Abstract
Cellular interactions in development of the kidney are used as a model of reciprocal inductive events between epithelium and mesenchyme. Time- and labor-intensive methods have been developed to study this phenomenon. For example, in mice, the targeted disruption of genes in vivo has been used to modify the genetic program directing kidney development. However, gene targeting is a resource-intensive approach and alternative strategies for gene and protein modification in the kidney need to be developed. Herein, we have developed an efficient system for the delivery of antisense morpholino to alter normal protein expression. We describe the use of Endo-Porter to effectively deliver morpholinos to all parts and regions of the kidney explant. Also, we definitively show via confocal microscopy and Western blot analysis that the use of Endo-Porter in delivering antisense morpholinos is robust throughout the entire kidney explant, providing efficient suppression of protein expression. This method saves time and cost when compared with targeted disruption and is an improvement upon previous kidney organ culture methods.
Cellular interactions in development of the kidney are used as a model of reciprocal inductive events between epithelium and mesenchyme. Murine kidney development begins at embryonic day 10.5 (E10.5) with a series of reciprocal interactions between the epithelial cells of the ureteric bud and the mesenchyme. This interaction induces dichotomous branching that is partially regulated by the activity of p38 mitogen-activated protein kinase (p38MAPK) (1) of the ureteric bud and mesenchyme condensation resulting in the formation of the collecting duct system and nephrons, respectively (2,3).
In mice, targeted disruption of genes in vivo has been used to modify the genetic program directing kidney development. However, as the kidney is amenable to ex vivo culture, the effects of growth factors and gene silencing (by siRNA or function-blocking antibodies) on maturation and differentiation have been studied. These approaches are sometimes difficult to interpret because the developing kidney is composed of distinct populations with differing biological functions, differentiation potentials, and gene expression patterns. Moreover, the use of inhibitory antibodies is limited by the availability of such reagents. Consequently, this method has only been applied to a few genes, such as WT1 (4). To complement existing strategies, we have developed an efficient system for morpholino-mediated (5) protein inactivation in kidney organ culture. Herein, we describe the use of Endo-Porter (Gene-Tools, Philomath, OR, USA) to effectively deliver morpholinos to kidney explant cultures in order to decrease expression of the apoptotic regulator and p38MAPK modulator, NRAGE (6,7). Endo-Porter is a small peptide that increases the permeability of membranes of acidic endocytic vesicles, releasing endocy-tosed molecules into the cytosol (8). The combination of Endo-Porter and morpholinos results in effective knockdown of protein expression in kidney organ culture for up to 72 h. This is a useful modification of established culture methods to augment specific protein expression throughout the kidney explant.
The method of ex vivo culturing of developing kidneys was first described by Grobstein (9), which was modified by Avner (10) to use a defined medium. This method has been used to repress specific proteins to measure their effects on development using either antisense oligonucleotides (11–13) or morpholinos (14). It is important to establish that all cells in the explant are receiving equal amounts of morpholino, otherwise protein inhibition will occur in a mosaic pattern throughout the kidney explant, making interpretations of the results difficult. In our modification, we use Endo-Porter to facilitate endosomal releases so that cells of the ureteric bud and the mesenchyme receive equal amounts of morpholino.
We cultured E11.5 ICR mouse (Taconic, Hudson, NY, USA) kidney explants for upwards of 72 h with 10 µM NRAGE morpholino (6) and 4 µM Endo-Porter. Explants were placed onto 40 µm polycarbonate filters (Millipore, Billerica, MA, USA) and cultured in a Netwell well (Corning, NY, USA). The wells were transferred to a 12-well plate with Richter’s Modified IMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10 µg/mL of Holo-transferrin (Sigma-Aldrich, St. Louis, USA) to a total volume of 750 µl. This arrangement allowed for diffusion of the culture media into the kidney explant through the filter without submerging the explant. The 12-well dish was placed in a humidified incubator at 37°C and 5% CO2 for the times described. After the incubation period, the kidney explants were lysed with 20 µl of SDS lysis buffer (6). Protein concentration was determined using the bicinchoninic acid protein (Pierce Biotechnology, Rockford, IL, USA) and samples were loaded equally onto a 10% SDS-PAGE gel and subjected to Western blot analysis. We used a morpholino (5′-GGTTTCTGAGCCATAGCTCTCGTC-3′) designed to inhibit the translation of NRAGE mRNA, as previously described (6). As seen in Figure 1, NRAGE protein expression decreased in the presence of NRAGE morpholino as opposed to the negative control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′, sequence from Gene-Tools). The decrease in protein expression was dependent upon both the presence of the NRAGE-specific morpholino and Endo-Porter.
Figure 1. Western blot analysis of lysates collected from kidney explants treated with NRAGE antisense morpholino or a negative control morpholino.
Western blot analysis of lysates collected from kidney explants treated with NRAGE antisense morpholino or a negative control morpholino (72 h incubation) and probed with antibodies to anti-NRAGE and anti-βactin. The use of NRAGE morpholino (10 µM) alone without Endo-Porter showed little to no decrease in protein expression.
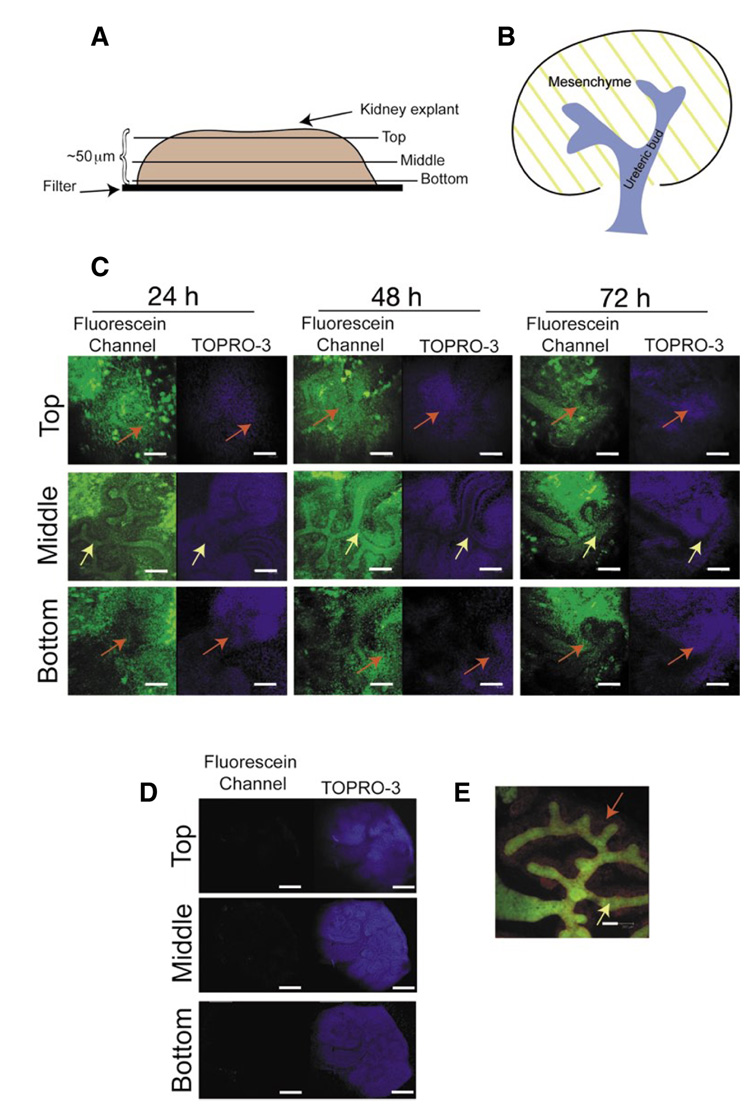
Next, we used 10 µM of a fluorescein-conjugated negative control morpholino to treat E11.5 kidney explants to ensure that the morpholino was delivered to both the ureteric bud and the surrounding mesenchyme. After a 72-h incubation period, kidneys were transferred to a 12-well dish and fixed with 4% paraformaldehyde, 1 h at room temperature, and washed with PBS for 15 min. The kidneys were placed into a fresh 12-well dish containing the nuclear stain TOPRO-3 (Molecular Probes, Carlsbad, CA, USA) in PBS for 4 h at room temperature. Finally, the kidneys were transferred to a glass microscope slide and submerged into Prolong-Gold (Molecular Probes, USA) mounting media. Analysis, using a Leica TCS-SP confocal microscope, demonstrated that a significant amount of fluorescein-conjugated morpholino had penetrated the center of the kidney explant as well as the surrounding mesenchyme (Figure 2). Gene-Tools also supplies a lissamine-conjugated negative control morpholino, which in our hands appeared to limit the viability of the kidney explants. Specifically, kidney explants treated with the lissamine-conjugated negative control morpholino (10 µM) failed to develop, whereas all kidneys treated with fluorescein-conjugated morpholinos underwent normal development as rigorous as compared with untreated kidney explants. One reason for this decrease in viability is due to the lower solubility of a lissamine-conjugated morpholino, which may lead to complexing of the morpholino. This, in turn, would result in a concentration of the morpholino higher than 10 µM and therefore could have a toxic effect on the kidney explants, decreasing viability (personal communication, Jon Moulton, Gene-Tools).
Figure 2. Confocal microscopy analysis of kidney explants treated with fluorescein-conjugated negative control morpholino.
(A) Schematic diagrams of kidney explant and (B) an E12 kidney. (C) Optical sections (2 µm) of kidney explants cultured with fluorescein-conjugated negative control morpholino (green) and Endo-Porter (TOPRO-3, blue). (D) E11.5 kidney explant cultured without fluorescein-conjugated morpholino (72 h) to demonstrate the level of autofluorescence. (E) E11.5 Hoxb7-EGFP (15) kidney cultured (untreated, 72 h) and probed with the anti-Pax2 antibody (Zymed; Alexa fluor 546 [red]), illustrating the ureteric bud (green) and mesenchyme (red). Scale bar: 100 microns. Yellow arrows demarcate the ureteric bud; red arrows demarcate the metanephric mesenchyme.
In summary, the use of Endo-Porter can effectively deliver morpholinos to the center of kidney explants resulting in a significant decrease in gene expression. This method is cost-effective in comparison with targeted disruption and is an improvement upon previous kidney organ culture methods.
ACKNOWLEDGMENTS
G.N.N. is supported by a predoctoral fellowship from the American Heart Association. L.O. is supported by a National Institutes of Heatlh (NIH) grant (no. P20 RR18789). I.P. is supported by the NIH’s Center of Biomedical Research Excellence (COBRE) grant in Vascular Biology (grant no. P20 RR15555). J.M.V. is supported by the NIH (grant no. R01 NS055304).
Footnotes
To purchase reprints of this article, contact: Reprints@BioTechniques.com
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
REFERENCES
- 1.Hu MC, Wasserman D, Hartwig S, Rosenblum ND. p38MAPK acts in the BMP7-dependent stimulatory pathway during epithelial cell morphogenesis and is regulated by Smad1. J. Biol. Chem. 2004;279:12051–12059. doi: 10.1074/jbc.M310526200. [DOI] [PubMed] [Google Scholar]
- 2.al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832–1842. doi: 10.1046/j.1523-1755.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- 3.Piscione TD, Rosenblum ND. The molecular control of renal branching morphogenesis: current knowledge and emerging insights. Differentiation. 2002;70:227–246. doi: 10.1046/j.1432-0436.2002.700602.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies JA, Ladomery M, Hohenstein R, Michael L, Shafe A, Spraggon L, Hastie N. Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum. Mol. Genet. 2004;13:235–246. doi: 10.1093/hmg/ddh015. [DOI] [PubMed] [Google Scholar]
- 5.Summerton J, Stein D, Huang SB, Matthews P, Weller D, Partridge M. Morpholino and phosphorothioate antisense oligomers compared in cell-free and in-cell systems. Antisense Nucleic Acid Drug Dev. 1997;7:63–70. doi: 10.1089/oli.1.1997.7.63. [DOI] [PubMed] [Google Scholar]
- 6.Kendall SE, Battelli C, Irwin S, Mitchell JG, Glackin CA, Verdi JM. NRAGE mediates p38 activation and neural progenitor apoptosis via the bone morphogenetic protein signaling cascade. Mol. Cell. Biol. 2005;25:7711–7724. doi: 10.1128/MCB.25.17.7711-7724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi AH, Roux PP, Kubu CJ, Zeindler C, Bhakar A, Tannis LL, Verdi JM, Barker PA. NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron. 2000;27:279–288. doi: 10.1016/s0896-6273(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 8.Summerton JE. Endo-Porter: a novel reagent for safe, effective delivery of substances into cells. Ann. N.Y. Acad. Sci. 2005;1058:62–75. doi: 10.1196/annals.1359.012. [DOI] [PubMed] [Google Scholar]
- 9.Grobstein C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature. 1953;172:869–870. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- 10.Avner ED, Ellis D, Temple T, Jaffe R. Metanephric development in serum-free organ culture. In Vitro. 1982;18:675–682. doi: 10.1007/BF02796422. [DOI] [PubMed] [Google Scholar]
- 11.Kanwar YS, Ota K, Yang Q, Wada J, Kashihara N, Tian Y, Wallner EI. Role of membrane-type matrix metal-loproteinase 1 (MT-1-MMP), MMP-2, and its inhibitor in nephrogenesis. Am. J. Physiol. 1999;277:F934–F947. doi: 10.1152/ajprenal.1999.277.6.F934. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZZ, Wada J, Kumar A, Carone FA, Takahashi M, Kanwar YS. Comparative role of phosphotyrosine kinase domains of c-ros and c-ret protooncogenes in metanephric development with respect to growth factors and matrix morphogens. Dev. Biol. 1996;178:133–148. doi: 10.1006/dbio.1996.0204. [DOI] [PubMed] [Google Scholar]
- 13.Quaggin SE, Vanden Heuvel GB, Igarashi P. Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech. Dev. 1998;71:37–48. doi: 10.1016/s0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- 14.Gross I, Morrison DJ, Hyink DP, Georgas K, English MA, Mericskay M, Hosono S, Sassoon D, et al. The receptor tyrosine kinase regulator Sprouty1 is a target of the tumor suppressor WT1 and important for kidney development. J. Biol. Chem. 2003;278:41420–41430. doi: 10.1074/jbc.M306425200. [DOI] [PubMed] [Google Scholar]
- 15.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev. Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]