Abstract
Recent evidence suggests that a rare population of self-renewing cancer stem cells (CSC) is responsible for cancer progression and therapeutic resistance. Chronic myeloid leukemia (CML) represents an important paradigm for understanding the genetic and epigenetic events involved in CSC production. CML progresses from a chronic phase (CP) in hematopoietic stem cells (HSC) that harbor the BCR-ABL translocation, to blast crisis (BC), characterized by aberrant activation of β-catenin within granulocyte-macrophage progenitors (GMP). A major barrier to predicting and inhibiting blast crisis transformation has been the identification of mechanisms driving β-catenin activation. Here we show that BC CML myeloid progenitors, in particular GMP, serially transplant leukemia in immunocompromised mice and thus are enriched for leukemia stem cells (LSC). Notably, cDNA sequencing of Wnt/β-catenin pathway regulatory genes, including adenomatous polyposis coli, GSK3β, axin 1, β-catenin, lymphoid enhancer factor-1, cyclin D1, and c-myc, revealed a novel in-frame splice deletion of the GSK3β kinase domain in the GMP of BC samples that was not detectable by sequencing in blasts or normal progenitors. Moreover, BC CML progenitors with misspliced GSK3β have enhanced β-catenin expression as well as serial engraftment potential while reintroduction of full-length GSK3β reduces both in vitro replating and leukemic engraftment. We propose that CP CML is initiated by BCR-ABL expression in an HSC clone but that progression to BC may include missplicing of GSK3β in GMP LSC, enabling unphosphorylated β-catenin to participate in LSC self-renewal. Missplicing of GSK3β represents a unique mechanism for the emergence of BC CML LSC and might provide a novel diagnostic and therapeutic target.
Keywords: blast crisis chronic myeloid leukemia, wnt pathway, xenograft, self-renewal, cancer stem cells
Chronic myeloid leukemia (CML) was the first cancer shown to be initiated at the hematopoietic stem cell (HSC) level by a pathognomonic chromosomal abnormality the Philadelphia chromosome, which produces a constitutively active protein tyrosine kinase —P210BCR-ABL (1–6). CML was also the first malignancy treated with a molecularly targeted agent imatinib, which inhibits the BCR-ABL tyrosine kinase (3). However, most CML patients treated with BCR-ABL inhibitors harbor cells with low-level BCR-ABL transcripts, suggesting that these cells may be susceptible to further transforming events that promote relapse (3).
Several studies indicate that relapse and disease progression derive from a rare population of cancer stem cells (CSC), the only cells within the cancer that can recapitulate the tumor in transplant models (1, 7–16). Recent evidence suggests that CSC are generated by a sequence of heritable events, both epigenetic and via mutations that alter progenitor self-renewal, survival, and differentiation (16). To date, CSC have been identified in human acute myelogenous leukemia (AML), breast cancer, several brain tumors, head and neck squamous cell carcinomas, pancreatic, and colon cancer (1, 7–16). Preliminary studies suggest that CSC of the hematopoietic system, also called leukemia stem cell (LSC), have subverted the properties normally ascribed to HSC such as self-renewal capacity (1, 9–11, 16). By studying 100 CML blood and marrow samples, we previously discovered that a cell population sharing the same immunophenotype as granulocyte-macrophage progenitors (GMP) expressed high levels of BCR-ABL and had activated the Wnt/β-catenin self-renewal pathway (1). Candidate LSC had enhanced replating capacity, an in vitro surrogate measure of self-renewal potential that was inhibited by a specific Wnt pathway antagonist-axin (1).
Here we studied (i) the capacity of candidate blast crisis (BC) LSC to self-renew in immunocompromised mice and (ii) the mechanisms driving Wnt/β-catenin self-renewal pathway activation.
Results
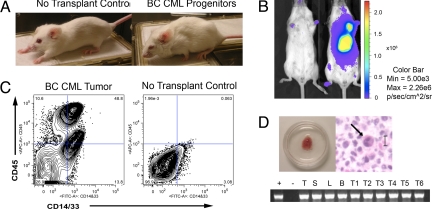
We developed an in vivo bioluminescent BC LSC model via intrahepatic transplantation of candidate BC LSC transduced with lentiviral luciferase (GLF) into neonatal immunocompromised (RAG2−/−γc−/−) mice (17). Controls included CML HSC and blast (Lin+) cells, as well as normal HSCs and progenitors (Tables S1 and S2A). Weekly in vivo bioluminescence imaging demonstrated enhanced engraftment of GLF-transduced BC progenitors (CD34+CD38+Lin−) which, compared to CD34+CD38−Lin− cells, yielded twice as many myeloid progenitors, prominent myeloid engraftment in all hematopoietic tissues, and 1.7-fold higher β-catenin expression (Figs. S1 and S2a). Moreover, mice transplanted with lentiviral luciferase (GLF)-transduced BC CML progenitors developed signs of leukemia, including wasting, piloerection, and lethargy (Fig. 1A). These mice expressed BCR-ABL in all hematopoietic tissues and formed bioluminescent BCR-ABL+ myeloid cell-enriched tumors typical of BC granulocytic sarcomas (Fig. 1 B, C, and D). While only one tumor formed in mice (n = 8) transplanted with the HSC enriched 34+38−Lin− fraction, 9 tumors arose in mice (n = 15) transplanted with BC CML progenitors and no tumors arose in mice (n = 4) transplanted with Lin+ cells (Fig. S2b).
Fig. 1.
BC CML progenitors (CD34+CD38+Lin−) transplant leukemia. (A) Mice transplanted with progenitors show signs of leukemia including wasting, piloerection, and lethargy by 6 weeks posttransplantation. (B) Transplantation of progenitors resulted in prominent tumor bioluminescence as demonstrated at 7 weeks posttransplantation. (C) FACS analysis of tumors derived from BC CML progenitors demonstrated 87.4% human engraftment consisting of 29% human myeloid cells (n = 3 experiments). (D Upper) Tumor derived from a mouse transplanted with 1 × 105 progenitor cells. Hematoxylyn-eosin-stained tumor tissue revealed prominent infiltration with human myeloid cells as typified by the human immature granulocyte characteristic of a BC CML granulocytic sarcoma. (Lower) RT-PCR P210 BCR-ABL analysis of hematopoietic tissues including thymus (T), spleen (S), liver (L), bone marrow (B) and tumors (T1-T6) from CD34+CD38+ transplanted mice (n = 4).
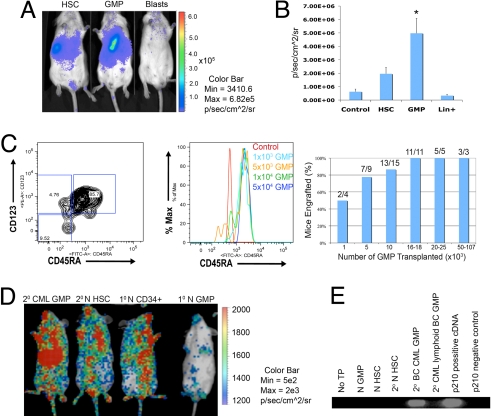
Because the progenitor fraction of BC CML blood and marrow is composed primarily of GMP, we examined whether this population also produces leukemia (1). Notably, myeloid BC GMP promoted engraftment more frequently than BC HSC and blasts (Fig. S3). Normal human HSC populations also engrafted long-term, whereas normal committed progenitors, including GMP, did not (Fig. S3). These results suggested that self-renewing LSC were enriched within the GMP fraction of myeloid BC CML. To further investigate the leukemic transplantation capacity of BC HSC, GMP, or blasts, weekly in vivo bioluminescence imaging was performed following GLF-transduction and transplantation (Fig. 2A). In these studies, BC GMP (LSC) showed significantly greater engraftment capacity by bioluminescence than BC HSC or blasts compared with untransplanted controls (Fig. 2B). Secondary BC CML progenitor transplantation (n = 9) gave rise to a preponderance of GMP (Fig. 2C). Subsequent titration experiments revealed that as few as 1,000 BC GMP were sufficient to engraft 50% of transplanted mice while approximately 10-fold more cells resulted in leukemic engraftment in the majority of transplant recipients (Fig. 2C). Self-renewal potential was assessed by serially transplanting human CD45+ cells derived from hematopoietic tissues of RAG2−/−γc−/− mice transplanted with hematopoietic subpopulations (17). Serial transplantation capacity of BC GMP was compared with that of BC HSC and blasts as well as normal GMP and HSC. Both normal HSC and BC GMP demonstrated serial engraftment potential that was absent in committed normal progenitors (Fig. 2D). These experiments suggest that BCR-ABL expressing-BC GMP aberrantly gain self-renewal capacity resulting in LSC generation (Fig. 2E).
Fig. 2.
Leukemia stem cells are enriched in the BC GMP population. (A) In 8 experiments involving normal bone marrow or cord blood (n = 30 mice) and 12 BC CML experiments (n = 43 mice) equivalent numbers (103-4 × 105) of HSC, progenitor and blast (Lin+) cells per experiment were transplanted. Bioluminescence imaging demonstrated that BC CML GMP had the greatest engraftment potential. (B) In 3 experiments, quantitative bioluminescence engraftment analysis demonstrated that GMP had a higher level of bioluminescence (P = 0.02; asterisk in figure; two-tailed Student's t test) than HSC (P = 0.06) or Lin+ (P = 0.35). (C Left) FACS analysis of tumors (n = 9) from mice transplanted with 2° BC CML progenitors demonstrated a preponderance of GMP (66.4%; P = 8.6 × 10−9; two-tailed Student's t test) while common myeloid progenitors (CMP) (16.3%) and megakaryocyte-erythroid progenitors (MEP) (2.2%) represented a minority of cells. (Middle) Tertiary (3°) BC CML GMP transplantation of 1 × 103, 5 × 103, 1 × 104 and 5 × 104 resulted in engraftment of a CD45RA positive progenitor population in transplanted mice (n = 3 experiments). (Right) Graph of 3° BC CML GMP titration experiments. (D) Bioluminescence imaging was performed 9 weeks posttransplantation and demonstrated that both 2° normal HSC and 2° BC CML GMP (n = 6 mice) had long-term engraftment capacity but 1° normal GMP did not. Primary normal cord blood CD34+ cells served as a positive control for engraftment. (E) RT-PCR analysis of P210 BCR-ABL expression in livers from transplanted mice revealed that 2° myeloid BC GMP harbored P210 BCR-ABL transcripts. There were no detectable BCR-ABL transcripts in mice that were untransplanted or those that were transplanted with normal GMP, normal HSC, 2° normal HSC, or lymphoid BC GMP from P190 BCR-ABL-expressing marrow.
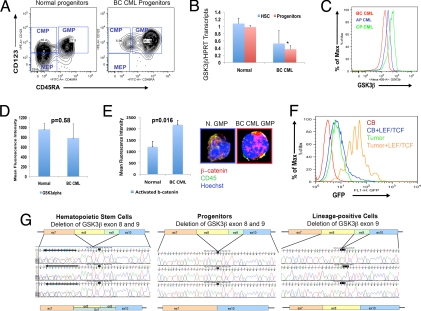
Previous research demonstrated activation of components of the Wnt/β-catenin self-renewal pathway in CML progenitors during progression to myeloid BC (1, 6). Thus, we sought possible molecular mechanisms driving β-catenin activation and expansion of the BC GMP pool (Fig. 3A). Quantitative RT-PCR analysis of key Wnt/β-catenin pathway gene transcript levels revealed a significant decrease in glycogen synthase kinase 3β (GSK3β) transcripts in BC CML progenitors compared with their normal counterparts (Fig. 3B). Moreover, FACS analysis demonstrated decreased GSK3β protein expression by CML progenitors during progression from chronic phase (CP) and accelerated phase (AP) to myeloid BC (Fig. 3C). There was no significant (P = 0.58) change in GSK3α expression in BC (n = 5) compared with normal peripheral blood (n = 5) progenitor samples (Fig. 3D). Confocal fluorescence microscopic as well as FACS analysis and LEF/TCF-GFP reporter assays showed that GSK3β-depleted BC GMP and progenitor-derived tumors had higher levels of activated β-catenin than normal GMP indicative of elevated β-catenin transcriptional activity in the LSC population (Fig. 3 E and F).
Fig. 3.
Aberrant GSK3β expression by BC CML progenitors. (A) FACS plot demonstrating characteristic expansion of the GMP compartment in BC CML compared with normal blood and marrow samples. FACS analysis performed on normal (n = 9) and CML CP (n = 5), CML AP (n = 6), and BC CML (n = 6) blood and bone marrow samples revealed that, while the proportion of HSC did not expand with progression to BC, there was a significant increase in GMP compared with normal controls (P = 1.93 × 106; two-tailed Student's t test). (B) HSC and progenitors were FACS sorted from normal or BC CML CD34+ (n = 3) blood samples and GSK3β transcript levels measured by quantitative RT-PCR. There was a significant difference (P < 0.05; two-tailed Student's t test) in GSK3β transcript levels between normal (mean 0.98 ± S.E.M. 0.05) and BC CML progenitors (0.36 ± S.E. 0.04). (C) FACS histograms of CML CP (n = 3), AP (n = 2), and BC (n = 3) progenitors revealed a decrease in GSK3β protein expression with progression to BC. (D) FACS analysis performed on normal blood (n = 5), and BC CML (n = 5) revealed that there was not a significant difference (P = 0.58; two-tailed Student's t test) in GSK3α protein expression as measured by mean fluorescence intensity ± SEM in BC CML samples compared with normal blood. Mean fluorescence intensity of isotype control (Rabbit IgG) was subtracted from all of the samples. (E Left) FACS analysis performed on normal blood (n = 6), and BC CML (n = 5) revealed that there was a significant difference (P = 0.016; two-tailed Student's t test) in activated β-catenin levels as measured by mean fluorescence intensity ± SEM in BC CML compared with normal blood. Isotype control (Mouse IgG1) was subtracted from all of the samples. (Right) Confocal fluorescence microscopic analysis revealed that normal GMP had little activated nuclear β-catenin whereas BC CML GMP expressing misspliced GSK3β had high levels of nuclear β-catenin (green: CD45 membrane marker, blue: Hoechst nuclear stain, red: activated β-catenin). (F) In 6 experiments, BC GMP from CML samples (n = 2) or tumor (n = 1) derived from BC GMP transplanted mice and normal cord blood GMP (n = 3) were transduced with a lentiviral LEF/TCF GFP reporter for activated β-catenin. BC GMP samples had significantly higher GFP expression (P = 0.037, two-tailed Student's t test) than normal cord blood GMP (n = 3) treated in the same manner. Results are expressed as percentage of maximum fluorescence intensity. (G Left) BC CML HSC in 5 of 8 patient samples subjected to cDNA sequencing analysis had demonstrable misspliced GSK3β transcripts. Nucleotide sequence data represents 2 species of GSK3β transcript in HSC: misspliced GSK3β and FL-GSK3. (Middle) BC CML progenitors in 5 of 8 samples had prominent misspliced GSK3β transcripts in the ORF of the cDNA. (Right) BC CML lineage-positive (blast) cells showing a deletion of GSK3β exon 9 in the ORF of the cDNA that was also detectable in normal samples.
To elucidate the genetic and epigenetic events responsible for decreased GSK3β expression during CML progression and to determine whether other Wnt pathway mediators were aberrantly regulated, 15 normal (Table S2A), 4 chronic phase, 1 accelerated phase, and 8 myeloid BC CML (Table S2B) samples were subjected to direct DNA sequencing to identify mutations in critical Wnt/β-catenin signaling pathway genes including β-catenin, GSK3β, axin 1, adenomatous polyposis coli (APC), cyclin D1, lymphoid enhancer factor-1 (LEF-1), and c-myc (18–25). A comprehensive cDNA sequencing analysis revealed a novel exon 8 and 9 deleted misspliced isoform of GSK3β (m-GSK3β) in progenitors in 4 of 7 myeloid BC CML samples and 1 of 4 CP CML samples (Table S2 A and B). In addition to its role in regulation of metabolic pathways such as insulin signaling, GSK3β is a critical component of the β-catenin destruction complex and thus, GSK3β deregulation would be expected to enhance β-catenin activation (19, 21–23). While cDNA sequencing analysis demonstrated that m-GSK3β was a prominent isoform in BC GMP, BC HSC harbored lower levels of m-GSK3β (Table S2B and Fig. 3G). These m-GSK3β transcripts lacking the FRAT and axin binding domains encoded by exons 8 and 9 were not detected by sequencing in CML blasts or normal sample populations (Tables S2 A and B) (23). Splice isoform specific Q-PCR confirmed higher levels of m-GSK3β transcripts in BC GMP compared with CD34+CD38− cells, which may explain, in part, the functional hierarchy in leukemic transplantation potential. This analysis also detected low levels of m-GSK3β in BC Lin+ cells and 3 additional CP progenitor samples (Fig. S4 A and B). Alternative GSK3β splice isoforms, deleted in exon 9, exon 11, or exon 9 and 11, were detected in the blasts as well as CP CML and normal peripheral blood, marrow, and cord blood, where they represented the predominant transcripts (Tables S2 A and B and Fig. S5). While exon 9- and 11-deleted GSK3β splice isoforms were previously identified in neurons of Parkinson's disease patients (18), exon 8 and 9 truncated transcripts found in CML progenitors have not been described to date. The capacity to down regulate β-catenin signaling may have been impaired as a result of both the absence of axin 2 expression (Fig. S4C) and deregulation of GSK3β. These data suggest that in the molecular context of LSC, an m-GSK3β isoform predominates that cannot phosphorylate β-catenin.
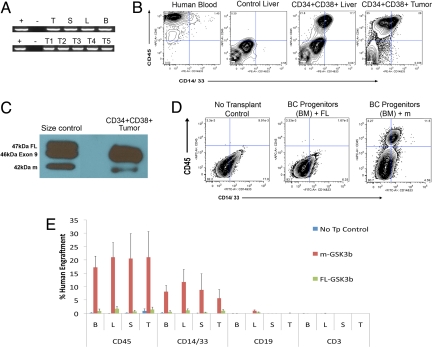
Transplantation of m-GSK3β-expressing BC progenitors produced high levels of BCR-ABL+ myeloid cell engraftment in hematopoietic tissues and tumors in primary and secondary recipient mice (Fig. 4 A and B). Western blot analysis demonstrated that m-GSK3β protein expression in tumors derived from BC progenitor transplanted mice (Fig. 4C). While lentiviral m-GSK3β transduction of CP CML progenitors led to increased levels of activated β-catenin expression, lentivirally-enforced expression of full-length GSK3β reduced β-catenin expression. In addition, CP CML progenitors lentivirally cotransduced with GLF and misspliced GSK3β had increased replating capacity (Fig. S4E). Conversely, lentiviral overexpression of full-length GSK3β reduced activated β-catenin expression (Fig. S4D) and leukemic engraftment by BC progenitors in all hematopoietic tissues (Fig. 4 D and E). Thus, deregulation of GSK3β through missplicing may be a key event in the evolution of LSC.
Fig. 4.
Enhanced engraftment of misspliced GSK3β-expressing CML progenitors. (A) P210 BCR-ABL RT-PCR transcript levels in hematopoietic tissues from mice transplanted with human CD45+ cells sorted from BC CML progenitor transplanted mice (105 cells; n = 7 mice). Tissues include thymus (T), spleen (S), liver (L), bone marrow (B) and tumors (T1-T5) (B) FACS analysis of 2° myeloid progenitor engrafted mice transplanted with human CD45+ cells sorted from bone marrow, liver, and spleen of mice that was originally transplanted with BC CML progenitor cells (n = 3 experiments). (C) Western blot of K562 cells (size control, left lane) transduced with GSK3β full length (FL), GSK3β exon 9 deleted (Exon 9), or GSK3β exon 8 and 9 deleted (m) and of 34+CD38+ CML BC derived tumor (right lane), shows that mGSK3β protein is expressed in the tumor cells. (D) Representative FACS plots of long-term (week 11–12) human CD45 and CD14/33 bone marrow (BM) engraftment in no transplant control (Left), BC progenitors transduced with FL-GSK3β (Middle) and misspliced GSK3β (n = 3) transduced BC CML progenitor transplanted mice (Right). (E) FACS analysis revealed a decreased percentage of long-term human CD45 cells in the PI negative fraction ± S.E.M. in hematopoietic organs including bone marrow (B; P = 0.019), liver (L; P = 0.025), spleen (S; P = 0.0109), and thymus (T; P = 0.10) of mice transplanted with FL-GSK3β (n = 3) compared with m-GSK3β transduced (n = 3) BC CML progenitors. Reduced human CD14 and CD33 engraftment was also noted in bone marrow (P = 0.03), liver (P = 0.08), spleen (P = 0.024), and thymus (P = 0.22) while there was no appreciable engraftment of human CD3 or CD19 cells in BC CML progenitor transplanted mice.
These studies suggest that decreases in functional GSK3β at specific stages of hematopoiesis may represent an important mechanism triggering aberrant β-catenin activation, nuclear entry, and enhanced self-renewal capacity (18–23). In CML, the combined effects of BCR-ABL-mediated stabilization of β-catenin (24) and possibly decreased expression of other negative regulators of the Wnt/β-catenin pathway, such as axin 2, may accentuate the effects of m-GSK3β on β-catenin activation (Fig. S4C).
Discussion
Our finding that enforced overexpression of full-length (FL) GSK3β decreases β-catenin expression and engraftment of m-GSK3β expressing BC progenitors validates the potential of repaired splicing to have some therapeutic benefit. GSK3β acts in different cells and has many important substrates; here we show that in the leukemic progression of an HSC clone and its GMP progeny, one effect of decreasing its activity is to enhance the activity of β-catenin in these cells. Extrapolation of this activity in these cells to other situations might be enhanced by an assessment of the other activities of the enzyme in the target cells.
Recently, loss of β-catenin was shown to inhibit engraftment of mouse myeloid leukemia and normal stem cells, underscoring the importance of β-catenin in normal and LSC self-renewal (25). Previous studies in mice revealed that a master regulator of hematopoiesis, Ikaros, has splice isoforms specific to HSC, progenitors, and progeny cells (26). The mechanism(s) responsible for alternative splicing of GSK3β have yet to be elucidated. Previously, SNPs were shown to create alternative splice acceptor sites responsible for generating GSK3β splice isoforms (18). Alternatively, these transcripts could arise through epigenetic fixation of splice isoforms, mutations in a yet unknown splicing element, or spliceosomal errors (26–28). Of particular significance to the pathogenesis of CML, enforced expression of BCR-ABL in cord blood progenitors was previously reported to induce increased expression of a number of genes involved in alternative splicing (27). Changes in splicing have been shown to play a functionally significant role in tumorigenesis, either by inactivating tumor suppressors or by gain of function of proteins promoting tumor development (28). In addition, oncogenic splicing events may generate novel epitopes that can be recognized by the host's immune system as cancer-specific and may serve as targets for immunotherapy. Finally, the identification of LSC-specific splice isoforms of GSK3β may be a useful indicator of disease progression and should be evaluated as a therapeutic target for eradicating the reservoir of LSC in advanced phase CML.
Materials and Methods
FACS Analysis and Sorting.
Normal blood and marrow samples were purchased from the San Diego Blood Bank or All Cells. CML blood and marrow samples were donated by Stanford University and MD Anderson Cancer Center patients according to Institutional Review Board (Princess Margaret Hospital) approved protocols. HSC, progenitor, and Lin+ cells were purified via FACS as previously described (1).
Lentiviral Transduction and Transplantation.
Equal numbers of normal or CML HSC, progenitors and Lin+ cells were FACS sorted, transduced with lentiviral vectors according to established methods (1, 29), and transplanted intrahepatically into neonatal RAG2−/−γc−/− mice (1, 17). Weekly bioluminescence imaging was performed with an in vivo imaging system (IVIS 200; Caliper Inc.). When moribund or at 8–12 weeks posttransplantation, mice were euthanized and single cell suspensions of hematopoietic organs and tumors were analyzed for human cell engraftment via FACS (SI Methods). BCR-ABL transcripts in transplanted mouse hematopoietic tissues were detected by PCR with a one step RT-PCR kit (Qiagen) (1, 30) BCR-ABL product sizes for b2a2 and b3a2 were 383bp and 458bp, respectively (30).
GSK3α, GSK3β, β-catenin, and Target Gene Analysis.
Normal or CML CD34+ cells were stained with a rabbit anti-human GSK3α (#9338, Cell Signaling Technology), rabbit anti-human GSK3β (#9315, Cell Signaling Technology) or anti-activated β-catenin monoclonal antibody (clone 8E7, Upstate Technologies) (1). To further assess β-catenin activation, sorted GMP (700–5,000 cells/well) were transduced with a lentiviral LEF/TCF GFP reporter and analyzed for GFP expression by FACS after 6–7 days in culture (2). Quantitative RT-PCR to detect axin2, c-myc, GSK3β transcripts, and the GSK3β isoforms in normal versus CML HSC and progenitors was performed with SYBR Greener two-Step Q-RT-PCR Kit (Invitrogen) (SI Methods) (1). To determine misspliced GSK3β protein expression, primary BC CML progenitors, tumors from BC progenitor transplanted mice or 293FT cells transduced with lentiviral FL-GSK3β or m-GS3β for 48 h, were collected and 5 μg of the whole cell lysate were probed with GSK3β antibody (Cell Signaling) in Western blot analysis.
Wnt Pathway Mutation Screening.
Genomic DNA mutation and cDNA analysis of β-catenin, APC, axin 1, c-myc, LEF-1, cyclin D1, and GSK3β was conducted with SURVEYOR mismatch cleavage analysis using the WAVE-HS System (Transgenomic) followed by bidirectional sequence analysis on an ABI 3100 sequencer (Applied Biosystems, Inc.) (SI Methods).
Statistical Analysis.
Statistical analyses were performed with the aid of FlowJo, Caliper, and Excel software.
Supplementary Material
Acknowledgments.
We are indebted to Dr. Karl Willert for his kind gift of the lentiviral LEF/TCF-GFP reporter and to Dr. Sheldon Morris for expert assistance with statistical analysis. We would also like to thank Dr. David Voehringer and Dr. Andrea Tu from Cell Biosciences for expert phosphoproteomic advice. This research was also enhanced by discussions with Roel Nusse, our longtime collaborator and advisor in this field. We are grateful to Dennis Young of the University of California at San Diego Moores Cancer Center FACS facility for his expert assistance with FACS Aria analysis and sorting, Dr. Julie Beth Sneddon and Daniel Goff for expert assistance with sample preparation, Dr. Oded Singer for help with lentivirus techniques, and Libuse Jerabek for excellent laboratory management. We would also like to thank Lenn Fechter, Dr. Steven Coutre, Dr. Stanley Schrier, Dr. Caroline Berube, Dr. Diana Laird, Dr. Robin Wright, and Dr. Lawrence Leung at Stanford for providing normal and CML blood and marrow samples, and, most of all, the patients who made this research possible. This research was supported by grants (CA086065 and CA86017, to Dr. Weissman; 2PO1CA49605, to Ms. Jones and Dr. Zehnder; and K23 HL04409, to Dr. Gotlib) from the National Institutes of Health, Dr. Weissman was supported by a de Villiers grant from the Leukemia Society, as well as the Walter and Beth Weissman Fund, the Smith Family Fund, and holds the Virginia K and Daniel Ludwig Professorship at Stanford. Dr. Jamieson and this research were supported by a grant from the California Institute of Regenerative Medicine (RS1–00228–1). Dr. Keating holds the Gloria and Seymour Epstein Chair in Cell Therapy and Transplantation at the University Health Network and the University of Toronto.
Footnotes
Conflict of interest statement: I.L.W. has equity ownership in Cellerant, Inc. and Stemcells, Inc. C.H.M.J consults for Wintherix Inc.
References
- 1.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 2.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Fialkow PJ, Jacobson RJ, Papayannopoulou T. Chronic myelocytic leukemia: Clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet, and monocyte/macrophage. Am J Med. 1977;63:125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- 5.Hungerford DA. The Philadelphia Chromosome and Some Others. Ann Intern Med. 1964;61:789–793. doi: 10.7326/0003-4819-61-4-789. [DOI] [PubMed] [Google Scholar]
- 6.Radich JP, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 11.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 12.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 14.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.Weissman I. Stem cell research: Paths to cancer therapies and regenerative medicine. JAMA. 2005;294:1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 17.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 18.Kwok JB, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 19.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 21.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12(6):957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, et al. Glycogen synthase kinase 3 alpha and 3 beta have distinct functions during cardiogenesis of zebrafish embryo. BMC Dev Biol. 2007;7:93. doi: 10.1186/1471-213X-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dajani R, et al. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 2003;22:494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coluccia AM, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klug CA, et al. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc Natl Acad Sci USA. 1998;95:657–662. doi: 10.1073/pnas.95.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salesse S, Dylla SJ, Verfaillie CM. p210BCR/ABL-induced alteration of pre-mRNA splicing in primary human CD34+ hematopoietic progenitor cells. Leukemia. 2004;18:727–733. doi: 10.1038/sj.leu.2403310. [DOI] [PubMed] [Google Scholar]
- 28.Pajares MJ, et al. Alternative splicing: An emerging topic in molecular and clinical oncology. Lancet Oncol. 2007;8:349–357. doi: 10.1016/S1470-2045(07)70104-3. [DOI] [PubMed] [Google Scholar]
- 29.Breckpot K, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–667. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- 30.Emig M, et al. Accurate and rapid analysis of residual disease in patients with CML using specific fluorescent hybridization probes for real time quantitative RT-PCR. Leukemia. 1999;13:1825–1832. doi: 10.1038/sj.leu.2401566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.