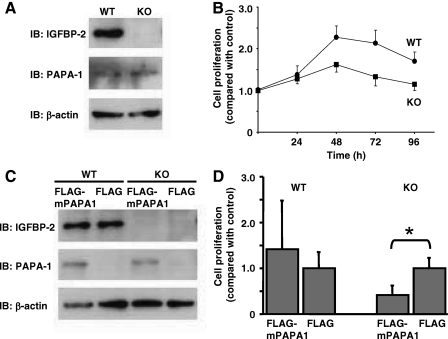
To confirm the functional link between IGFBP-2 and PAPA-1, we obtained mouse embryonic fibroblasts (MEFs) derived from the IGFBP-2 knockout mouse (27,28). Both IGFBP-2 knockout and control MEFs expressed PAPA-1; however, only the control MEFs expressed IGFBP-2 (Fig. 6A). As expected due to the proliferative role of IGFBP-2, knockout MEFs had a slower proliferation rate than wild-type controls (Fig. 6B). Overexpression of PAPA-1 in wild-type MEFs had no significant effect on the cell proliferation, as assessed by BrdU incorporation. In contrast, PAPA-1 inhibited the proliferation of MEFs derived from the IGFBP-2 knockout mouse (P < 0.001, Fig. 6D), suggesting that IGFBP-2 abrogates the growth-inhibitory effect of PAPA-1.
Figure 6.
PAPA-1 inhibition of cell proliferation is IGFBP-2 dependent. A, The expression of IGFBP-2, PAPA-1, and β-actin (loading control) in IGFBP-2 knockout and wild-type MEFs was assessed by immunoblotting (IB). B, Cell proliferation of IGFBP-2 knockout and wild-type MEFs was compared after 96 h by MTS assay. C, Expression of IGFBP-2, PAPA-1, and β-actin (loading control) was assessed in MEFs transiently transfected with FLAG-PAPA-1 or empty vector control. D, BrdU incorporation as a measure of cell proliferation was assessed in MEFs transfected as in C. Data are presented compared with control, means ± sd. *, Significance that mean is different from 1: P < 0.05.
Discussion
We cloned PAPA-1 as a novel partner protein of IGFBP-2. IGFBP-2 and PAPA-1 were colocalized predominantly in the nucleus, and the interaction between IGFBP-2 and PAPA-1 was confirmed by both GST pull downs and immunoprecipitation in vitro.
PAPA-1 was originally identified in HeLa nuclear extracts as a binding partner for PAP-1, a protein phosphorylated by Pim-1 (29,30). Ectopic expression of PAPA-1 was reported to inhibit cell growth by causing cell cycle arrest, an effect dependent on its nucleolar localization. Consistent with these data, we observed inhibition of IGFBP-2-induced cell proliferation by PAPA-1, although PAPA-1 expression alone had no significant effects of cell growth or viability. Interestingly, we observed PAPA-1 localization throughout the entire nucleus of LNCaP cells rather than specific nucleolar expression. This suggests that PAPA-1 may exert effects that are cell-type or localization specific and provides a possible explanation as to why PAPA-1 alone had no effect in LNCaP cells.
Although very little is known about the biological function of PAPA-1, the activator of its nucleolar binding protein is Pim-1, a protooncogene commonly used as a prognostic marker in prostate cancer. This suggests a link between this group of proteins and prostate carcinogenesis and suggests that regulation of IGFBP-2 action by PAPA-1 may be an important mechanism in the control of cell growth within the prostate. Such a role is supported by our observation that the expression of PAPA-1 is regulated by androgens. Androgen treatment decreased the expression of total and cytoplasmic PAPA-1 and IGFBP-2 in a dose- and time-dependent manner. Interestingly, however, nuclear PAPA-1 and IGFBP-2 levels were relatively maintained after androgen treatment, suggesting that it is cytoplasmic, and not nuclear, expression that is specifically regulated by androgens.
The nuclear localization of IGFBP-3 and -5 is well established and occurs via importin-β and a consensus nuclear localization signal (31,32). Although there have been reports of perinuclear and nuclear IGFBP-2 (24,25), the mechanism of its nuclear uptake remains to be elucidated because IGFBP-2 does not possess a classical nuclear localization signal. However, because nonclassical nuclear localization signals have also been identified (33), the lack of a classical nuclear localization signal in the sequence of IGFBP-2 does not rule out its specific intracellular targeting. The generation of a nonnuclear IGFBP-2 would be a useful tool in studying IGFBP-2-PAPA-1 interactions, particularly in determining whether the interaction occurs in the nucleus or cytoplasm.
In addition to integrins, several IGFBP-2-binding proteins have been identified. Invasion inhibitory protein 45 (IIp45), identified as a binding partner by yeast two-hybrid screening from a human fetal brain cDNA library, inhibits IGFBP-2-stimulated glioma cell invasion (34). Cyclin-dependent kinase inhibitor p21CIP1/WAF1 was demonstrated to interact with IGFBP-2 in mouse lung epithelial MLE-12 cells (23). Intriguingly, p21 and IGFBP-2 colocalized in growth-arrested cells, and IGFBP-2 secretion and nuclear localization were increased upon p21 induction, suggesting that IGFBP-2 may have cell-specific and opposing actions on cell proliferation. Importantly, each of these partner proteins, including PAPA-1, plays a role in the inhibition of cell growth. In our study, we demonstrated the stimulation of cell proliferation by IGFBP-2 and revealed that the growth-promoting effect of IGFBP-2 is antagonized by PAPA-1. Taken with previous data, this would suggest that the proliferative actions of IGFBP-2 in many cell types is tightly regulated by its interaction with numerous cell factors, which may occur in a cell- or tissue-specific manner.
Materials and Methods
Reagents
Human PAPA-1 cDNA, FLAG-mPAPA-1 vector, and rat anti-mPAPA-1 monoclonal antibody, which recognizes both human and mouse PAPA-1, were generously provided by Prof. Ariga (Hokkaido University, Japan). Charcoal dextran-treated fetal bovine serum was purchased from Omega Scientific, Inc. (Tarzana, CA), and other cell culture reagents were from Invitrogen (Carlsbad, CA). Goat antihuman and antimouse IGFBP-2 antibodies were purchased from R&D Systems, Inc. (Minneapolis, MN); anti-IGFBP-2 (rabbit antiserum) was from Upstate (Lake Placid, NY), and rabbit anti-Myc tag polyclonal antibody was from Cell Signaling Technology (Danvers, MA). The mouse monoclonal anti-HSP60, mouse monoclonal anti-PARP, and mouse monoclonal anti-β-actin antibodies were purchased from Sigma (St. Louis, MO). Anti-TATA-binding protein antibody was purchased from Abcam (Cambridge, MA). Fluorescein-conjugated AffiniPure donkey antirabbit IgG and Texas Red dye-conjugated AffiniPure donkey antirat IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). R-1881 (methyltrienolone) was purchased from PerkinElmer Life Sciences (Wellesley, MA). Recombinant human IGFBP-2 protein was purchased from GroPep (Adelaide, Australia). SiGENOME SMARTpool reagent (siRNA for hPAPA-1 gene) and siCONTROL nontargeting siRNA pool were purchased from Dharmacon, Inc. (Lafayette, CO).
Yeast two-hybrid screen
A yeast two-hybrid screen was performed according to the Clontech Yeast Protocols Handbook for the MATCHMAKER GAL4 Two-Hybrid System 3 (Clontech, Mountain View, CA). Briefly, cDNA encoding mature human IGFBP-2 was inserted into the EcoRI-BamHI sites of the vector pGBKT7 containing the GAL-4 DNA-binding domain (pGBKT7-hIGFBP-2). A human prostate Matchmaker cDNA library with the activation domain of the GAL4 gene (pACT2-X) was screened by cotransforming yeast (AH109 strain) with both plasmids. Positive clones were identified under high-stringency conditions and were defined as clones that exhibited growth on the amino acid-deficient selective media: Trp-negative, Leu-negative, His-negative, Ade-negative; and were also positive for galactosidase activity. After confirmation under the same high-stringency conditions, genes encoding IGFBP-2-binding proteins were isolated by plasmid recovery followed by Escherichia coli transformation (ElectroMAX DH5α-E) (Invitrogen). Isolated plasmids were finally sequenced and compared with known sequences in GenBank by BLAST search.
Cell culture
The LNCaP human prostate cancer cell line was cultured in RPMI 1640 medium enriched with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Only cells with a passage number lower than P25 were used for experiments. MEF cells were cultured in DMEM containing 10% newborn calf serum. Only cells younger than P5 were used in experiments.
Whole-cell lysates
Cells incubated and treated as indicated were harvested into lysis buffer containing 20 mm Tris-HCl (pH 7.5), 140 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA (pH 8.0), 1% Triton X-100, and 10% glycerol with protease inhibitor cocktail set III (Calbiochem, San Diego, CA) and phosphatase inhibitor cocktail set II (Calbiochem). Lysates were sonicated two times at output 5 for 10 sec and centrifuged at 14,000 rpm for 30 min. The supernatant was taken as whole-cell lysate.
Cell fractionation
The cells were fractionated into the cytoplasmic and the nuclear fractions using CelLytic NuCLEAR extraction kit (Sigma, St. Louis, MO) following the manufacturer’s instructions. The validity of separation was determined by the following immunoblot with anti-HSP60 antibody for the cytoplasmic fraction and anti-PARP or anti-TATA-binding protein for the nuclear fraction.
Western blot
Cell lysates separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane. Membranes were blocked with 0.2% I-Block (Applied Biosystems, Foster City, CA) in PBS containing 0.1% Tween 20 for 3 h at room temperature and then probed with the appropriate primary and secondary antibodies. Antibody-antigen complexes were visualized by Western Lightning chemiluminescence reagents (PerkinElmer Life and Analytical Sciences, Inc., Waltham, MA) and autoradiography.
GST pull-down assay
The pGEX4T1-hPAPA-1 vector encoding GST-hPAPA-1 fusion protein was generated by inserting hPAPA-1 cDNA into the EcoRI-NotI site of the vector pGEX4T1 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). E. coli BL21 (DE3) were transformed with pGEX4T1-hPAPA-1 vector or pGEX4T1 empty vector. After induction with 1 mm isopropyl β-d-1-thiogalactopyranoside (GIBCO BRL, Grand Island, NY) overnight at 22 C, bacteria were disrupted by sonication in 10 ml PBS containing 1 mm dithiothreitol, 1 mm EDTA, and protease inhibitor cocktail Set III (Calbiochem, San Diego, CA). GST fusion proteins were isolated from the supernatant using glutathione Sepharose 4B (GE Healthcare Bio-Sciences) and eluted four times in 0.3% reduced glutathione (GIBCO BRL). Finally, the proteins were dialyzed against the elution buffer [50 mm Tris-HCl (pH 7.5), 5 mm MgCl2, 100 mm NaCl, 10% glycerol, 5 mm β-mercaptoethanol] in Slide-A-Lyzer dialysis cassette (extra strength) (Pierce, Rockford, IL).
Fifty micrograms of GST-hPAPA-1 fusion protein or GST alone were mixed with 25 ng recombinant human IGFBP-2 protein or 500 μg LNCaP whole-cell lysate or subcellular fraction. GST-hPAPA-1 fusion protein and GST were captured by incubation with glutathione Sepharose and then eluted by 25 μl eluting solution (10 mm glutathione in elution buffer). IGFBP-2 protein bound to GST-hPAPA-1 fusion protein was analyzed by Western blot.
Coimmunoprecipitation
Myc-hPAPA-1 vector was generated by inserting hPAPA-1 cDNA into the EcoRI-KpnI site of the vector pCMV-myc. LNCaP cells were transiently transfected using LipofectAMINE 2000 transfection reagent (Invitrogen) following the manufacturer’s instructions. For immunoprecipitations, 500 μg whole-cell lysate or subcellular fractions were pretreated with protein A agarose Fast Flow (Upstate, Lake Placid, NY) in 500 μl nonreducing immunoprecipitation buffer [50 mm Tris-HCl (pH 7.5), 5 mm MgCl2, 100 mm NaCl, 10% glycerol] for 1 h at 4 C and incubated with 10 μl anti-IGFBP-2 (rabbit antiserum; Upstate) overnight at 4 C. One hundred microliters of 50% slurry protein A agarose Fast Flow were added, and the samples were rotated for 1 h at 4 C. After washing, coimmunoprecipitated proteins were eluted by boiling in Laemmli sample buffer and analyzed by Western blot.
Immunofluorescence confocal microscopy
LNCaP cells (4 × 104 cells) were plated onto the four-chamber plate and cultured for 48 h. Cells were fixed in 1% paraformaldehyde for 15 min at room temperature and permeabilized in 0.2% Triton X-100 in PBS for 15 min. Cells were incubated with anti-IGFBP-2 (1:200 dilution, rabbit antiserum) followed by rat anti-mPAPA-1 (1:250) monoclonal antibody, each for 1 h at room temperature. Cells were incubated with fluorescein-conjugated AffiniPure donkey antirabbit IgG and Texas Red dye-conjugated AffiniPure donkey antirat IgG for 40 min at room temperature. Hoechst (10 μg/ml) was used for the nuclear staining. The stained cells were analyzed by using an Upright confocal microscope operated by Leica Confocal Software.
siRNA treatment
LNCaP cells at 30–50% confluent status were transfected with siGENOME SMARTpool reagent (siRNA for hPAPA-1 gene) or siCONTROL nontargeting siRNA pool using LipofectAMINE 2000 transfection reagent following the manufacturer’s instructions. Cells were harvested 72 h after transfection. The efficacy of the knockdown of the gene was evaluated at mRNA level by RT-PCR. Briefly, the cDNA was synthesized from total RNA by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) following the manufacturer’s instructions. RT-PCR for hPAPA-1 was performed with a primer set of 5′-tggttgtggataatgaagagg-3′ and 5′-agcagagctcgctgtcgagc-3′. DNA was denatured initially at 94 C for 5 min and cycling conditions were as follows: 94 C for 1 min, 63 C for 1 min, and 72 C 1 min for a total of 30 cycles. Final extension was allowed to proceed at 72 C for 10 min. RT-PCR for β-actin as control was also performed with a primer set of 5′-caccttctacaatgagctgc-3′ and 5′-aaggtagtttcgtggatgcc-3′. The PCR products were subjected to electrophoresis on a 1% agarose gel. Knockdown at protein level was evaluated by Western blot as described above.
To assess cell proliferation after siRNA treatment, 5000 LNCaP cells were plated on a 96-well plate and were cultured for 1 d to attach to the plate. On the next day, cells were transfected with siGENOME SMARTpool reagent (siRNA for hPAPA-1 gene) or siCONTROL nontargeting siRNA pool as described above and incubated for 24 h. Culture media were changed to serum-free media with or without 10 nm androgen for 24 h before 48 h incubation with 1 μg/ml recombinant human IGFBP-2. The cell viability/proliferation was analyzed by CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) as described below.
Cell proliferation assay
To assess cell viability/proliferation, cells growing in 96-well plates were treated as appropriate and analyzed by CellTiter 96 AQueous One Solution Cell Proliferation Assay following the manufacturer’s instructions. The data were analyzed as an average of three samples in each experiment.
BrdU cell proliferation assay of LNCaP
BrdU incorporation in to cells growing on 96-well plates and treated as appropriate was assessed using BrdU Cell Proliferation Assay (Calbiochem, San Diego, CA) following the manufacturer’s instructions. The data were analyzed as an average of five samples in each experiment.
Statistical analysis
Data were statistically analyzed by unpaired t test using StatView software and were presented as mean ± sd. Differences were considered statistically significant when P < 0.05.
Footnotes
This work was supported in part by the Grants 1R01CA100938 and P50CA92131 from the National Institutes of Health, and Department of Defense (DOD) Grant PC050485 (to P.C.) and a DOD fellowship award to L.C.
Disclosure Summary: None of the authors have any to declare.
First Published Online December 18, 2008
Abbreviations: BrdU, Bromodeoxyuridine; GST, glutathione S-transferase; HSP60, heat-shock protein 60; IGFBP, IGF-binding protein; IGF-IR, IGF type I receptor; MEF, mouse embryonic fibroblast; PAP, Pim-1-associated protein-1; PAPA-1, PAP-1-associated protein-1; PARP, poly (ADP-ribose) polymerase; siRNA, small interfering RNA.
References
- Wetterau LA, Moore MG, Lee KW, Shim ML, Cohen P 1999 Novel aspects of the insulin-like growth factor binding proteins. Mol Genet Metab 68:161–181 [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC 2002 Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854 [DOI] [PubMed] [Google Scholar]
- Ho PJ, Baxter RC 1997 Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol (Oxf) 46:333–342 [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG 1993 Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab 76:1031–1035 [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS 1997 Elevated serum insulin-like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab 82:2308–2313 [DOI] [PubMed] [Google Scholar]
- el Atiq F, Garrouste F, Remacle-Bonnet M, Sastre B, Pommier G 1994 Alterations in serum levels of insulin-like growth factors and insulin-like growth-factor-binding proteins in patients with colorectal cancer. Int J Cancer 57:491–497 [DOI] [PubMed] [Google Scholar]
- Muller HL, Oh Y, Lehrnbecher T, Blum WF, Rosenfeld RG 1994 Insulin-like growth factor-binding protein-2 concentrations in cerebrospinal fluid and serum of children with malignant solid tumors or acute leukemia. J Clin Endocrinol Metab 79:428–434 [DOI] [PubMed] [Google Scholar]
- Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemisto A, Kavanagh JJ, Lee JH, Zhang W 2005 Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer 4:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Zhu XQ, Zhao GH, Xia YB, Zhang YS 2006 Expression of insulin-like growth factor binding protein-2 in gastric carcinoma and its relationship with cell proliferation. World J Gastroenterol 12:6285–6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama S, Morrison K, Zellweger T, Akbari M, Cox M, Yu D, Miyake H, Gleave ME 2003 Castration-induced increases in insulin-like growth factor-binding protein 2 promotes proliferation of androgen-independent human prostate LNCaP tumors. Cancer Res 63:3575–3584 [PubMed] [Google Scholar]
- Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW 2004 Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol 32:859–868 [DOI] [PubMed] [Google Scholar]
- Wang GK, Hu L, Fuller GN, Zhang W 2006 An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin α5 is essential for IGFBP2-induced cell mobility. J Biol Chem 281:14085–14091 [DOI] [PubMed] [Google Scholar]
- Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, Rossjohn J, Price JT 2004 Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with αvβ3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res 64:977–984 [DOI] [PubMed] [Google Scholar]
- Moore MG, Wetterau LA, Francis MJ, Peehl DM, Cohen P 2003 Novel stimulatory role for insulin-like growth factor binding protein-2 in prostate cancer cells. Int J Cancer 105:14–19 [DOI] [PubMed] [Google Scholar]
- Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL 2007 Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci USA 104:5563–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM 2007 IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene 26:5966–5972 [DOI] [PubMed] [Google Scholar]
- Hettmer S, Dannecker L, Foell J, Elmlinger MW, Dannecker GE 2005 Effects of insulin-like growth factors and insulin-like growth factor binding protein-2 on the in vitro proliferation of peripheral blood mononuclear cells. Hum Immunol 66:95–103 [DOI] [PubMed] [Google Scholar]
- Shi Z, Henwood MJ, Bannerman P, Batista D, Horvath A, Guttenberg M, Stratakis CA, Grimberg A 2007 Primary pigmented nodular adrenocortical disease reveals insulin-like growth factor binding protein-2 regulation by protein kinase A. Growth Horm IGF Res 17:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Park ES, Soloff MS 2004 Proliferation of DU145 prostate cancer cells is inhibited by suppressing insulin-like growth factor binding protein-2. Int J Urol 11:876–884 [DOI] [PubMed] [Google Scholar]
- Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H 2007 Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem 282:18634–18644 [DOI] [PubMed] [Google Scholar]
- Dunlap SM, Celestino J, Wang H, Jiang R, Holland EC, Fuller GN, Zhang W 2007 Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci USA 104:11736–11741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer KW, Reichenmiller K, Schutt BS, Hoeflich A, Ranke MB, Dodt G, Elmlinger MW 2006 IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol 37:13–23 [DOI] [PubMed] [Google Scholar]
- Terrien X, Bonvin E, Corroyer S, Tabary O, Clement A, Henrion Caude A 2005 Intracellular colocalization and interaction of IGF-binding protein-2 with the cyclin-dependent kinase inhibitor p21CIP1/WAF1 during growth inhibition. Biochem J 392:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich A, Reisinger R, Schuett BS, Elmlinger MW, Russo VC, Vargas GA, Jehle PM, Lahm H, Renner-Muller I, Wolf E 2004 Peri/nuclear localization of intact insulin-like growth factor binding protein-2 and a distinct carboxyl-terminal IGFBP-2 fragment in vivo. Biochem Biophys Res Commun 324:705–710 [DOI] [PubMed] [Google Scholar]
- Besnard V, Corroyer S, Trugnan G, Chadelat K, Nabeyrat E, Cazals V, Clement A 2001 Distinct patterns of insulin-like growth factor binding protein (IGFBP)-2 and IGFBP-3 expression in oxidant exposed lung epithelial cells. Biochim Biophys Acta 1538:47–58 [DOI] [PubMed] [Google Scholar]
- Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P 2000 Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem 275:33607–33613 [DOI] [PubMed] [Google Scholar]
- Wood TL, Rogler LE, Czick ME, Schuller AG, Pintar JE 2000 Selective alterations in organ sizes in mice with a targeted disruption of the insulin-like growth factor binding protein-2 gene. Mol Endocrinol 14:1472–1482 [DOI] [PubMed] [Google Scholar]
- Demambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ 2008 Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology 149:2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda TS, Maita H, Tabata T, Taira T, Kitaura H, Ariga H, Iguchi-Ariga SM 2004 A novel nucleolar protein, PAPA-1, induces growth arrest as a result of cell cycle arrest at the G1 phase. Gene 340:83–98 [DOI] [PubMed] [Google Scholar]
- Maita H, Harada Y, Nagakubo D, Kitaura H, Ikeda M, Tamai K, Takahashi K, Ariga H, Iguchi-Ariga SM 2000 PAP-1, a novel target protein of phosphorylation by pim-1 kinase. Eur J Biochem 267:5168–5178 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC 2000 Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin β-subunit. J Biol Chem 275:23462–23470 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Young TF, Firth SM, Baxter RC 1998 Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem 273:18347–18352 [DOI] [PubMed] [Google Scholar]
- Christophe D, Christophe-Hobertus C, Pichon B 2000 Nuclear targeting of proteins: how many different signals? Cell Signal 12:337–341 [DOI] [PubMed] [Google Scholar]
- Song SW, Fuller GN, Khan A, Kong S, Shen W, Taylor E, Ramdas L, Lang FF, Zhang W 2003 IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci USA 100:13970–13975 [DOI] [PMC free article] [PubMed] [Google Scholar]