Abstract
Pregabalin is one of the latest antiepileptic drugs introduced for the treatment of partial epilepsy. Its efficacy and safety as adjunctive therapy in refractory partial epilepsy have been established in four double-blind placebo-controlled trials (n = 1396) and 4 long-term open-label studies (n = 1480). In 3 fixed-dose trials, the proportion of patients with a ≥50% reduction in seizure frequency across the effective dose-range (150–600 mg/day) ranged between 14% and 51%, with a clear dose-response relationship. Suppression of seizure activity could be demonstrated as early as day 2. The most frequently reported CNS-related adverse events included dizziness, somnolence, ataxia and fatigue, were usually mild or moderate, and tended to be dose related. In long-term studies, weight gain was reported as an adverse event by 24% of patients. When pregabalin dose was individualized to according to response within the 150 to 600 mg/day dose range, tolerability was considerably improved compared with use of a high-dose, fixed-dose regimen (600 mg/day) without titration. In long-term studies up to 4 years, no evidence of loss efficacy was identified. During the last year on pregabalin, 3.7% of patients were seizure-free. Pregabalin appears to be a useful addition to the therapeutic armamentariun for the management of refractory partial epilepsy.
Keywords: pregabalin, antiepileptic drugs, adjunctive therapy, partial seizures, efficacy, tolerability, clinical trials
Introduction
Pregabalin was granted a license as an antiepileptic drug (AED) for the adjunctive treatment of refractory partial seizures in the European Union in 2004 and in the United States in 2005. Since then, three other AEDs, namely stiripentol, rufinamide and lacosamide, have received regulatory approval in the European Union. This implies that physicians treating epilepsy can now utilize an armamentarium of almost 20 AEDs. Since each of these drugs differs from the others, the opportunity of tailoring AED choice to the individual characteristics of the patient has never been greater. On the other hand, physicians also face complex knowledge challenges, because optimal use of these drugs requires a good understanding of their activity against different seizure types, pharmacokinetic characteristics, titration and dosing regimens, adverse effects profile and potential drug-drug interactions (Perucca 2002). The present article will review the most relevant pharmacological and clinical properties which are available for pregabalin.
Most clinical data discussed in this review are derived from prospective randomized controlled trials. These are undoubtedly the most important source of information about the value of a drug, since they provide an objective perspective of their efficacy, tolerability and safety. During the pre-registration development of an AED, however, clinical trials are designed primarily to meet the requirements of regulatory authorities, eg, to document efficacy in terms of superiority over placebo and an acceptable risk to benefit ratio. Physicians and patients, however, are more concerned about long-term clinical utility, and, most importantly, about efficacy and tolerability in comparison with other AEDs which are available for the same indication(s). In the case of pregabalin, only limited long-term data are available, and trials comparing this agent with other AEDs have not yet been completed.
Pharmacological profile
Pregabalin displays potent anticonvulsant activity in a variety of experimental models of seizures in rodents, including seizure induced by maximal electroshock and seizures provoked by chemoconvulsants such as pentylenetetrazole, bicuculline, and picrotoxin (Ben-Menachem 2004; Warner and Figgit 2005; Taylor and Vartanian 1997). Pregabalin is also active in animal models of epilepsy, being effective in protecting against audiogenic seizures in genetically seizure-susceptible DBA/2 mice and against kindled seizures in rats. The latter model is considered to be predictive of clinical efficacy against partial-onset seizures. Pregabalin does not suppress spontaneous seizures in the Genetic Absence Epilepsy Rat from Strasbourg (GAERS), a model of absence epilepsy.
In other preclinical studies, pregabalin has been found to be effective in animal models of anxiety and neuropathic pain (Shneker et al 2005; Vartanian et al 2006). These observations have led to clinical trials and demonstration of efficacy in patients with generalized anxiety disorder, postherpetic neuralgia and painful diabetic peripheral neuropathy (Frampton and Scott 2004; Shneker et al 2005; Bandelow et al 2007; Blommel and Blommel 2007; Owen 2007; Tassone et al 2007).
Pregabalin is structurally related to both γ-aminobutyric acid (GABA) and gabapentin. However, as for gabapentin, the primary mechanism underlying its pharmacological action does not appear to involve the GABA system. In particular, pregabalin does not bind to GABAA, GABAB or benzodiazepine receptors and is neither metabolically converted to GABA or to a GABA agonist, nor it has any effect on the uptake or degradation of GABA (Errante and Petroff 2002; Ben-Menachem 2004). In fact, the primary mode of action of pregabalin appears to involve inhibition of depolarization-induced calcium influx at P, Q and N-type voltage-gated calcium channels, resulting in decreased release of excitatory neurotransmitters such as glutamate from nerve terminals. At molecular level, this action results from pregabalin binding to the α-2-δ subunit of calcium channels (Ben-Menachem 2004; Warner and Figgit 2005; Fink et al 2002; Dooley et al 2002; Li et al 2005). Although the mechanism of action of pregabalin does not appear to differ from that of gabapentin, the affinity of pregabalin for the α-2-δ modulatory site is much greater than that of gabapentin. This explains why pregabalin is 3- to 6-fold more potent than gabapentin in animal models of seizures and epilepsy, and also in models of anxiety and neuropathic pain.
Clinical pharmacokinetics
Unlike gabapentin, which shows dose-dependent pharmacokinetics due to decreasing oral bioavailability with increasing dosages, pregabalin exhibits dose-independent pharmacokinetics (Ben-Menachem 2004; Warner and Figgit 2005). This implies that, within the effective dose-range, plasma pregabalin concentrations are linearly related to the prescribed daily dose.
Pregabalin is rapidly and virtually completely absorbed from the gastrointestinal tract, with peak plasma concentrations being observed after about 1 hour of drug intake (Ben-Menachem 2004). The extent of absorption is not affected by concomitant intake of food. Like gabapentin, pregabalin is absorbed from the upper intestine by an amino acid carrier system with affinity for large neutral amino acids (Jezyk et al 1999), although there seem to be differences in regional distribution and sodium dependence between the carrier system for pregabalin and the carrier system for gabapentin (Piyapolrungroj et al 2001). Most importantly, the amino acid carrier responsible for the active uptake of pregabalin in the intestine does not become saturated within the clinically used dose range. Therefore, the oral bioavailability of pregabalin remains above 90% throughout the effective dose range. The same does not apply to gabapentin, which has incomplete bioavailability, is absorbed in only a limited region of the small intestine, and undergoes saturable absorption within the therapeutic dose range, resulting in dose-dependent pharmacokinetics, high inter-patient variability, and potentially ineffective drug exposure (McLean 1994; Cundy et al 2008).
Pregabalin is not bound to plasma proteins and crosses efficiently the blood–brain barrier, although with some delay compared with its appearance into the bloodstream (Feng et al 2001). Pregabalin is negligibly metabolized (<2% of the dose) and is eliminated primarily in urine in unchanged form. The half-life of pregabalin is about 6 hours on average, and is independent of dose and duration of administration (Ben-Menachem 2004). Animal studies, however, suggest that there is a temporal dissociation between plasma pregabalin levels and anticonvulsant action, and that duration of effect may be longer than expected from the half-life of the drug in plasma (Feng et al 2001).
There is little information on the influence of age on pregabalin pharmacokinetics, although preliminary findings suggest that the clearance of the drug decreases in elderly patients (May et al 2007), as anticipated because of the known reduction in renal function in old age (Perucca 2006). As expected, pregabalin clearance is reduced in patients with renal insufficiency (Randinitis et al 2003). A 50% reduction in daily dose is recommended for patients with creatinine clearance between 30 and 60 mL/min, compared with doses used in patients with creatinine clearance >60 mL/min. Further dose reductions are indicated in the presence of more severe renal insufficiency. Since pregabalin is removed extensively by hemodialysis, supplemental doses may be required after a dialysis session (Randinitis et al 2003).
Drug interactions
Since pregabalin is eliminated virtually entirely by renal excretion and does not influence the activity of drug metabolizing enzymes (Ben-Menachem 2004), clinically significant pharmacokinetic drug-drug interactions are not expected to occur with pregabalin. Indeed, results of clinical studies are in agreement with this prediction. In particular, pregabalin does not appear to affect the plasma concentration of concomitantly administered carbamazepine, phenytoin, phenobarbital, valproic acid, gabapentin, lamotrigine, topiramate, oral contraceptives, oral hypoglycemics, diuretics and insulin (Ben-Menachem 2004; Brodie et al 2005; Janiczek-Dolphin et al 2005). Likewise, pregabalin pharmacokinetic does not appear to be affected by co-administration of other AEDs, although a recent study provided suggestive evidence that enzyme-inducing agents such as carbamazepine may cause a 20% to 30% reduction in plasma pregabalin concentrations at steady-state (May et al 2007).
Pregabalin has been shown to exert additive effects on the alterations in cognitive and gross motor functions caused by oxycodone, and to potentiate the central nervous system (CNS) effects of ethanol and lorazepam (Ben-Menachem 2004).
Clinical efficacy against partial-onset seizures
Design of randomized controlled trials
Because it is considered unethical to treat active epilepsy in monotherapy with an agent whose clinical efficacy is as yet unknown (Perucca 2008), new AEDs are initially evaluated as adjunctive therapy in patients whose seizures were not controlled by available treatments. These early trials are usually conducted in patients with partial-onset seizures, mainly because partial epilepsies represent the most prevalent refractory seizure disorder in an adult population.
Pregabalin was no exception to this rule, and to date randomized controlled trials with this agent have been largely confined to double-blind placebo-controlled studies in which the drug has been administered as adjunctive therapy to patients already receiving up to 3 concomitant AEDs. Overall, the patients enrolled in these trials were highly refractory in that they had a mean duration of epilepsy of 25 years and a median seizure frequency of 10 seizures per month, despite treatment over the years with a variety of AEDs (Brodie 2004; Elger et al 2005). Demonstrating efficacy in such a refractory population may be regarded as a more challenging objective than performing a monotherapy study in a newly diagnosed population, because it requires achievement of additive seizure control in a population that is more likely to develop adverse drug effects due to presence of concomitant AEDs often administered at the highest tolerated dose.
Four randomized, double-blind, placebo-controlled parallel-group adjunctive-therapy trials of pregabalin have been completed in a total of 1396 patients with refractory partial-onset seizures, with or without secondary generalization (French et al 2003; Arroyo et al 2004; Beydoun et al 2005; Elger et al 2005; Brodie 2004; Ryvlin 2005; Warner and Figgit 2005; Lozsadi et al 2008) (Table 1).
Table 1.
Overview of randomized placebo-controlled double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization
| Study | Total pregabalin dose (mg/day) | Frequency of administrationa | Titration period | Trial duration | Number of patients (ITT) |
|---|---|---|---|---|---|
| French et al 2003 | 50 | bid | None | 8 weeks baseline | 453 |
| 150 | bid | 12 weeks double-blind | |||
| 300 | bid | ||||
| 600 | bid | ||||
| Arroyo et al 2004 | 150 | tid | 1 week | 8 weeks baseline | 287 |
| 600 | tid | 12 weeks double-blind | |||
| Beydoun et al 2005 | 600 | bid | 1 week | 8 weeks baseline | 312 |
| 600 | tid | 12 weeks double-blind | |||
| Elger et al 2005 | Fixed 600 | bid | None | 6 weeks baseline | 341 |
| Flexible 150–600 | bid | Flexible 12 weeks | 12 weeks double-blind |
bid, twice daily; tid, three times daily.
In 3 fixed-dose studies, which enrolled a total of 1052 patients, different dosing regimens were simultaneously evaluated (Table 1). All studies used a similar parallel-group design which involved an 8-week prospective baseline period followed by randomization to placebo and 2 to 4 different dosing regimens. Eligibility criteria for these studies included (i) a history of failure to obtain seizure control in spite of a trial of at least two AEDs at maximally tolerated doses; (ii) ongoing treatment with 1 to 3 AEDs; and (iii) occurrence of at least 6 partial seizures and no more than 4 weeks without seizures during an 8-week baseline phase (French et al 2003; Arroyo et al 2004; Beydoun et al 2005). In the first study, which enrolled 453 patients aged 12 years and older in North America, patients were randomized to placebo or 50, 150, 300, or 600 mg/day pregabalin using a twice daily dosing regimen without titration (French et al 2003). The second study, conducted in Europe, South Africa and Australia, randomized 287 patients aged 18 years and older to placebo or pregabalin 150 or 600 mg/day with a 3 times daily dosing regimen with up to 1 week of titration (Arroyo et al 2004). In the third study, conducted in North America, 312 patients (≥18 years old) were randomized to placebo, pregabalin 600 mg/day on a twice daily regimen or pregabalin 600 mg/day on a 3 times daily regimen, each with up to 1 week of titration (Beydoun et al 2005).
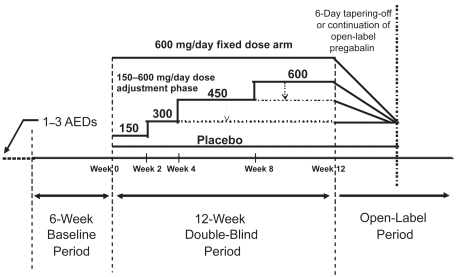
Unlike the other trials, the fourth trial aimed at assessing the potential of pregabalin given with a flexible dose regimen resembling routine clinical practice in comparison with a high-dose, fixed-dose regimen (Elger et al 2005). In this 12-week placebo-controlled trial, conducted at 53 centers in Europe and Canada, 341 patients aged 18 years and older were enrolled based on inclusion criteria that required concomitant treatment with up to 3 AEDs and occurrence of at least 4 partial-onset seizures with no more than 4 weeks without seizures during a 6-week baseline phase. Patients were then randomized to receive placebo, a fixed pregabalin dose of 600 mg/day without titration or a flexible pregabalin regimen with dosages individually adjusted within the 300 to 600 mg/day dose range (Table 1 and Figure 1). In the flexible-dose group, pregabalin was started at 150 mg/day for 2 weeks followed by 300 mg/day for the subsequent 2 weeks; thereafter, dosage could be increased to 450 mg/day for 4 weeks and to 600 mg/day for another 4 weeks if a patient showed acceptable tolerability and was not seizure-free in each 4-week period. If intolerable adverse effects developed at 450 or 600 mg/day in the flexible-dose group, dosage could be brought back to the previous level. In both groups, the total daily dose was given in two divided administrations. To maintain the blind, all patients could reduce the actual number of capsules of study medication, but only those in the flexible-dose group received an actual drug reduction.
Figure 1.
Trial design used in a pregabalin randomized flexible-dose (150–600 mg/day) versus fixed-dose (600 mg/day) double-blind adjunctive-therapy trial in patients with refractory partial-onset seizures, with or without secondary generalization. Adapted from Elger et al 2005.
Efficacy endpoints
Complete seizure freedom is the ultimate goal of antiepileptic treatment for both physicians and patients alike. However, since the typical patient populations recruited in adjunctive therapy trials of new AEDs are highly refractory, complete seizure control is not really a realistic expectation in these patients (Walker and Sander 1996). Thus, in common with other adjunctive therapy trials and as recommended by FDA and EMEA guidelines (Committee for Proprietary Medicinal Products 2000;French 2001; Mohanraj and Brodie 2003), the primary endpoints employed in the add-on pregabalin trials focused on changes in seizure frequency between the baseline period and the treatment period.
Traditionally, changes in seizure frequency are determined by counting seizures during the baseline and treatment period and then comparing the results (expressed in terms of frequency, eg, seizure counts per 28-day epoch) in patients receiving the drug with those obtained in patients receiving placebo. A problem with analyzing seizure frequency data, however, relates to the non-normal distribution of the data and the high degree of variation both between subjects and within subjects (French 2001). In particular, while seizure frequency can only be reduced by a maximum of 100%, there is no limit to the percentage increase that this parameter can undergo. In the pregabalin trials, this problem has been resolved by using the Response Ratio (RRatio) as the primary measure of seizure reduction. The RRatio is defined as the difference between the 28-day seizure rates during treatment (T) and during baseline (B), divided by the sum of baseline and treatment seizure rates, and multiplied by 100:
The advantage of the RRatio is that all values fall within the range between −100 and +100, and can be analyzed using parametric statistical methods (Mohanraj and Brodie 2003). Negative values of the RRatio represent an improvement in seizure rate (for example, a value of −33 is equivalent to a 50% reduction in seizure frequency versus baseline), whereas positive values reflect a deterioration. The percent change in seizure frequency from baseline can be derived from the RRatio by using the following formula (Arroyo et al 2004):
Since percent change in seizure frequency reflects more directly the magnitude of response and is a more familiar measure to physicians, in the current review efficacy results have been converted to this parameter. Additionally, as required by EMEA guidelines (Committee for Proprietary Medicinal Products 2000), efficacy data were also expressed by categorizing patients into responders and non-responders, with responders being conventionally defined as those patients who had a reduction in seizure frequency by at least 50% compared with baseline.
Experience with previous drug trials in epilepsy has shown that only few drug-refractory patients achieve seizure freedom after starting adjunctive-therapy with a new AED. Therefore, seizure freedom is unsuitable for use as a primary endpoint, although it may be assessed as a secondary endpoint. As discussed in detail in a recent publication (Leppik et al 2006), there are different ways to define freedom from seizures. One aspect of the definition relates to the minimum length of time without seizure that is required to categorize a patient as seizure-free. In the pregabalin trials, the last 28 days of double-blind treatment was selected as the period during which freedom from seizures was evaluated, a choice justified by the high median seizure frequency of the enrolled patients (about 10 seizures per month) and the requirement that no patient could have a 28-day seizure-free interval during the baseline. There is also scope in assessing the proportion of patients free from seizures during the entire double-blind treatment period, which is likely to be a more clinically meaningful measure. However, choice of this endpoint raises the question of how patients who discontinued the study early during treatment should be evaluated, and which patients should be included in the analysis. For example, if a patient discontinued treatment after 7 days because of adverse effects and had no seizure during that brief period, should that patient be counted as seizure-free in the calculation of seizure-free rates? In the conventional intent-to-treat (ITT) analysis, responses during the available observation period are extrapolated to the entire treatment period (the so-called last-observation-carried-forward analysis), and therefore any patient discontinuing treatment prematurely prior to occurrence of any seizure would be regarded as seizure-free. It is clear, however, that this method of calculation leads to overestimation of actual seizure-free rates, particularly in groups randomized to doses which are associated with high discontinuation rates. A conservative approach that avoids this problem is to consider as seizure free only those patients who complete the entire treatment period without seizure, and calculating seizure-free rates by using the entire ITT population as denominator (Leppik et al 2006). A comparison of seizure freedom rates in fixed-dose pregabalin rates using these two methods (eg, last-observation-carried-forward ITT analysis versus ITT analysis in which only seizure-free completers are considered) has been conducted (Gazzola et al 2007). Additionally, an intermediate approach has also been used, in which an ITT last-observation-carried-forward analysis was performed but only patients who had been on medication for at least 28 days and completed at least 75% of their seizure diary could be considered seizure-free.
Another type of seizure freedom analysis used in AED trials involves counting the number of seizure-free days in each treatment group (French and Arrigo 2005). This type of analysis considers each day individually for each patient and examines whether or not a seizure has occurred: proportion of patients free from seizures on any treatment day can then be compared across treatment groups. This approach, which has the advantage of providing information on speed of onset of anticonvulsant activity and potential changes in magnitude of response over time, was used in a post-hoc analysis of pooled data from fixed-dose pregabalin trials (Perucca et al 2002).
Efficacy results from fixed-dose trials
The three fixed-dose placebo-controlled studies provide valuable information for the characterization of dose-response relationships, including identification of a minimum effective dose and of a maximal tolerated dose, and exploration of optimal dosing intervals. In each of these studies, pregabalin, given either 2 or 3 times daily, exhibited clear efficacy in reducing seizure frequency when administered as adjunctive therapy in patients with refractory partial-onset seizures (French et al 2003; Arroyo et al 2004; Beydoun et al 2005).
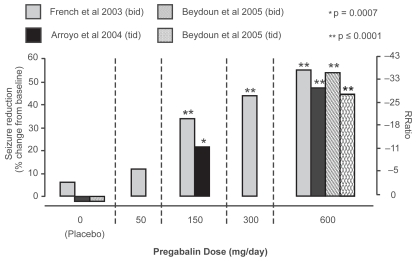
At baseline, patients had a mean seizure rate of 24.4 seizures per month (median 11.2 per month), and 73% were receiving a combination of least 2 AEDs (Brodie 2004; Ryvlin 2005). RRatios and corresponding percent reductions in seizure frequency as a function of the allocated dose in each of the three trials are shown in Figure 2. Seizure frequency was significantly reduced, in comparison to placebo, at doses of 150, 300 and 600 mg/day, whereas response at the 50 mg/day dose did not differ significantly from placebo (French et al 2003; Brodie 2004; Ryvlin 2005). The minimal effective dose was therefore established at 150 mg/day. The decrease in seizure frequency over the assessed dose range was also found to be dose-dependent, irrespective of the dosing regimen used (twice or three times daily). Responder rates (proportion of patients with ≥50% reduction in seizure frequency compared with baseline) also increased with increasing dose, and ranged from 14% to 51% in pregabalin groups compared to 6% to 14% in the placebo groups (Figure 3).
Figure 2.
Seizure reduction in short-term fixed-dose pregabalin adjunctive therapy studies. Dose response relationship for seizure reduction (shown as response ratio [RRatio] on right y axis and percent change from baseline as calculated from RRatio on left y-axis) is shown for each of the three short-term fixed-dose pregabalin studies (French et al 2000; Arroyo et al 2004; Beydoun et al 2005). P values shown represent a significant difference from placebo in the same study. Adapted from Brodie et al 2004.
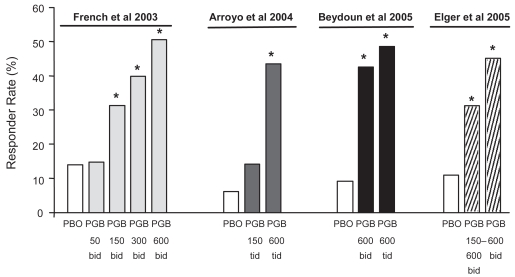
Figure 3.
Responder rates (proportion of patients with ≥50% reduction in seizure frequency compared with baseline) in each of four randomized adjunctive-therapy double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization. The first three trials (French et al 2003; Arroyo et al 2004; Beydoun et al 2005) included fixed-dose pregabalin treatment groups whilst the fourth trial (Elger et al 2005) included both flexible-dose (150–600 mg/day) and fixed-dose (600 mg/day) groups. *Significantly different from placebo in the same study.
Abbreviations: PGB, pregabalin; PBO, placebo.
A recent additional post-hoc analysis of pooled data from the three fixed-dose trials examined the influence of pregabalin on secondarily generalized tonic-clonic seizures (Briggs et al 2008). In this analysis, a 600 mg/day dose was found to be significantly superior to placebo in reducing the absolute frequency of secondarily generalized tonic-clonic seizures, but the effect appeared to be related to suppression of partial-onset seizures rather than to inhibition of secondary generalization.
Across the fixed-dose trials, between 3% and 17% of patients randomized to effective doses were seizure-free during the last 28 days of treatment, and seizure-free rates increased with increasing doses (Brodie 2004). In comparison, no more than 1% of patients allocated to the placebo groups were free from seizures during the corresponding period. In the ITT last-observation-carried-forward analysis of all patients randomized to pregabalin in each trial (all doses), seizure-free rates across the entire treatment period ranged across trials from 3.7% to 7.9% (Gazzola et al 2007). However, in the conservative ITT analysis in which only completers could be considered seizure-free (ie, patients withdrawing from the trial were considered not seizure-free, even when no seizures had occurred up to the time of withdrawal), seizure freedom rates decreased to 1.3% to 1.4% (Gazzola et al 2007). In the modified ITT analysis in which the last-observation-carried-forward extrapolation was applied only to patients who had been on medication for at least 28 days and had completed at least 75% of their seizure diary, significantly higher seizure-free rates on pregabalin than on placebo were found for the 300 mg/day dose (1 study – French et al 2003) and for the 600 mg/day dose (3 studies – French et al 2003; Arroyo et al 2004; Beydoun et al 2005).
Seizure-free days analysis on the pooled dataset including five doses (150–600 mg/day) in fixed-dose studies showed that the onset of pregabalin action is rapid, with a reduction in seizure activity compared with placebo being already statistically significant on the second day of treatment (Perucca et al 2002). The reduction in seizure activity documented by seizure-free day analysis persisted throughout the 12-week evaluation period.
In conclusion, the fixed-dose studies have shown that adjunctive therapy with pregabalin is effective in reducing dose-dependently the frequency of partial-onset seizures within the 150 to 600 mg/day dose range. Efficacy was comparable with either twice or three times daily dosing, was rapid in onset and persisted for the full duration of assessment. These findings provide the rationale for the implementation of a monotherapy development programme, which is underway.
Efficacy results from the flexible-dose trial
The efficacy of pregabalin in reducing the frequency of partial-onset seizures was confirmed in the trial that compared fixed (600 mg/day) with flexible dosing (150–600 mg/day) (Elger et al 2005). The percent reduction in the frequency of seizures was statistically significant (p < 0.01) both in the group allocated to a fixed dose (49.3%) and in the group allocated to a flexible dose (35.4%), compared with the group allocated to placebo (10.6%). Proportions of patients with a >50% seizure reduction were 45.3% in the fixed-dose group (p < 0.001 versus placebo) and 31.3% in the flexible-dose group (p < 0.001), compared with 11.0% in the placebo group. These rates are comparable to those found in the fixed-dose trials (Figure 3). Seizure freedom rates during the last 28 days of treatment were 12.4% in the fixed-dose group, 12.2% in the flexible-dose group, and 8.2% in the placebo group.
Comparison with results from randomized controlled trials with other second generation AEDs
Responder rates with pregabalin in adjunctive-therapy randomized controlled trials compare favorably with those reported in similarly designed trials of other second generation AEDs in patients with refractory partial-onset seizures (Cramer et al 1999; Brodie 2004). However, when comparing data from different trials, differences in responder rates in the groups assigned to placebo should also be considered. Therefore, rather than comparing absolute responder rates, it may be more relevant to compare the relative risk (RR) of being a responder on drug treatment in relation to the responder rate on placebo. Indirect comparisons should also take into account systematic bias such as the differences in placebo response rates between pediatric and adult populations (Rheims et al 2008). In a systematic review of all randomized controlled AED trials in adults with refractory partial epilepsy, in which the above confounders were taken into account, pregabalin ranked second in efficacy among the 10 most recently developed AEDs (Ryvlin et al 2006). When ineffective dosages were excluded from the analysis, pregabalin was associated with the highest RR for 50% responder rates, followed in decreasing order of responder rates by levetiracetam, topiramate, oxcarbazepine, tiagabine, zonisamide, vigabatrin, gabapentin, and lamotrigine (Ryvlin et al 2006). However, 95% confidence intervals of RRs overlapped for all the assessed drugs, indicating that differences among AEDs failed to reach statistical significance. This is more likely to reflect the low statistical power of the comparisons, rather than equivalence of responder rates among AEDs.
Long-term efficacy data
Most patients with epilepsy need to continue treatment for many years, sometimes for a lifetime. Therefore, it is imperative that the long-term efficacy and safety of any new AEDs be carefully investigated. Since it would be unethical to keep patients who are prone to seizures on placebo treatment for several years under double-blind conditions for comparative purposes, data on long-term efficacy and safety must be obtained in open-label follow-up studies. It is usual for these studies to be an extension of placebo-controlled trials, and this was also the case for most follow-up studies with pregabalin.
In clinical studies with a follow-up of several years, seizure freedom is probably the most useful indication of a drug’s efficacy, since this is the ultimate goal of AED treatment (Mohanraj and Brodie 2003). The most meaningful seizure freedom measure under these conditions is probably represented by the proportion of patients who are free from seizures over a pre-defined period. Additional outcome measures that may be used to evaluate clinical benefit over time include the number of seizure-free days over different intervals, and the proportion of responders (patients with a reduction in seizure frequency by 50% compared with pre-treatment) at different time points during follow-up. Whatever outcome measure is selected, results need to be interpreted cautiously not only because of the uncontrolled nature of these observations, but also because of confounders such as concomitant changes in underlying AED treatment, difficulties with accounting for patients who discontinue the study drug (resulting in an “enriched population”, whereby only those patients who appear to benefit from the drug remain on it), and the potential bias caused by missing data from patients lost to follow-up.
In the case of pregabalin, an assessment of long-term outcome has been made based on results in 4 open-label studies in a total of 1480 patients, representing a cumulative exposure of 3150 patient-years (Pfizer, data on file). Of these patients, 968 took part in earlier randomized double-blind trials or in a small short-term in-patient monotherapy trial, while 512 started open-label treatment de novo. Follow-up of this population has been complicated by the fact that about 2 to 3.5 years after enrolment, the Food and Drug Administration (FDA) stipulated that patients in the United States could remain in these studies only if they were refractory to other AEDs and had shown a favorable response to pregabalin, defined as a reduction by at least 30% in the frequency of their seizures relative to pre-treatment.
At the time of completion of the open-label studies, approximately 20% of patients had received pregabalin for ≥5 years, 35% for ≥2 years, 59% for ≥1 year and 77% for ≥24 weeks. Overall, 71% and 48% of patient years were exposed to pregabalin doses ≥450 mg/day and 600 mg/day, respectively. At the time of enrolment in the open-label period, about 50% of the patients were receiving two concomitant AEDs. The FDA requalification of the criteria for remaining in the study led to withdrawal of 188 patients (13% of those initially enrolled, and 44% of the 431 patients that were assessed for requalification). Since the requalification process complicates the interpretation of long-term outcome by allowing continuation in the study only for patients who were responding favorably, the data collected after the time of initial requalification were not included in the analysis of changes in seizure frequency. Assessment of seizure freedom data, however, was done also done on all data.
After exclusion of data collected after requalification, the mean number of seizure-free days per 28-day period increased from 18.3 days at pre-treatment to 21.6 days during pregabalin treatment, with an overall mean 39% increase in seizure-free days in individual patients. Of 892 patients with a follow-up of at least 6 months prior to requalification, 69 (7.7%) were seizure-free during their last 6 months on pregabalin treatment, whereas of 710 patients with a follow-up of at least 1 year, 26 (3.7%) were seizure-free during the last 12 months (Table 2). When the same analysis was done on all ITT data (including those collected after requalification), the mean number of seizure-free days per 28-day period increased from 18.3 days at pre-treatment to 21.5 days during pregabalin treatment, with a mean 41.6% increase in number of seizure-free days in individual patients. In this enlarged analysis, the proportions of patients seizure-free during the last 6 and 12 months of treatment were 9.2% (103/1119) and 8.1% (71/877), respectively (Table 2).
Table 2.
Seizure freedom rates in patients treated with long-term adjunctive-therapy pregabalin
| Elapsed time between last seizure and final observation | All patients, all data
|
Data up to initial requalificationa |
||||
|---|---|---|---|---|---|---|
| Number assessed | Number seizure-free | % seizure-free | Number assessed | Number seizure-free | % seizure-free | |
| At least 1 month | 1423 | 311 | 21.9% | 1115 | 219 | 19.6% |
| At least 2 months | 1361 | 203 | 14.9% | 1064 | 145 | 13.6% |
| At least 3 months | 1300 | 156 | 12.0% | 1018 | 105 | 10.3% |
| At least 4 months | 1250 | 128 | 10.2% | 984 | 83 | 8.4% |
| At least 6 months | 1119 | 103 | 9.2% | 892 | 69 | 7.7% |
| At least 1 year | 877 | 71 | 8.1% | 710 | 26 | 3.7% |
| At least 2 years | 511 | 42 | 8.2% | 192 | 6 | 3.1% |
| At least 3 years | 354 | 26 | 7.3% | 59 | 1 | 1.7% |
Notes: Results are pooled data from four open-label studies using both the entire ITT population (all data) and the ITT population with only data obtained before a patient’s initial requalification.
Patients from the US were required to undergo requalification to determine eligibility to continue in the trial, and only data collected prior to initial requalification were included for these patients (see text). For unaffected patients, data for the entire open-label treatment period were included.
In the subgroup of evaluable patients who had participated in fixed-dose double-blind trials, assessment of seizure frequency during the first 12 weeks of open-label treatment indicated that 37% had a seizure-reduction of at least 50% compared with baseline. For those patients who had remained in the study for 2 years, the responder rate during the initial 12-week open-label period was 52% and remained in the range of 50% to 58% during subsequent intervals. For the cohorts of patients that had remained in the study for 6 months or 1 year, a similar pattern of sustained reduction in seizure frequency compared with baseline was observed. These data suggest that response to pregabalin is maintained during long-term treatment, although, as discussed above, interpretation should be cautious because an influence of several potential bias cannot be excluded in open-label long-term follow-up studies.
Safety and tolerability profile
Results from randomized-controlled trials
A useful assessment of adverse effects associated with pregabalin treatment and their relationship to dose in patients with refractory partial-onset seizures can be derived from the four double-blind adjunctive-therapy studies conducted in this population (French et al 2003; Arroyo et al 2004; Brodie 2004; Beydoun et al 2005; Elger et al 2005; Ryvlin 2005; Warner and Figgitt 2005). Table 3 lists the most common adverse events in patients allocated to each pregabalin dose group and to placebo in a pooled analysis of these trials (French et al 2003; Arroyo et al 2004; Beydoun et al 2005; Elger et al 2005).
Table 3.
Most common adverse events reported in randomized placebo-controlled double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization
| Frequency (%)
|
||||||
|---|---|---|---|---|---|---|
| Pregabalin daily dose
|
||||||
| Adverse event | 50 mg | 150 mg | 300 mg | 600 mg | Flexible, 150–600 mg | Placebo |
| n = 88 | n = 187 | n = 90 | n = 533 | n = 131 | n = 294 | |
| Dizziness | 9.1 | 17.6 | 31.1 | 33.8 | 24.4 | 10.5 |
| Somnolence | 10.2 | 11.2 | 17.8 | 25.5 | 19.1 | 10.9 |
| Ataxia | 3.4 | 5.9 | 10 | 19.9 | 9.2 | 4.1 |
| Fatigue | 5.7 | 10.7 | 12.2 | 18 | 16.8 | 8.2 |
| Headache | 6.7 | 7.5 | 5.6 | 10.1 | 13.7 | 11.6 |
| Weight gaina | 1.1 | 4.8 | 6.7 | 17.1 | 19.1 | 1.4 |
| Withdrawal for adverse events | 6.9 | 5.9 | 14.4 | 24.2 | 12.2 | 6.3 |
Notes: Data were pooled from four studies (French et al 2003; Arroyo et al 2004; Beydoun et al 2005; Elger et al 2005). Some patients reported >1 adverse event.
Weight gain spontaneously reported as an adverse event by patient.
In fixed-dose trials, dizziness was the most frequently reported adverse event and showed a clear relationship with dose, being recorded in about one third of patients at 300 and 600 mg/day, compared with one tenth of patients allocated to placebo. Somnolence, ataxia and fatigue also increased in frequency with increasing doses, whereas headache occurred in all dose groups at a frequency comparable with that recorded in patients randomized to placebo. Adverse events were generally mild to moderate, they tended to occur mostly in the first 2 weeks of treatment, and they often resolved without adjustment in dose. Serious treatment-related adverse events occurred in 4 cases among pregabalin-treated patients and in 2 cases treated with placebo (Brodie 2004). In fixed-dose studies, 15% of patients allocated to pregabalin discontinued treatment prematurely because of adverse events, compared with 6% of patients allocated to placebo (Brodie 2004; Ryvlin 2005). At 50 and 150 mg/day, discontinuation rates were comparable to those recorded in the placebo group, whereas at higher doses discontinuation rates increased dose-dependently. At the highest dose of 600 mg/day, 24% of patients withdrew prematurely for adverse events (Table 3).
The possible relationship between adverse events and dosing frequency was assessed in a double-blind trial in which groups were randomized to the same total daily dose (600 mg/day) given in either 2 or 3 divided daily administrations (Beydoun et al 2005). There was no clear evidence of either dosing regimen being superior to the other in terms of efficacy or tolerability. The most common adverse event, dizziness, occurred in 41.7% of patients allocated to the twice daily regimen and in 37.8% of those allocated to the 3 times daily regimen. Somnolence was slightly more common in the twice daily group than in the three times daily group (30.1% vs 23.4%). Discontinuation rates for adverse events were also slightly higher with the twice daily regimen than in the 3 times daily regimen (26% vs 19%, respectively), suggesting that some patients may tolerate the latter regimen more favorably.
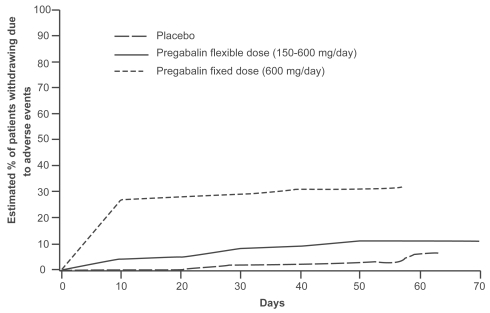
In the flexible-dose study, gradual up-titration of dose, associated with dose reduction if intolerable adverse events occurred, was found to have a clearly favorable impact on pregabalin’s tolerability (Elger et al 2005). In this study, dizziness was the most common adverse event in both groups, but it occurred with a much lower frequency in patients allocated to flexible-dosing (150–600 mg/day) than in those allocated to 600 mg/day fixed-dose without titration (24.4% vs 43.1%, respectively). Ataxia and weight gain were the other most commonly reported adverse events in the fixed-dose group, and occurred in 21.2% and 20.4% of patients, respectively. Other adverse events in the flexible-dose group included somnolence and weight gain, each occurring in 19.1% of patients. The overall improvement in tolerability in the group allocated to flexible-dosing was best reflected by the finding that only 12.2% of patients in this group discontinued treatment because of adverse events, a proportion only slightly higher than that reported in the group allocated to placebo (6.8%). Conversely, about one third of patients (32.8%) allocated to the fixed-dose group withdrew because of adverse events. As shown in Figure 4, discontinuation of treatment, which was mostly caused by adverse events, occurred mostly during the first weeks of treatment, particularly in the fixed-dose group. In the first week of treatment, only 3% of patients in the flexible dose group withdrew due to adverse events, compared with 24% of those allocated to the fixed-dose group. In summary, the data indicate that pregabalin tolerability is improved by gradual titration from a starting dose of 150 mg/day, with subsequent adjustment according to clinical response. It is reasonable to expect that, in most situations, such a flexible regimen will reflect the optimal mode of use of the drug. However, the fact that the majority of patients in the fixed-dose group tolerated the maximum dose (600 mg/day) from the outset indicates that initiation with high doses without titration is a feasible option, particularly in special cases where rapid attainment of seizure control is an utmost priority.
Figure 4.
Kaplan-Meier analysis of time to discontinuation due to adverse events with flexible (150–600 mg/day) versus fixed (600 mg/day) pregabalin dosing. Patients in the fixed-dose group discontinued from the study due to adverse events earlier than those in the flexible-dose group. Adapted from Elger et al 2005.
An adverse event that appears to be unrelated to speed of titration is an increase in body weight. Overall, in double-blind studies, weight gain was spontaneously reported as an adverse event in 10.4% of pregabalin-treated patients compared with 1.4% of patients treated with placebo. However, only 0.4% of patients in double-blind studies discontinued treatment as a result of weight gain. In fixed-dose trials, the proportion of patients reporting increased body weight as an adverse event increased with increasing dose, from 1.1% at 50 mg/day to 4.8% at 150 mg/day, 6.7% at 300 mg/day and 17.1% at 600 mg/day (Table 3). In all double-blind trials, body weight was measured at baseline and at the last study visit. By defining as significant an increase by ≥7% over baseline, a significant gain in weight was observed in 18% of pregabalin-treated patients, compared with 2.1% of those treated with placebo. In the majority of these patients, the weight increase did not exceed 10% of the baseline weight. Change in weight were not associated with changes in lipids or loss of glycemic control.
No deaths were recorded during pregabalin double-blind epilepsy trials. In trials conducted in other indications, the tolerability profile of pregabalin was similar to that reported in patients with epilepsy, except for peripheral oedema which occurred more commonly in studies conducted in neuropathic pain than in epilepsy trials (Freeman et al 2008). Pregabalin has been designated as a Schedule V controlled substance in the United States because of concerns about possible abuse and dependence. These concerns, however, do not appear to be of significance for the use of pregabalin as an antiepileptic drug.
Results from long-term follow-up studies
Adjunctive-therapy with pregabalin was in general relatively well tolerated in long-term open-label studies, and no new safety concerns have been identified during extended follow-up. Of 1480 patients included in the four open-label studies discussed above, 23 died during or after the studies for reasons that the investigator did not consider related to the drug. In 5 cases of these cases, death appeared to be related to seizures (Pfizer, data on file). There were 246 patients with serious adverse events, but in only 15 cases (1.0% of the total population) were these considered to be related to pregabalin. Overall, 193 patients (13%) withdrew due to adverse events, which were considered as treatment-related in 160 (11%) patients.
Most adverse events during long-term studies were comparable to those that emerged in randomized controlled studies. Adverse events recorded during long-term treatment in ≥5% of patients included dizziness, somnolence, weight gain, ataxia, visual disturbances, difficulties with attention/concentration, nausea, tremor, amnesia, depression, insomnia, nervousness, anxiety, and confusion. Most of these events were mild or moderate in intensity and often resolved with continued treatment. Accidental injury, infection, headache, asthenia, and pain were also recorded in an appreciable proportion of patients (Table 4), but they were generally not considered to be drug-related. Infrequently reported adverse events include myoclonus (Huppertz et al 2001; Hellwig et al 2008; Kalviainen et al 2008; Modur and Milteer 2008), painful gynecomastia (Málaga and Sanmarti 2006), and erectile dysfunction (Hitiris et al 2006). Very rarely did adverse events lead to discontinuation of treatment (Table 4).
Table 4.
Most common adverse events (reported by ≥10% of all patients) among 1480 patients treated with long-term adjunctive-therapy pregabalin based on pooled data from 4 open-label studies
| Adverse event | Frequency (%) | Withdrawals due to event (%) |
|---|---|---|
| Dizziness | 33.9 | 1.4 |
| Accidental injury | 28.6 | 0.3 |
| Somnolence | 27.4 | 1.7 |
| Weight gaina | 23.6 | 2.0 |
| Infection | 22.4 | 0 |
| Headache | 20.0 | 0.6 |
| Asthenia | 19.9 | 1.2 |
| Pain | 17.8 | 0.1 |
| Ataxia | 14.3 | 0.8 |
| Amblyopia | 14.1 | 0.5 |
| Diplopia | 11.8 | 0.2 |
| Thinking abnormalb | 11.7 | 0.9 |
| Nausea | 10.5 | 0.6 |
Weight gain spontaneously reported as an adverse event by patient.
Coded term used to indicate difficulties with attention or concentration.
The proportion of patients reporting weight gain as an adverse event was 24%. The mean weight gain at study termination compared with baseline was approximately 5 kg, with 44% of patients gaining ≥7% of their initial weight. In contrast to short-term trials which suggested a relationship between weight gain and pregabalin dose, in long-term studies weight gain did not appear to correlate with absolute dose (mg/day), weight-normalized dose (mg/kg/day), or categorized dosage (more or less than 300 mg/day) (Hoppe et al 2008). In one study, extended patient counseling was not found to be effective in preventing the occurrence of weight gain (Hoppe et al 2008).
Conclusions
Although the pregabalin clinical trial program was primarily designed to provide proof of efficacy and safety for regulatory purposes, the findings also provide relevant information for the practicing physician. Four randomized-placebo-controlled studies have demonstrated that pregabalin is effective as adjunctive therapy in the management of adults with partial-onset seizures, with or without secondary generalization (Ryvlin et al 2006;Beydoiun et al 2008; Lozsadi et al 2008). Since pregabalin in these studies did not affect the plasma levels of concomitantly administered drugs, its efficacy cannot be explained by pharmacokinetic interactions with underlying AEDs.
In short-term trials, pregabalin reduced seizure frequency significantly at doses between 150 and 600 mg/day, with a clear dose-response relationship and a rapid onset of action. Efficacy appeared to be maintained during long-term treatment, with no clear evidence of tolerance developing over a follow-up period of up to 4 years. In long-term open-label studies, close to 8% of pregabalin-treated patients were free from seizures during the last 6 months of observation, and 3.7% were seizure-free during the previous 12 months.
In fixed-dose studies, dizziness and sedation were the most common dose-limiting adverse events. Up to a dosage of 300 mg/day, discontinuation rates for adverse events were similar or only slightly higher than those reported in placebo-treated patients, but at a dosage of 600 mg/day a quarter of patients withdrew prematurely due to adverse events. Although most patients can tolerate doses as high as 600 mg/day without titration, tolerability is clearly improved by gradual dose titration and dose adjustments according to clinical response (Elger et al 2005). A twice daily dosing regimen appears to be generally appropriate, although a 3 times daily regimen may be considered if treatment is suboptimally tolerated, particularly in patients receiving doses in the upper range.
Based on available data, adjunctive pregabalin will be a useful therapeutic tool for clinicians concerned with the long-term management of patients with refractory partial epilepsy. Given the efficacy of pregabalin in the treatment of generalized anxiety disorder and neuropathic pain, patients with these comorbidities appear to be particularly suitable candidates to pregabalin treatment. Pregabalin has not been found to be useful in generalized epilepsies, and may even aggravate seizures when used in patients with certain generalized epilepsy syndromes such as progressive myoclonic epilepsies (Kalviainen et al 2008). Among patients with partial epilepsy, no specific factors have been identified that may be used to predict responsiveness to pregabalin. In most cases, an already effective starting dose can be 150 mg/day, given in 2 divided administrations, without the need for titration. If needed for additional efficacy, doses of 300 and 600 mg/day may be used, and increasing dose flexibly in relation to efficacy and tolerability appears to be the best strategy to ensure an optimal clinical response.
Footnotes
Disclosures
Philippe Ryvlin has received speaker’s or consultancy fees from the manufacturers of carisbamate and topiramate (Johnson and Johnson), ethosuximide, gabapentin, phenytoin and pregabalin (Pfizer), lamotrigine (GSK), lacosamide and levetiracetam (UCB Pharma), retigabine (Valeant), tiagabine, valproic acid and vigabatrin (Sanofi-Aventis), and rufinamide and zonisamide (Eisai).
Emilio Perucca has received speaker’s or consultancy fees and/or research grants from the manufacturers of carbamazepine and oxcarbazepine (Novartis), carisbamate and topiramate (Johnson and Johnson), eslicarbazepine (BIAL), ethosuximide, gabapentin, phenytoin and pregabalin (Pfizer), lamotrigine (GSK), lacosamide and levetiracetam (UCB Pharma), retigabine (Valeant), tiagabine, valproic acid and vigabatrin (Sanofi-Aventis), and rufinamide and zonisamide (Eisai).
Sylvain Rheims has received speaker’s fees from the manufacturer of ethosuximide, gabapentin, phenytoin and pregabalin (Pfizer).
References
- Arroyo S, Anhut H, Kugler AR, et al. Pregabalin add-on treatment: a randomized double-blind placebo-controlled dose-response study in adults with partial seizures. Epilepsia. 2004;45:20–7. doi: 10.1111/j.0013-9580.2004.31203.x. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Wedekind D, Leon T. Pregabalin for the treatment of generalized anxiety disorder: a novel pharmacologic intervention. Expert Rev Neurother. 2007;7:769–81. doi: 10.1586/14737175.7.7.769. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl 6):13–8. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
- Beydoun A, Uthman BM, Kugler AR, et al. Safety and efficacy of two pregabalin regimens for partial epilepsy: an add-on, placebo-controlled trial. Neurology. 2005;64:475–80. doi: 10.1212/01.WNL.0000150932.48688.BE. [DOI] [PubMed] [Google Scholar]
- Beydoun A, Nasreddine W, Atweh S. Efficacy and tolerability of pregabalin in partial epilepsy. Expert Rev Neurother. 2008;8:1013–24. doi: 10.1586/14737175.8.7.1013. [DOI] [PubMed] [Google Scholar]
- Blommel ML, Blommel AL. Pregabalin: an antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm. 2007;64:1475–82. doi: 10.2146/ajhp060371. [DOI] [PubMed] [Google Scholar]
- Bockbrader HN, Miller R, Frame B, et al. The concomitant use of pregabalin and oral contraceptives does not affect the efficacy of either agent. Epilepsia. 2005;46(Suppl 8):170. [Google Scholar]
- Briggs DE, Lee CM, Spiegel K, et al. Reduction of secondarily generalized tonic-clonic (SGTC) seizures with pregabalin. Epilepsy Res. 2008 doi: 10.1016/j.eplepsyres.2008.07.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Brodie MJ. Pregabalin as adjunctive therapy for partial seizures. Epilepsia. 2004;45(Suppl 6):19–27. doi: 10.1111/j.0013-9580.2004.455004.x. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Wilson EA, Wesche DA, et al. Pregabalin drug interaction studies: lack of effect on the pharmacokinetics of carbamazepine, phenytoin, lamotrigine, andvalproate in patients with partial epilepsy. Epilepsia. 2005;46:1407–13. doi: 10.1111/j.1528-1167.2005.19204.x. [DOI] [PubMed] [Google Scholar]
- Committee for Proprietary Medicinal Products (CPMP) Note for guidance on clinical investigation of medicinal products in the treatment of epileptic disorders. CPMP/EWP/566/98rev1. London. [Accessed on 25 August 2006];2000 URL: www.emea.eu.int/pdfs/human/ewp/056698en.pdf.
- Cramer JA, Fisher R, Ben-Menachem E, et al. New antiepileptic drugs: comparison of key clinical trials. Epilepsia. 1999;40:590–600. doi: 10.1111/j.1528-1157.1999.tb05561.x. [DOI] [PubMed] [Google Scholar]
- Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of Gabapentin. J Clin Pharmacol. 2008 Sep 30; doi: 10.1177/0091270008322909. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Meder WP, et al. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse. 2002;45:171–90. doi: 10.1002/syn.10094. [DOI] [PubMed] [Google Scholar]
- Elger CE, Brodie MJ, Anhut H, et al. Pregabalin add-on treatment in patients with partial seizures: a novel evaluation of flexible-dose and fixed-dose treatment in a double-blind, placebo-controlled study. Epilepsia. 2005;46:1926–36. doi: 10.1111/j.1528-1167.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- Errante LD, Petroff OA. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2002;12:300–6. doi: 10.1016/s1059-1311(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Feng MR, Turluck D, Burleigh J, et al. Brain microdialysis and PK/PD correlation of pregabalin in rats. Eur J Drug Metab Pharmacokinet. 2001;26:123–8. doi: 10.1007/BF03190385. [DOI] [PubMed] [Google Scholar]
- Ferrendelli JA. Concerns with antiepileptic drug initiation: safety, tolerability, and efficacy. Epilepsia. 2001;42(Suppl 4):28–30. doi: 10.1046/j.1528-1157.2001.0420s4028.x. [DOI] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Frampton JE, Scott LJ. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–20. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31:1448–54. doi: 10.2337/dc07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J, Arrigo C. Rapid onset of action of levetiracetam in refractory epilepsy patients. Epilepsia. 2005;46:324–6. doi: 10.1111/j.0013-9580.2005.31504.x. [DOI] [PubMed] [Google Scholar]
- French JA, Kugler AR, Robbins JL, et al. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. 2003;60:1631–7. doi: 10.1212/01.wnl.0000068024.20285.65. [DOI] [PubMed] [Google Scholar]
- Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48:1303–7. doi: 10.1111/j.1528-1167.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- Hellwig S, Amtage F. Pregabalin-induced cortical negative myoclonus in a patient with neuropathic pain. Epilepsy Behav. 2008;13:418–20. doi: 10.1016/j.yebeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Hitiris N, Barrett JA, Brodie MJ. Erectile dysfunction associated with pregabalin add-on treatment in patients with partial seizures: five case reports. Epilepsy Behav. 2006;8:418–21. doi: 10.1016/j.yebeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Rademacher M, Hoffmann JM, et al. Bodyweight gain under pregabalin therapy in epilepsy: Mitigation by counseling patients? Seizure. 2008;17:327–32. doi: 10.1016/j.seizure.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Feuerstein TJ, Schulze-Bonhage A. Myoclonus in epilepsy patients with anticonvulsive add-on therapy with pregabalin. Epilepsia. 2001;42:790–2. doi: 10.1046/j.1528-1157.2001.44000.x. [DOI] [PubMed] [Google Scholar]
- Janiczek-Dolphin N, Corrigan BW, Bockbrader HN. Diuretics, oral hypoglycemic agents, and insulin do not alter pregabalin pharmacokinetics. Epilepsia. 2005;46(Suppl 6):115. [Google Scholar]
- Jezyk N, Li C, Stewart BH, et al. Transport of pregabalin in rat intestine and Caco-2 monolayers. Pharm Res. 1999;16:519–26. doi: 10.1023/a:1018866928335. [DOI] [PubMed] [Google Scholar]
- Kälviäinen R, Khyuppenen J, Koskenkorva P, et al. Clinical picture of EPM1-Unverricht-Lundborg disease. Epilepsia. 2008;49:549–56. doi: 10.1111/j.1528-1167.2008.01546.x. [DOI] [PubMed] [Google Scholar]
- Leppik I, De Rue K, Edrich P, et al. Measurement of seizure freedom in adjunctive therapy studies in refractory partial epilepsy: the levetiracetam experience. Epileptic Disord. 2006;8:118–30. [PubMed] [Google Scholar]
- Li Z, Donevan S, Taylor, et al. High-affinity binding of pregabalin at alpha-2-delta subunits of voltage-gated calcium channel: Contribution to anticonvulsant action. Epilepsia. 2005;46(Suppl 6):280. [Google Scholar]
- Lozsadi D, Hemming K, Marson AG. Pregabalin add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2008;1:CD005612. doi: 10.1002/14651858.CD005612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málaga I, Sanmarti FX. Two cases of painful gynecomastia and lower extremity pain in association with pregabalin therapy. Epilepsia. 2006;47:1576–9. doi: 10.1111/j.1528-1167.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- May T, Rambeck B, Neb R, et al. Serum concentrations of pregabalin in patients with epilepsy: The influence of dose, age, and comedication. Ther Drug Monit. 2007;29:789–94. doi: 10.1097/FTD.0b013e31815d0cd5. [DOI] [PubMed] [Google Scholar]
- McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44(Suppl 5):S17–22. [PubMed] [Google Scholar]
- Modur PN, Milteer WE. Adjunctive pregabalin therapy in mentally retarded, developmentally delayed patients with epilepsy. Epilepsy Behav. 2008;13:554–6. doi: 10.1016/j.yebeh.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Mohanraj R, Brodie MJ. Measuring the efficacy of antiepileptic drugs. Seizure. 2003;12:413–43. doi: 10.1016/s1059-1311(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Owen RT. Pregabalin: its efficacy, safety and tolerability profile in generalized anxiety. Drugs Today. 2007;43:601–10. doi: 10.1358/dot.2007.43.9.1133188. [DOI] [PubMed] [Google Scholar]
- Perucca E. What can we learn from clinical trials of anticonvulsant drugs in epilepsy? Eur J Pain. 2002;6(Suppl A):35–44. doi: 10.1053/eujp.2001.0320. [DOI] [PubMed] [Google Scholar]
- Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age. Clin Pharmacokinet. 2006;45:351–63. doi: 10.2165/00003088-200645040-00002. [DOI] [PubMed] [Google Scholar]
- Perucca E. Designing clinical trials to assess antiepileptic drugs as monotherapy: difficulties and solutions. CNS Drugs. 2008;22:917–38. doi: 10.2165/00023210-200822110-00003. [DOI] [PubMed] [Google Scholar]
- Perucca E, Robbins JL, Kugler, et al. Pregabalin demonstrates anticonvulsant activity onset by the second day. Epilepsia. 2002;43(Suppl 8):48. [Google Scholar]
- Piyapolrungroj N, Li C, Bockbrader H, et al. Mucosal uptake of gabapentin (neurontin) vs. pregabalin in the small intestine. Pharm Res. 2001;18:1126–30. doi: 10.1023/a:1010970809090. [DOI] [PubMed] [Google Scholar]
- Randinitis EJ, Posvar EL, Alvey CW, et al. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003;43:277–83. doi: 10.1177/0091270003251119. [DOI] [PubMed] [Google Scholar]
- Rheims S, Cucherat M, Arzimanoglou A, et al. Greater response to placebo in children than in adults: a systematic review and meta-analysis in drug-resistant partial epilepsy. PLoS Med. 2008;5(e166):1223–36. doi: 10.1371/journal.pmed.0050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P. Defining success in clinical trials – profiling pregabalin, the newest AED. Eur J Neurol. 2005;(Suppl 4):12–21. doi: 10.1111/j.1468-1331.2005.01327.x. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, Semah F, et al. Meta-Analysis of add-on treatment in drug resistant partial epilepsy: a comprehensive study of 41 randomized controlled trials among 10 AEDs. Neurology. 2006;66(Suppl 2):A36. [Google Scholar]
- Shneker BF, McAuley JW. Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005;39:2029–37. doi: 10.1345/aph.1G078. [DOI] [PubMed] [Google Scholar]
- Tassone DM, Boyce E, Guyer J, et al. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48. doi: 10.1016/j.clinthera.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Vartanian MG. Profile of the anticonvulsant activity of CI-1008 (pregabalin) in animal models. Epilepsia. 1997;38(Suppl 8):8. [Google Scholar]
- Vartanian MG, Radulovic LL, Kinsora JJ, et al. Activity profile of pregabalin French JA (2001). Proof of efficacy trials: endpoints. Epilepsy Res. 2006;45:53–6. doi: 10.1016/s0920-1211(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Walker MC, Sander JW. The impact of new antiepileptic drugs on the prognosis of epilepsy: seizure freedom should be the ultimate goal. Neurology. 1996;46:912–4. doi: 10.1212/wnl.46.4.912. [DOI] [PubMed] [Google Scholar]
- Warner G, Figgit DP. Pregabalin as adjunctive treatment of partial seizures. CNS Drugs. 2005;19:265–72. doi: 10.2165/00023210-200519030-00007. [DOI] [PubMed] [Google Scholar]