Abstract
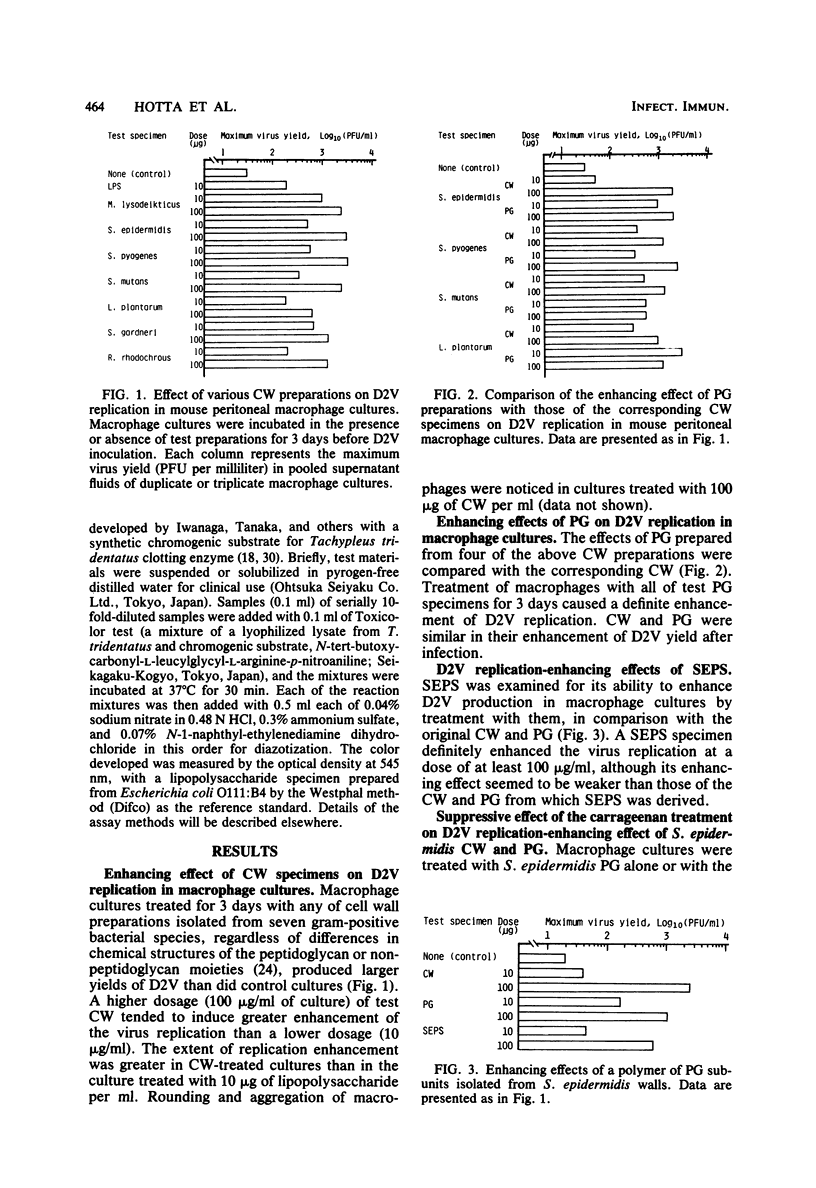
The effects of bacterial cell walls, peptidoglycans, and a water-soluble polymer of peptidoglycan subunits on dengue virus type 2 replication in cultured mouse peritoneal macrophages were studied. Pretreatment of macrophage cultures with all of test cell walls isolated from seven bacterial species for 3 days significantly enhanced the virus production in the cultures. Peptidoglycans prepared from four of the above cell walls also exerted the virus production-enhancing effects in a similar manner as the walls. A water-soluble polymer of peptidoglycan subunits which was prepared by treatment of Staphylococcus epidermidis wall peptidoglycan with an interpeptide bridge-splitting enzyme (endopeptidase) also definitely enhanced the virus production in macrophage cultures, although its activity was weaker than that of the original wall and peptidoglycan. Macrophage cultures from athymic nude mice, when treated with cell walls and peptidoglycans of S. epidermidis and Lactobacillus plantarum for 3 days, also showed an increased ability to support dengue virus type 2 replication. The infectious center assay demonstrated that the virus replication enhancement by S. epidermidis cell wall and peptidoglycan was primarily due to an increase in the number of virus-infected cells. This finding did not seem to be in conflict with the observation that macrophages treated with the above cell wall or peptidoglycan phagocytized more latex particles than did untreated macrophages. The conclusions based on the above experiments are that the treatment of mouse peritoneal macrophage cultures with bacterial cell walls and their components increases the take of dengue virus type 2 by macrophages and thus raises the virus production in the macrophage cultures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Brandt W. E., McCown J. M., Top F. H., Jr, Bancroft W. H., Russell P. K. Effect of passage history on dengue-2 virus replication in subpopulations of human leukocytes. Infect Immun. 1979 Nov;26(2):534–541. doi: 10.1128/iai.26.2.534-541.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam A. R., Dutko F. J., Olding L. B., Oldstone M. B. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J Gen Virol. 1979 Aug;44(2):349–359. doi: 10.1099/0022-1317-44-2-349. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Brandt W. E., McCloud T. G., Shapiro D., Russell P. K. Immunological and biophysical separation of dengue-2 antigens. J Virol. 1971 Jan;7(1):15–23. doi: 10.1128/jvi.7.1.15-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Schwartz H. J., Graham R. C., Jr Spectrum and possible mechanism of carrageenan cytotoxicity. Am J Pathol. 1971 Aug;64(2):387–404. [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Hadden J. W., Englard A., Sadlik J. R., Hadden E. M. The comparative effects of isoprinosine, levamisole, muramyl dipeptide and SM1213 on lymphocyte and macrophage proliferation and activation in vitro. Int J Immunopharmacol. 1979;1(1):17–27. doi: 10.1016/0192-0561(79)90026-2. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Kotani S., Takada H., Tsujimoto M., Hirachi Y., Kusumoto S., Shiba T., Kawata S., Yokogawa K., Nishimura H. Liberation of serotonin from rabbit blood platelets by bacterial cell walls and related compounds. Infect Immun. 1982 Sep;37(3):1181–1190. doi: 10.1128/iai.37.3.1181-1190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta H., Hotta S. Dengue virus multiplication in cultures of mouse peritoneal macrophages: effects of macrophage activators. Microbiol Immunol. 1982;26(8):665–676. doi: 10.1111/j.1348-0421.1982.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Hotta H., Murakami I., Miyasaki K., Takeda Y., Shirane H., Hotta S. Inoculation of dengue virus into nude mice. J Gen Virol. 1981 Jan;52(Pt 1):71–76. doi: 10.1099/0022-1317-52-1-71. [DOI] [PubMed] [Google Scholar]
- Hotta H., Murakami I., Miyasaki K., Takeda Y., Shirane H., Hotta S. Localization of dengue virus in nude mice. Microbiol Immunol. 1981;25(1):89–93. doi: 10.1111/j.1348-0421.1981.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Hotta S., Fujita N., Maruyama T. Research on dengue in tissue culture. I. Plaque formation in an established monkey kidney cell line culture. Kobe J Med Sci. 1966 Sep;12(3):179–187. [PubMed] [Google Scholar]
- Imai K., Tanaka A. Effect of muramyldipeptide, a synthetic bacterial adjuvant, on enzyme release from cultured mouse macrophages. Microbiol Immunol. 1981;25(1):51–62. doi: 10.1111/j.1348-0421.1981.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Iwanaga S., Morita T., Harada T., Nakamura S., Niwa M., Takada K., Kimura T., Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7(2-3):183–188. doi: 10.1159/000214260. [DOI] [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Harada K. Gelation of the amoebocyte lysate of Tachypleus tridentatus by cell wall digest of several gram-positive bacteria and synthetic peptidoglycan subunits of natural and unnatural configurations. Biken J. 1977 Mar;20(1):5–10. [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. M., Nisalak A., Cheamudon U., Seridhoranakul S., Nimmannitya S. Isolation of dengue viruses from peripheral blood leukocytes of patients with hemorrhagic fever. J Infect Dis. 1980 Jan;141(1):1–6. doi: 10.1093/infdis/141.1.1. [DOI] [PubMed] [Google Scholar]
- Takada H., Nagao S., Kotani S., Kawata S., Yokogawa K., Kusumoto S., Shiba T., Yano I. Mitogenic effects of bacterial cell walls and their components on murine splenocytes. Biken J. 1980 Jun;23(2):61–68. [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H., Obayashi T., Takagi K., Tanaka S., Nakahara C., Kawai T. Perchloric acid treatment of human blood for quantitative endotoxin assay using synthetic chromogenic substrate for horseshoe crab clotting enzyme. Thromb Res. 1982 Jul 1;27(1):51–57. doi: 10.1016/0049-3848(82)90277-8. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Imai K., Mori R. Macrophage activation by muramyl dipeptide as measured by macrophage spreading and attachment. Microbiol Immunol. 1980;24(6):547–557. doi: 10.1111/j.1348-0421.1980.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Yamamoto Y., Nagao S., Tanaka A., Koga T., Onoue K. Inhibition of macrophage migration by synthetic muramyl dipeptide. Biochem Biophys Res Commun. 1978 Feb 28;80(4):923–928. doi: 10.1016/0006-291x(78)91333-5. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]



