Abstract
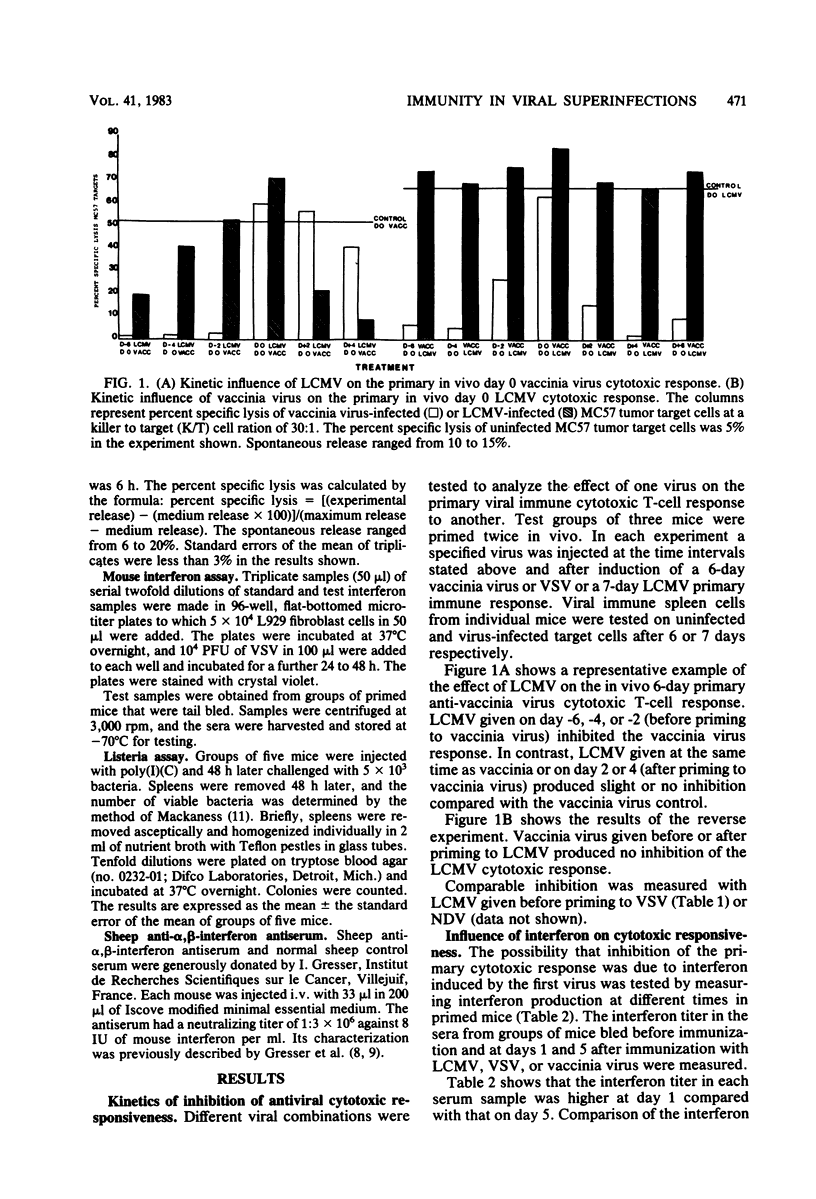
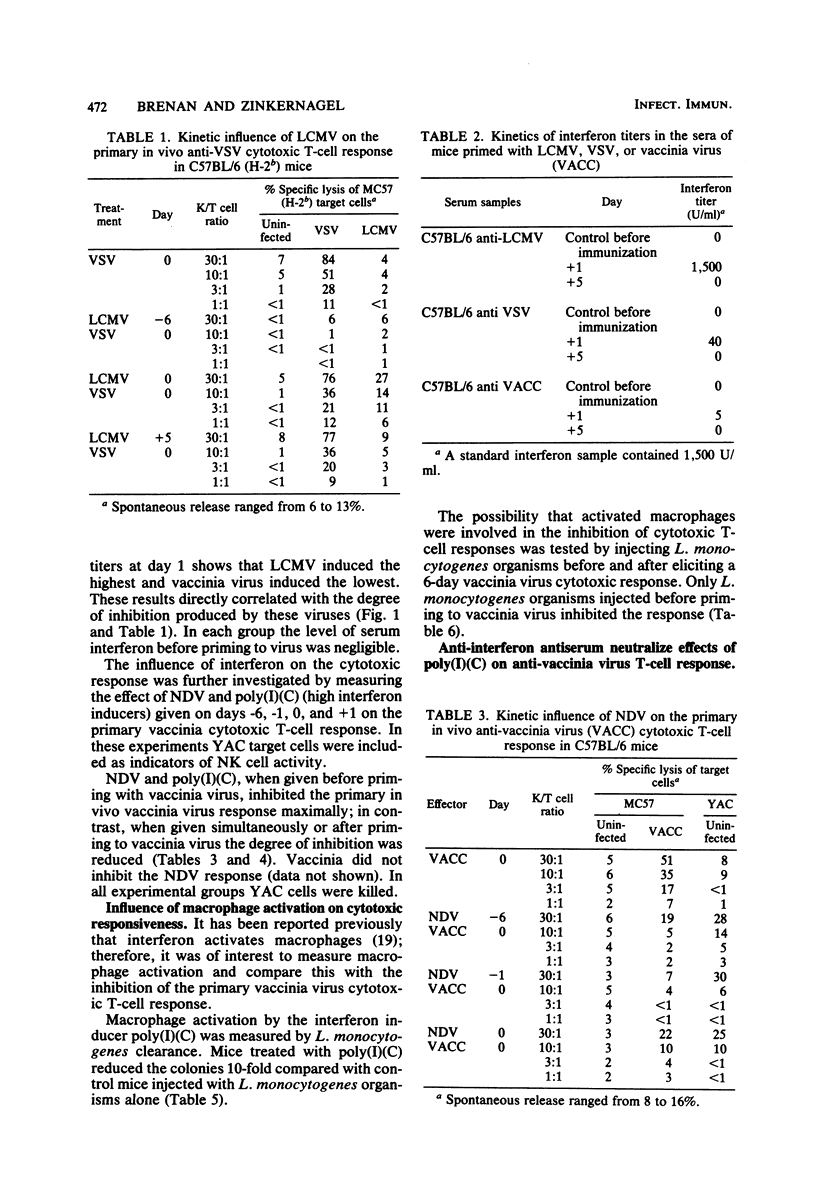
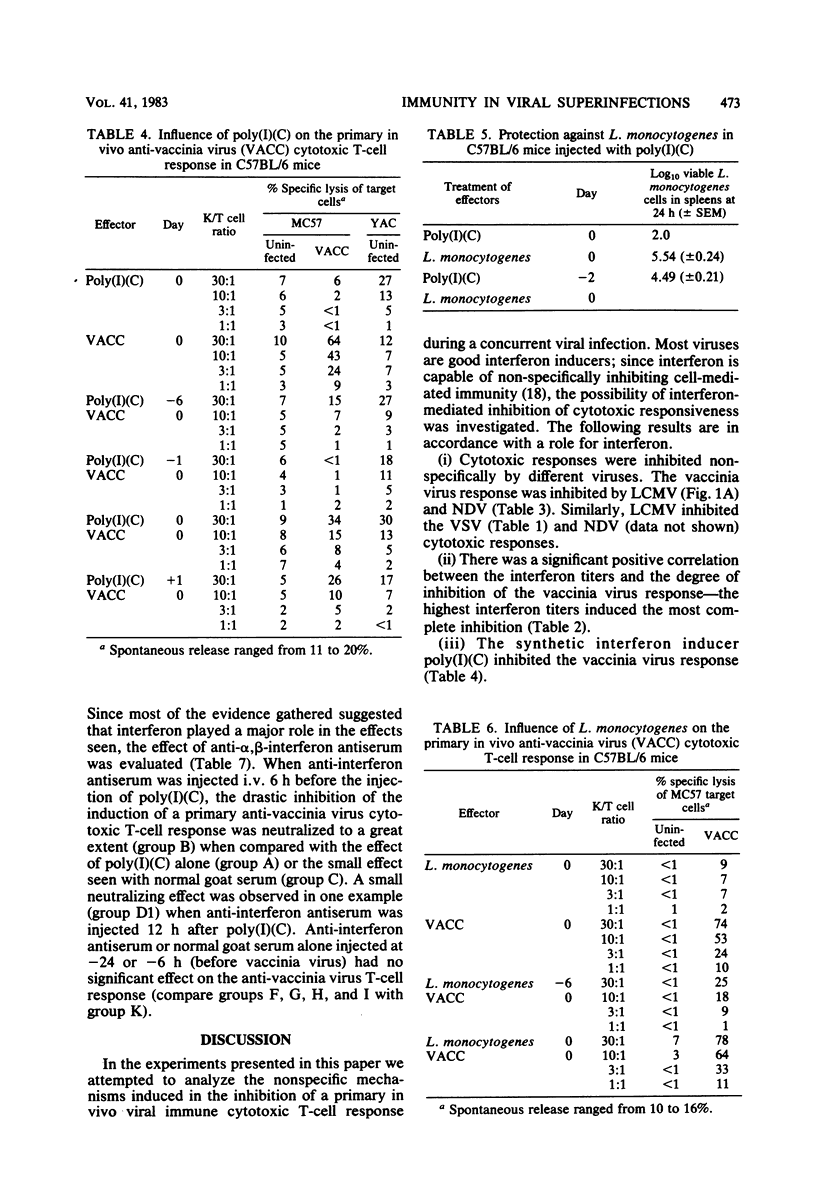
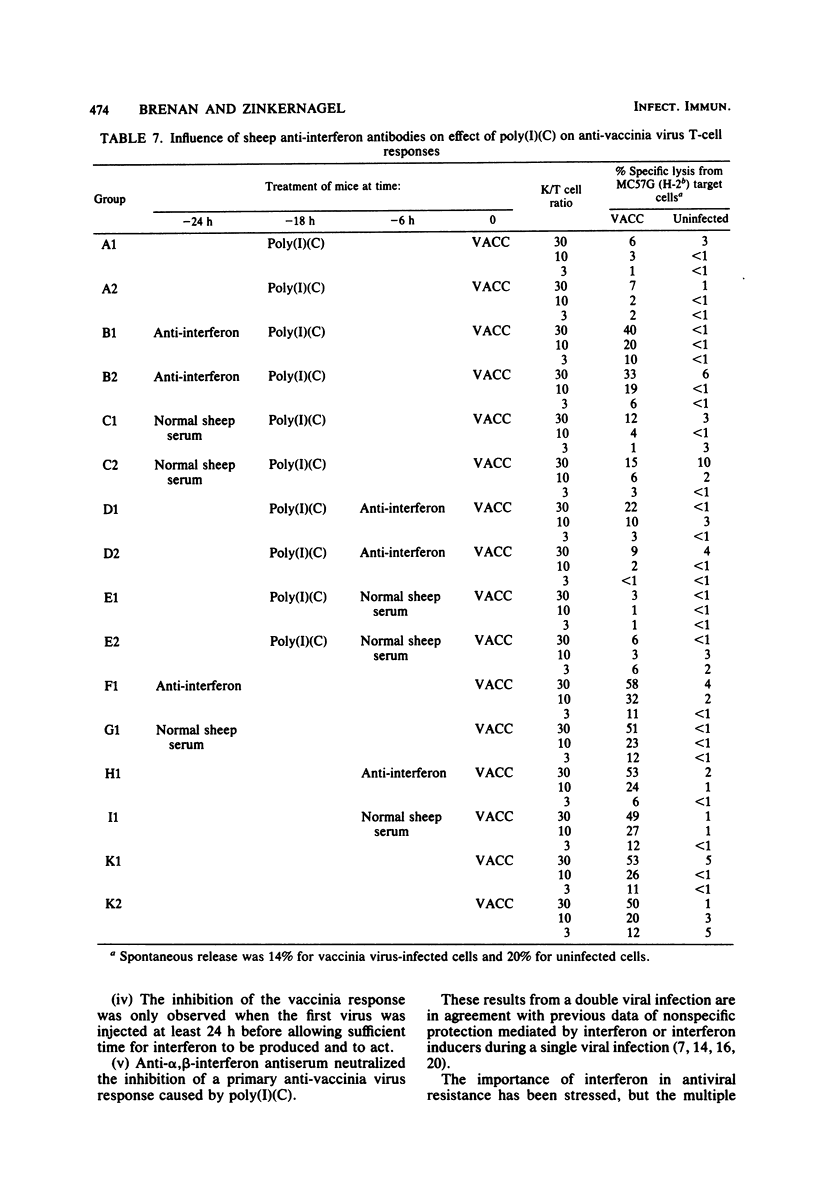
The influence of one virus on the in vivo cytotoxic T-cell response to a different concurrent viral infection was analyzed. Lymphocytic choriomeningitis and Newcastle disease viruses, known to induce high interferon titers, and the synthetic interferon inducer polyriboinosinic acid-polyribocytidylic acid inhibited the cytotoxic T-cell response against the second virus. In contrast, vaccinia and vesicular stomatitis viruses failed to induce inhibition. Inhibition directly correlated with the interferon titers; similarly, the interferon titers directly correlated with macrophage and natural killer cell activation. The involvement in vivo of interferon in macrophage and natural killer cell activation and the possible mechanisms of inhibition of the cytotoxic responses are shown by the inhibition of the effect by antibodies against interferon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armerding D., Simon M. M., Hämmerling U., Hämmerling G. J., Rossiter H. Function, target-cell preference and cell-surface characteristics of herpes-simplex virus type-2-induced non-antigen-specific killer cells. Immunobiology. 1981;158(4):347–368. doi: 10.1016/s0171-2985(81)80006-x. [DOI] [PubMed] [Google Scholar]
- Berry L. J., Smythe D. S., Colwell L. S., Schoengold R. J., Actor P. Comparison of the effects of a synthetic polyribonucleotide with the effects of endotoxin on selected host responses. Infect Immun. 1971 Mar;3(3):444–448. doi: 10.1128/iai.3.3.444-448.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Brenan M., Müllbacher A. Analysis of the cytotoxic T cell response to H-Y in CBA/H mice. J Immunol. 1981 Aug;127(2):681–685. [PubMed] [Google Scholar]
- Donahoe R. M., Huang K. Y. Neutralization of the phagocytosis-enhancing activity of interferon preparations by anti-interferon serum. Infect Immun. 1973 Mar;7(3):501–503. doi: 10.1128/iai.7.3.501-503.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A. Transfer of interferon-producing macrophages: new approach to viral chemotherapy. Science. 1970 Nov 20;170(3960):854–856. doi: 10.1126/science.170.3960.854. [DOI] [PubMed] [Google Scholar]
- Gresser I., Maury C., Bandu M. T., Tovey M., Maunoury M. T. Role of endogenous interferon in the anti-tumor effect of poly I-C and statolon as demonstrated by the use of anti-mouse interferon serum. Int J Cancer. 1978 Jan 15;21(1):72–77. doi: 10.1002/ijc.2910210113. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. D., Keller R. Natural cytotoxicity of murine cytomegalovirus-infected cells mediated by mouse lymphoid cells: role of interferon in the endogenous natural cytotoxicity reaction. Infect Immun. 1982 Jan;35(1):5–12. doi: 10.1128/iai.35.1.5-12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manejias R. E., Hamburg S. I., Rabinovitch M. Serum interferon and phagocytic activity of macrophages in recombinant inbred mice inoculated with Newcastle disease virus. Cell Immunol. 1978 Jun;38(1):209–213. doi: 10.1016/0008-8749(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968 Oct;101(4):717–724. [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Blazkovec A. A., Walker D. L. Immunosuppression during acute murine cytomegalovirus infection. J Immunol. 1968 Apr;100(4):835–844. [PubMed] [Google Scholar]
- Rodgers B. C., Mims C. A. Role of macrophage activation and interferon in the resistance of alveolar macrophages from infected mice to influenza virus. Infect Immun. 1982 Jun;36(3):1154–1159. doi: 10.1128/iai.36.3.1154-1159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Schultz R. M., Papamatheakis J. D., Chirigos M. A. Interferon: an inducer of macrophage activation by polyanions. Science. 1977 Aug 12;197(4304):674–676. doi: 10.1126/science.877584. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Jensen F. C. Cell-mediated immune response to lymphocytic choriomeningitis and vaccinia virus in rats. J Immunol. 1977 Oct;119(4):1242–1247. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


