Abstract
Background
Methylphenidate (MP) is a dopamine- and noradrenaline-enhancing agent beneficial for post-stroke depression (PSD) and stroke recovery due to its therapeutic effects on cognition, motivation, and mood; however, the neural mechanisms underlying its clinical effects remain unknown. This study used functional magnetic resonance imaging (f MRI) to investigate the effect of MP on brain activity in response to cognitive tasks in patients with PSD.
Methods
Nine stroke outpatients with DSM IV defined major depression underwent fMRI during two cognitive tasks (2-back and serial subtraction) on four occasions, on the first and third day of a three-day treatment of MP and placebo. Nine healthy control (HC) subjects matched for age and sex scanned during a single session served as normative data for comparison. The main outcome measure was cognitive task-dependent brain activity.
Results
For the 2-back task, left prefrontal, right parietal, posterior cingulate, and temporal and bilateral cerebellar regions exhibited significantly greater activity during the MP condition relative to placebo. Less activity was detected in rostral prefrontal and left parietal regions. For serial subtraction, greater activity was detected in medial prefrontal, biparietal, bitemporal, posterior cingulate, and bilateral cerebellar regions, as well as thalamus, putamen, and insula. Further, underactivation observed during the placebo condition relative to HC improved or reversed during MP treatment. No significant differences in behavioral measures were found between MP and placebo conditions or between patients and HC.
Conclusions
Short-term MP treatment may improve and normalize activity in cognitive neuronal networks in patients with PSD.
Keywords: methylphenidate, post-stroke depression, functional MRI, cognition
Depression is the most common and severe mood disorder following stroke. Approximately 30% to 50% of stroke patients may suffer from depression during the first two years after a stroke (Robinson 1998b). Depression following stroke adversely affects post-stroke physical and cognitive functioning and long term survival, which can be remedied by treating the depression (Gonzalez-Torrecillan et al 1995; Ramasubbu et al 1998; Kimura et al 2000; Jorge et al 2003). Hence, recognition and effective treatment of post-stroke depression (PSD) is crucial to reduce disease burden, morbidity and mortality in stroke patients. Enhancement of central catecholaminergic activity has been considered as a potential treatment strategy for PSD, based on the evidence from clinical and preclinical studies implicating catecholamine deficits in the etiology of PSD (Robinson and Bloom 1977; Barry and Dinan 1990; Bryer et al 1992).
One suggested approach to improve the catecholamine system in PSD is the use of psychostimulants, such as methylphenidate (MP), which is a potent dopamine and noradrenergic reuptake inhibitor (Kuczemski and Segal 1997). MP is widely used to treat children and adults with attention deficit hyperactivity disorder. It is also recommended in the treatment of depression in specific patient sub-groups, such as depression secondary to brain injury, geriatric depression and resistant depression, where cognitive and motivational deficits are predominant (Hallman and Lipsky 2000; Orr and Taylor 2007). MP has been successfully used to alleviate PSD and to enhance post-stroke recovery. A series of retrospective studies and one double-blind placebo-controlled study have suggested the efficacy and safety of MP in the treatment of PSD and also in the acceleration of stroke recovery (Masand et al 1991; Johnson et al 1992; Lazarus et al 1992, 1994; Grade et al 1998). MP treatment is advantageous over antidepressants as it produces rapid clinical response within three to ten days after the initiation of treatment at a mean dose of 17 mg/d (Masand et al 1991).
The therapeutic effects of MP in the treatment of depression have been attributed to its ability to improve mood, motivation and other cognitive functions including attention, working memory and executive functions (Hallman and Lipsky 2000). In support of its cognitive effects, preclinical and clinical studies have shown that MP is effective in improving working memory (WM), attention, and alertness (Hallman and Lipsky 2000; Wright and White 2003; Orr and Taylor 2007). Cognitive impairment is frequently associated with post stroke major depression and the nature of the relationship between cognitive impairment and post stroke depression remains complex (Robinson 1998a). Thus cognitive impairment may be a cause or effect or both (cause and effect) in relation to PSD. Furthermore, cognitive impairment and depression may be independent consequences of stroke. Given that major depression is frequently associated with cognitive impairment in stroke patients and MP is beneficial for both depression and related cognitive impairments, MP may have a role in the treatment of PSD with cognitive dysfunction (Robinson 1998b). However, the cognitive effects of MP in patients with PSD and their underlying neural mechanisms have not been well studied.
In this experiment, we examined the effects of short-term MP administration on behavioral performances and hemodynamic (blood oxygenation level-dependent; BOLD) responses during working memory and mental arithmetic (subtraction) cognitive tasks in patients with PSD, employing a double-blind placebo-controlled within-subject design. Based on previous observations (Mehta et al 2000; Volkow et al 2004), we predicted that in patients with PSD, MP short-term treatment, as compared to placebo, would improve and normalize BOLD responses in neuronal networks especially in parietal and frontal areas activated by the working memory and mental subtraction tasks as in healthy subjects. We also predicted that changes in BOLD responses induced by MP would be correlated with changes in task performance and mood.
Methods
This study was approved by the conjoint research ethics board governing the institution, and all subjects gave written informed consent prior to their participation.
Subjects
Nine right-handed patients (5 females, 4 males; mean age = 50.5 years; range 40–70 years) with DSM IV defined major depression due to stroke (APA 2000) participated in the study. The handedness was determined by the Edinburgh handedness inventory (Oldfield 1971). Patients had identifiable ischemic stroke lesions involving middle cerebral artery (MCA) and/or anterior cerebral artery (ACA) territories, as determined by clinical imaging. Subjects with PSD were recruited from a cohort of patients referred to the “Post-Stroke Depression Clinic” at the Foothills Medical Centre affiliated with the Faculty of Medicine, University of Calgary, Calgary, Canada. DSM IV diagnosis of major depression was determined by structured clinical interview (SCID) (First et al 1997). The Mini-Mental State Exam (MMSE) and Barthel Index were used to measure cognitive and physical functioning respectively (Mahoney and Barthel 1965; Folstein et al 1975). Lesion characteristics, medications and medical illnesses were obtained from medical charts. The exclusion criteria were: i) severe communication and cognitive deficits (MMSE < 20); ii) less than grade 12 education; iii) neurological conditions (epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, or severe head injury); iv) medical conditions (active cancer, renal or liver failure, unstable diabetes mellitus, or hypertension); v) psychiatric conditions other than depression (psychosis, current substance abuse, or obsessive compulsive disorder); vi) current use of antidepressants, antipsychotics or mood stabilizers; vii) patients with prestroke depression that continued during poststroke period; viii) contradictions for MRI. Since sleep deprivation may affect cognitive performance, patients were allowed to take Zopiclone (5 mg to 7.5 mg) for insomnia, if needed.
Nine right-handed healthy volunteers served as control subjects, matched for age and gender to the patient group. Subjects in the healthy comparison group were recruited from the local hospital environment, nursing and administrative staff. All subjects were free of any major neurological or medical problems, as determined by self-report and detailed interview. The absence of psychiatric illness in healthy controls was ascertained by SCID (First et al 1997).
Experimental design
Subjects with PSD participated in four f MRI sessions: on the first and third day of a three-day placebo treatment and on the first and third day of a three-day MP treatment. The study was conducted using a double-blind crossover design with sessions of placebo and MP treatment approximately one week apart. The order of placebo and MP conditions were counterbalanced across subjects to control for practice effects on the performance of cognitive tasks. Owing to the ethical considerations of giving MP to healthy elderly controls, subjects in the comparison group were scanned during a single session without a placebo or MP treatment.
Drug dose and administration
Patients with PSD were given oral MP 20 mg (slow release) and placebo (lactulose), presented in identical capsules. Patients were asked to take MP or placebo at a fixed time between 6 and 8 am in the morning, after a light breakfast and without any beverages containing caffeine for three consecutive days. Caffeine is a psychostimulant with a physiological half-life of 3.5 hours (Parsons and Neims 1978), and may augment the effect of MP, potentially complicating the findings of the study. Patients were advised to adhere to the same time schedule for MP and placebo intake for subsequent imaging sessions of the study. Approximately 4 hours after administration of MP or placebo, imaging was performed between 10 to 12 am for all subjects. Considering the rapid clinical response to MP in PSD (Masand et al 1991), this study design included only three days of treatment. Further, the short-term treatment was preferred to minimize the length of placebo exposure for ethical reasons and also to limit the carry-over effects of clinical recovery in a crossover design. The dose of 20 mg of MP selected for this study was based upon previous reports documenting its therapeutic efficacy at a mean dose of 17 mg per day in PSD (Masand et al 1991). We did not choose the dose based on weight, as there are no clear guidelines for weight-adjusted doses in elderly patients. Slow release was chosen as it allowed us to have a flexible drug latency period of 4 hours between MP administration and imaging. The time to peak for slow release MP is 4.7 hours (1.3 to 8.2 hrs) and 1.9 hrs (0.3 to 4.4 hrs) for regular MP tablets (Liu et al 2005).
Pre-scanning procedure
Subjects were requested to abstain from alcohol for at least 24 hours prior to the f MRI session, as ethanol interacts with MP, elevating metabolite levels and potentially adversely affecting cognitive function (Patrick et al 2007). All subjects were given instructions about the two cognitive tasks and were given off-line practice trials to familiarize themselves with the tasks. A different set of task stimuli were used for the scanning session. Blood pressure and pulse rate were measured before each scanning session (pulse rate during scanning could not be monitored due to technical problems). Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), Visual Analogue Scale (VAS) (Grunhaus et al 2002), and MMSE were used to measure depression severity and cognitive functioning prior to scanning.
Cognitive tasks
The N-back task is commonly used to investigate the neural basis of working memory. Mental subtraction task is a multi-component cognitive task to evaluate attention, calculation and working memory and executive functions. The purpose of using two cognitive tasks was to evaluate the neural networks of wide range of cognitive functions. Both tasks are known to activate a distributed network of brain areas including frontal and parietal regions (Rueckert et al 1996; Burbaud et al 1999; Owen et al 2005). Prior imaging studies have demonstrated abnormalities in frontal and parietal neural networks in patients with major depression during the performance of N-back and mental arithmetic tasks (Hugdahl et al 2004; Matsuo et al 2007).
Stimuli preparation
All task stimuli were created and presented using Presentation (Neurobehavioral Systems Inc., Albany, CA), and were viewed by subjects in the MR scanner using liquid crystal display goggles (Resonance Technology Inc., Parthenia, CA). Behavioral responses requiring a button press were recorded using an MR-compatible four-button keypad (Compumedics Neuroscan Inc., El Paso, TX).
2-back task
Subjects were presented with a series of single digits at 3-second intervals within 30-second blocks (Awh et al 1996). Subjects were asked to respond using the keypad when the current digit was the same as that presented two digits previously. Alternating 30-second blocks consisted of a blank grey screen with a fixation cross and the word “TAP” was presented at pseudo-random intervals to instruct the participant to respond with the keypad. Four blocks of task and five blocks of control task (TAP) were completed during each run, and two runs were performed for this task.
Mental subtraction task
In this task, subjects performed two types of subtraction, simple subtraction (SIMSUB) and serial subtraction (SERSUB), alternating in 30-second blocks. SIMSUB consisted of ten trials of visually presented subtraction operations (eg, 56-1), presented at 3-second intervals. A SERSUB block consisted a 3-second presentation of a two digit base number, and every 3 seconds thereafter, the operation to be performed appeared (eg, –1). Subjects were asked to perform this operation on the result of the previous operation. Each 30-second block of both tasks consisted of either subtracting the number 1 or the number 7 as a means to modulate the difficulty of the task. As a result, the blocks were in the following order: SIMSUB_1, SERSUB_1, SIMSUB_7, SERSUB_7, SIMSUB_1, SERSUB_1, SIMSUB_7, SERSUB_7, SIMSUB_1, SERSUB_1, SIMSUB_7, SERSUB_7, SIMSUB_1. During each block of ten trials, two pseudo-randomly chosen trials involved the subtraction of the number 2 to prevent subjects from anticipating the next operation. Subjects were asked to perform subtraction mentally without speaking or moving the tongue or lips, and to indicate with a button press when they completed a subtraction operation. Two runs of the task were performed.
Image acquisition
Scanning was performed using a 3 Tesla MR scanner (Signa Excite HD; GE Healthcare, Waukesha, WI) located at the Seaman Family MR Research Centre. Head movement was restricted with foam padding. An automated shimming routine was performed to optimize magnetic field homogeneity. A gradient echo T2*-weighted echo planar imaging (EPI) sequence was used to acquire f MRI data (TR/TE = 3000/30 ms, field of view = 24 cm, 96 × 96 matrix, flip angle = 50°, 20 6-mm thick slices). During the 2-back task, 90 volumes were collected, and during the mental subtraction task, 130 volumes were collected. A T1-weighted high-resolution volume using a 3D acquisition [spoiled gradient echo (SPGR) sequence: repetition time (TR) =21 ms, minimum echo time (TE), field of view (FOV) =24 × 18 × 12 cm, matrix size = 320 × 224 × 64] was used for anatomical registration of the f MRI data.
Image data analyses
Analysis involved four complete data sets from each of seven PSD patients, two incomplete data sets from each of two PSD patients (one session per drug condition), and one complete data set from each of nine healthy volunteers. Two PSD patients declined to participate in all four sessions because of the effects of depression and stress related to the cognitive tasks and imaging. Analysis of all scans was carried out using FEAT (F MRI Expert Analysis Tool) Version 5.4, part of FSL (F MRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). After motion correction using MCFLIRT (Jenkinson et al 2002) and spatial smoothing (Gaussian kernel FWHM 8 mm), time-series statistical analyses were carried out using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction (Woolrich et al 2001). Specifically, estimates of the average magnitude of activity within each image pixel in response to each type of trial block (ie, SIMSUB_1, SIMSUB_7, SERSUB_1, and SERSUB_7 for the subtraction task, and task versus rest for the 2-back task) were generated using models of the expected hemodynamic response (a normalized gamma function convolved with the binary timing of the corresponding block type and including the temporal derivative).
Higher-level planned comparisons were then carried out using a General Linear Mixed Effects using FLAME (FMRIB’s Local Analysis of Mixed Effects). For the 2-back data, mean activity during the task was determined for each of the placebo-treated, MP-treated, and control groups by determining clusters of brain activity exceeding Z = 2.3 (p = 0.01) and a corrected cluster size of k = 12. For the mental subtraction data, the contrast between the serial and simple subtraction conditions [ie, (SERSUB_1 + SERSUB_7) versus (SIMSUB_1 + SIM-SUB + 7)] was determined for each of the placebo-treated, MP-treated, and control groups by determining clusters of brain activity exceeding Z > 2.3 (p = 0.01) (uncorrected for multiple comparisons) and a corrected cluster size of k = 12. Further for the 2-back data, a within-group comparison was performed for the patients using regressors to model (1) a difference in activity between MP and placebo conditions, (2) a difference in activity between the first and second placebo condition, (3) a difference in activity between the first and second MP condition, and a regressor of noninterest to account for linear trend in activity across all four sessions. Contrast images for each of the regressors of interest were generated using significant clusters determined by Z > 2.3 (p = 0.01) (uncorrected for multiple comparisons) and a corrected cluster size of k = 12. An identical analysis was performed for the subtraction data using the SIMSUB versus SERSUB contrast estimate. Between-group comparisons were made between the healthy controls and each of the MP-treated and placebo-treated patient groups for mean activity during the 2-back task as well as for the SERSUB versus SIMSUB contrast.
Behavioral data analyses
Subject characteristics were analyzed using appropriate parametric (t-test) and nonparametric methods (Chi-square). A repeated measures analysis of variance (ANOVA) was used to analyze task performance data (number of errors and reaction time for 2-back task, reaction time for the subtraction task) with treatment condition (MP, placebo) and session (first, second) as factors. The interaction between these two factors was also investigated. Pulse rate, blood pressure (diastolic and systolic), and HAM-D, VAS, MMSE scores were analyzed in the same manner. T-tests were performed to analyze the differences in performance data between healthy control and PSD patients during placebo and MP treatment separately, corrected for multiple comparisons. All statistical analyses were performed using SPSS statistical software version 9 for Windows (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered as statistically significant.
Results
Sample characteristics
Sample characteristics are presented in Table 1. All PSD patients participating in the study had stroke lesions involving either right or left middle cerebral artery territories. Among these patients, four had right hemispheric lesions and four had left hemispheric lesions, and one had bilateral infarcts. The infarcts in these patients involved frontal, parietal, and temporal regions, as well as subcortical areas including the basal ganglia, internal capsule and periventricular regions. Seven out of nine depressed stroke patients had co-morbid medical conditions including hypertension and diabetes mellitus, and they were taking medications for their medical conditions. Three patients had personal history of depression and two patients had family history of depression in the first generation. None of the depressed patients were taking any antidepressants or psychotropic medications during the study. Only two patients were taking zopiclone 7.5 mg/d for insomnia, as needed. These patients had moderate to severe depression (HRSD scores: mean = 26.11, SD = 4.49), as well as minimal cognitive impairment (MMSE scores: mean = 28, SD = 2) and physical impairment (Barthel scores: mean = 87.78, SD =19.22) at the time of investigation. There were no significant differences in age [t(16) =−0.092, p = 0.93], gender (chi-square =0.22, df = 1, p = 0.64), and years of education [t(16) =−0.477, p = 0.64) between the PSD and healthy comparison groups.
Table 1.
Clinical characteristics of subjects with post-stroke major depression (PSD)
| Sex/Age, y | Years of education | Lesions | Time since stroke in months | Duration of PSD | Medical history | Medications | HRSD scores | Barthel scores | MMSE scores |
|---|---|---|---|---|---|---|---|---|---|
| F/35 | 13 | L. parietal occipital | 6 | 6 | – | Acetylsalicylic acid 81 mg/d | 28 | 100 | 30 |
| F/57 | 12 | R. basal ganglia | 8 | 8 | – | Baclofen 20 mg/d
Zopiclone 7.5 mg at bed time Calcium 1250 mg/d Gabapentin 900 mg/d |
27 | 50 | 28 |
| F/70 | 16 | L. basal ganglia | 12 | 10 | Hypertension osteoporosis | Ramipril 10 mg/d
Calcium 1250 mg/d |
22 | 90 | 27 |
| M/59 | 12 | L. frontal lobe
L. external capsule White matter lesions near left lateral ventricle |
19 | 17 | Hypertension | Ramipril 10 mg/d | 26 | 100 | 27 |
| F/56 | 16 | R. frontal
R. temporal R. lateral lenticulo- striatal infarct |
4 | 4 | – | Acetylsalicylic acid 81 mg/d
Zopiclone 7.5 mg at bed time |
28 | 100 | 27 |
| M/50 | 13 | R. frontal
Bifrontal lacunar infarcts |
2 | 2 | Hypertension
Diabetes mellitus Hypothyroidism |
Metformin 1500 mg/d
Clopidogrel 75 mg/d Levothyroxine 0.1 mg/d Irbesarton 300mg/d Acetylsalicylic acid 81 mg |
34 | 100 | 28 |
| M/58 | 14 | L. internal capsule infarction | 4 | 4 | Hypertension | Ramipril 10 mg/d
Acetylsalicyclic acid 81 mg/d |
24 | 90 | 30 |
| F/50 | 12 | R. periventricular
White matter infarcts |
12 | 12 | Hypertension | Acetylsalicylic acid 81 mg/d
Ramipril 5 mg/d |
18 | 100 | 30 |
| M/59 | 16 | R. internal
Capsule infarct |
17 | 12 | Hypertension osteoarthrosis | Zopiclone 7.5 mg at bed time
Acetylsalicyclic acid 81 mg/d Irbesartan 300 mg/d Simvastatin 40 mg/d |
28 | 60 | 25 |
Abbreviations: F, female; M, male; Y, years; PSD, post-stroke major depression; HRSD, Hamilton rating scale per depression; MMSE, mini-mental status examination; L, left; R, right; d, day.
Behavioral responses
The analysis of performance measures showed no effect of drug or time or drug × time interaction for both the 2-back and mental subtraction tasks in depressed stroke patients. For the 2-back task, the main effects of drug (F = 1.91, df = 1.6, p = 0.22) and time (F = 0.70, df = 1.6, p = 0.44), as well as drug × time interaction (F = 1.60, df = 1.6, p = 0.25) on the number of errors were nonsignificant. Further, there was no effect of drug (F = 0.01, df = 1.6, p = 0.87) or time (F = 0.08, df = 1.6, p = 0.79) or drug × time interaction (F = 2.05, df = 1.6, p = 0.20) on subject reaction time. Similarly, for the subtraction task, there was no effect of drug (F = 0.56, df = 1.6, p = 0.48) or time (F = 0, df = 1.6, p = 0.99) or drug × time interaction (F = 1.42, df = 1.6, p = 0.28) on reaction time; there was no accuracy measure for the subtraction task. The performance measures of control subjects were not significantly different from PSD patients in the placebo or MP conditions [for RT in the 2-back task: MP vs HC (t = 1.59, df = 16, p = 0.13), placebo vs HC (t = 1.51, df = 16, p = 0.15); for errors: MP vs HC (t = 0.54, df = 16, p = 0.60), placebo vs HC (t = 0.71, df = 16, p = 0.49); for RT in the serial subtraction task: MP vs HC (t = 0.71, df = 16, p = 0.49); placebo vs HC (t = 0.83, df = 16, p = 0.42); for RT in the simple subtraction task: MP vs HC (t = 0.90, df = 16, p = 0.38), placebo vs HC (t = 0.64, df = 16, p = 0.53)].
Clinical responses
Means were replaced for missing values in two patients who did not complete all 4 scanning sessions. There was no effect of drug (F = 0.844, df = 1.8, p = 0.39) or time (F = 2.99, df = 1.8, p = 0.12) or drug × time interaction (F = 1.843, df = 1.8, p = 0.21) on HRSD in PSD patients. Further, there was no effect of drug (F = 1.62, df = 1.8, p = 0.24) or drug × time interaction (F = 1.56, df = 1.8, p = 0.25), but there was an effect of time (F = 6.39, df = 1.8, p = 0.035) on VAS measures. VAS score on day 3 was lower than day 1 regardless of treatment condition (mean difference between day 1 and day 3 was 0.619, SE = 0.245). None of the patients reported any adverse effects with either placebo or MP. MMSE measures did not show significant effects of treatment (F = 1, df = 1.8, p = 0.35), time (F = 1, df = 1.8, p = 0.35) or drug × time interaction (F = 1, df = 1.8, p = 0.35).
Physiological responses
Means were replaced for missing values in two patients with incomplete data. Analysis of physiological measures in PSD patients showed a significant effect of drug on pulse rate and a nonsignificant effect on systolic and diastolic blood pressure. MP significantly increased pulse rate compared to placebo in both sessions. Thus, there was a main effect of drug on heart rate (F = 6.81, df = 1.8, p = 0.031), but no effect of time (F = 0.19, df = 1.8, p = 0.67) or drug × time interaction (F = 0.044, df =1.8, p = 0. 839) on the same measure. Regarding blood pressure responses, there was no effect of drug (F = 1.48, df = 1.8, p = 0.26; F = 0.61, df = 1.8, p = 0.46), or time (F = 2.694, df = 1.8, p = 0.14; F = 0.29, df = 1,8, p = 0.61), or drug × time interaction (F = 0.768, df = 1.8, p = 0.41; F = 0.34, df 1.8, p = 0.58) on systolic and diastolic blood pressure, respectively.
FMRI data
Effects of MP and placebo on task-dependent brain activity in depressed stroke
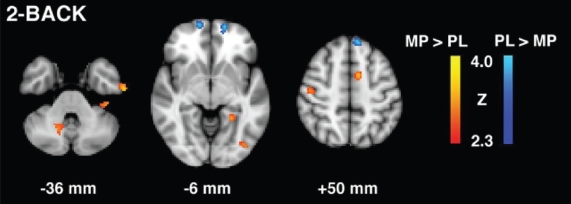
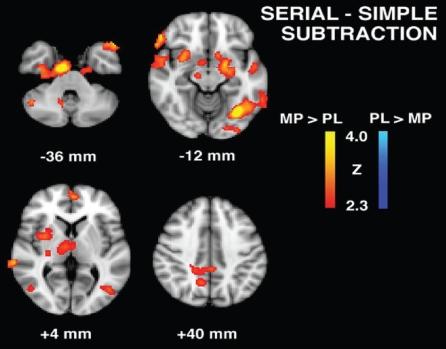
To test whether there were significant differences in task-dependent activity between MP and placebo conditions, we compared whole brain activations during specific task conditions between MP and placebo treatment. Figure 1 shows brain activity related to the 2-back task for MP vs placebo contrasts (regions are summarized in Table 2). During the MP condition, relative to placebo, significantly greater activity was detected in left prefrontal, right parietal, left temporal, posterior cingulate cortex (PCC), and bilateral cerebellar regions, and significantly less activity was detected in rostral prefrontal cortex and left parietal regions. Figure 2 shows brain activity related to the serial > simple subtraction condition for MP vs placebo contrasts (regions are summarized in Table 3). During the MP condition, relative to placebo, significantly greater activity was detected in left dorsolateral prefrontal cortex (DLPFC), biparietal, bitemporal, bilateral cerebellar regions as well as hippocampus, parahippocampus, thalamus, insula, and putamen. For both the 2-back and the serial > simple subtraction task conditions, there was significant activity during the MP condition in prefrontal, parietal, temporal and cerebellar regions, which was greater during the serial > simple subtraction task condition than during the 2-back task. Further, activations in subcortical structures were seen only for the serial > simple subtraction contrast.
Figure 1.
2-Back task group contrast maps for methylphenidate (MP) and placebo (PL) conditions in patients with post-stroke depression.
Table 2.
Group contrasts for brain activity during the 2-back task*
| Brain Regions | Brodmann area | Z Score | Talairach Coordinates (mm)
|
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| MP > Placebo | |||||
| L Superior frontal gyrus | 6 | 3.41 | −8 | −14 | 68 |
| L Medial frontal gyrus | 6 | 3.61 | −10 | 4 | 54 |
| L Precentral gyrus | 3 | 3.17 | −36 | −4 | 32 |
| R Postcentral gyrus | 3 | 3.72 | 40 | −26 | 58 |
| L Posterior cingulate | 31 | 3.37 | −26 | −64 | 20 |
| L Superior temporal gyrus | 39 | 3.14 | −42 | −50 | 24 |
| L Inferior temporal gyrus | 20 | 2.95 | −60 | 10 | −36 |
| L Parahippocampal gyrus | 37 | 3.33 | −24 | −46 | −6 |
| L Middle occipital gyrus | 18 | 3.58 | −40 | −80 | −8 |
| L Cerebellum | 3.44 | −8 | −60 | −26 | |
| R Cerebellum | 3.46 | −14 | −60 | −40 | |
| Placebo > MP | |||||
| L Superior frontal gyrus | 8 | 3.44 | −6 | 38 | 54 |
| L Superior frontal gyrus | 11 | 3.72 | −14 | 56 | −12 |
| L Medial frontal gyrus | 10 | 3.77 | −16 | 58 | −2 |
| R Medial fontal gyrus | 6 | 3.07 | 60 | 0 | 44 |
| L Superior parietal lobe | 7 | 3.55 | −14 | −74 | 58 |
| R Middle occipital gyrus | 19 | 3.52 | 44 | −74 | 8 |
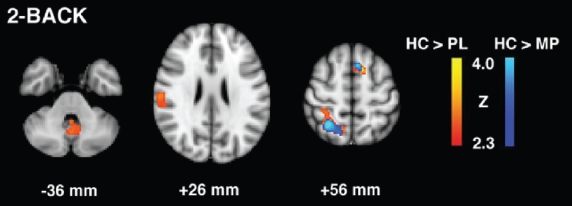
| HC > Placebo | |||||
| R Superior frontal gyrus | 6 | 3.12 | 4 | 8 | 64 |
| L Superior frontal gyrus | 6 | 4.2 | −10 | 16 | 50 |
| R Superior parietal lobule | 7 | 4.99 | 28 | −54 | 54 |
| R Postcentral gyrus | 3 | 3.28 | 62 | −20 | 36 |
| R Postcentral gyrus | 40 | 3.45 | 60 | −22 | 22 |
| R Superior temporal gyrus | 42 | 2.97 | 56 | −32 | 14 |
| L Cerebellum | 2.89 | −2 | −62 | −28 | |
| Placebo > HC | |||||
| L Superior frontal gyrus | 11 | 3.42 | −26 | 52 | −16 |
| HC > MP | |||||
| R Superior frontal gyrus | 6 | 3.29 | 4 | 8 | 64 |
| L Superior frontal gyrus | 6 | 4.41 | −10 | 16 | 50 |
| R Superior parietal lobule | 7 | 4.4 | 28 | −54 | 54 |
| R Postcentral gyrus | 40 | 2.95 | 60 | −22 | 22 |
| MP > HC | |||||
| L Superior frontal gyrus | 10 | 3.68 | −12 | 68 | −2 |
| L Superior frontal gyrus | 11 | 3.36 | −36 | 46 | −2 |
Abbreviations: MP, methylphenidate; HC, health control; R, right; L, left.
Notes: Activations were significant at the threshold Z = 2.3 (P = 0.01uncorrected for multiple comparisons), K = 12 voxels.
Figure 2.
Serial subtraction task group contrast maps for methylphenidate (MP) and placebo (PL) conditions patients with post-stroke depression.
Table 3.
Group contrasts for brain activity in the serial > simple condition*
| Brain Regions | Brodmann area | Z Score | Talairach coordinates (mm)
|
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| MP > Placebo | |||||
| L Medial frontal gyrus | 9 | 3.16 | −4 | 56 | 8 |
| R Precentral gyrus | 6 | 3.51 | 4 | −26 | 58 |
| L Superior parietal lobe | 40 | 3.19 | −32 | −4 | 60 |
| R Superior parietal lobe | 7 | 3.32 | 18 | −54 | 56 |
| L Posterior cingulate gyrus | 31 | 2.88 | −2 | −40 | 40 |
| R Precuneus | 7 | 2.87 | 12 | −58 | 40 |
| L Superior temporal gyrus | 42 | 2.81 | −56 | −32 | 18 |
| R Superior temporal gyrus | 22 | 3.14 | 66 | 0 | −2 |
| R Middle temporal gyrus | 21 | 3.46 | 68 | −36 | 0 |
| L Middle temporal gyrus | 37 | 2.59 | −60 | −50 | −10 |
| L Temporal pole | 38 | 3.53 | −46 | 16 | −30 |
| R Temporal pole | 38 | 2.56 | 44 | 22 | −30 |
| L Hippocampus | 4.07 | −22 | −14 | −20 | |
| R Parahippocampal gyrus | 19 | 3.55 | 32 | −20 | −24 |
| L Lateral occipital cortex | 19 | 3.02 | −24 | −74 | 34 |
| R Lateral occipital cortex | 18 | 3.63 | 44 | −74 | −4 |
| R Thalamus (medial dorsal nucleus) | 3.5 | 12 | −20 | 12 | |
| R Putamen | 3.68 | 32 | 2 | 0 | |
| R Insula | 13 | 3.2 | 30 | 10 | −2 |
| L Fusiform gyrus | 19 | 4.38 | −32 | −68 | −12 |
| R Anterior cerebellum | 3.47 | 20 | −46 | −22 | |
| R Posterior cerebellum | 3.72 | 20 | −86 | −26 | |
| L Anterior cerebellum | 2.94 | −42 | −48 | −28 | |
| Placebo > MP | - | – | – | – | – |
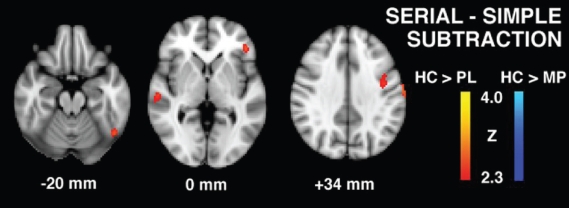
| HC > Placebo | |||||
| L Inferior frontal gyrus | 45 | 2.86 | −44 | 34 | 0 |
| L Precentral gyrus | 6 | 2.78 | −56 | −6 | 48 |
| R Postcentral gyrus | 1 | 3.03 | 60 | −18 | 48 |
| R Superior temporal gyrus | 42 | 2.61 | 68 | −30 | 12 |
| L Inferior temporal gyrus | 37 | 2.89 | −50 | −60 | −18 |
| L Lateral occipital cortex | 19 | 2.7 | −54 | −76 | 8 |
| R Inferior occipital cortex | 19 | 2.96 | 28 | −90 | −6 |
| L Caudate | 2.79 | −20 | 16 | 14 | |
| Placebo > HC | – | – | – | – | – |
| HC > MP | - | – | – | – | |
| MP > HC | |||||
| R Cuneus | 18 | 2.67 | 16 | −80 | 18 |
| L Hippocampus | 3.13 | −24 | −14 | −22 | |
| R Parahippocampal gyrus | 34 | 2.94 | 12 | 2 | −22 |
| L Anterior cerebellum | 2.65 | 12 | 2 | −22 | |
Abbreviations: MP, methylphenidate; HC, health control; R, right; L, left.
Notes: Activations were significant at the threshold Z = 2.3 (P = 0.01uncorrected for multiple comparisons), K = 12 voxels.
Effects of MP and placebo on task-dependent brain activity in depressed stroke compared with healthy controls
To examine whether short-term MP treatment improves or normalizes the baseline task-dependent impairments in PSD, we looked at the changes in task-dependent brain activity (increase, decrease or no signal changes) during each of the MP and placebo conditions compared to healthy controls. For the 2-back task (Table 2, Figure 3), relative to healthy controls, PSD patients during placebo treatment showed less activity in bilateral superior prefrontal, right superior parietal, and right superior temporal regions, as well as left cerebellum. However, during MP treatment, the baseline lesser activities in left cerebellum and the right superior temporal gyrus were normalized. Further, the baseline reductions in activities in parietal and prefrontal areas seemed to improve with MP treatment.
Figure 3.
2-Back task group contrast maps for healthy control (HC) vs. patients with post-stroke depression in placebo (PL) and methylphenidate (MP) conditions.
For the serial > simple subtraction task condition (Table 3, Figure 4), relative to healthy controls, PSD patients during placebo treatment showed less brain activity in left prefrontal, bitemporal, bioccipital, and left caudate regions, whereas during MP treatment, these baseline reductions in brain activity disappeared. Further, relative to healthy controls, greater activity was observed in right cuneus, hippocampus and anterior cerebellum in PSD patients.
Figure 4.
Serial subtraction task group contrast maps for healthy controls vs. patients with post-stroke depression in placebo (PL) and methylphenidate (MP) conditions.
Effect of time on task-dependent brain activity during MP and placebo conditions
Taking into account the effect of repeat testing on task related brain activity, we examined the changes in brain activity between the first session (day 1) and second session (day 3) during placebo and MP treatment separately (Table 4). In the MP condition, the second session of the serial > simple subtraction condition produced greater activity in the anterior cerebellum compared to first session, whereas in the placebo condition, there was greater activity in session 2 than session 1 in prefrontal occipital cortices and posterior cerebellar regions. There were no regions exhibiting less activity in session 2 compared to session 1 for either of the placebo or MP conditions. For the 2-back task, in the MP condition, there was greater activity in session 2 than session 1 within the lingual gyrus and less activity in several brain regions including prefrontal (BA 6,9,44,47), parietal regions (BA 7,40), anterior cingulate cortex, temporal regions (BA 22,21,38), thalamus, hippocampus, parahippocampus substantia nigra and amygdala. However, in the placebo condition, there was greater activity in session 2 compared to session 1 in prefrontal (BA 6, 9) and temporal regions (BA 21,22), as well as cerebellum and insula, and less activity in prefrontal regions (BA 6,8,10), parietal area (BA 7,40), and precuneus. In summary, there was a differential effect of repeated sessions on task specific brain activity between MP and placebo conditions.
Table 4.
Effects of repeated tests on brain activity in placebo and MP conditions*
| Anatomy | Brodmann area | Z Score | Talairach coordinates (mm)
|
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1. Serial > Simple Task | |||||
| Placebo (Day 1 >Day 3) | |||||
| No activations | − | − | − | − | − |
| Placebo (Day 3 > Day 1) | |||||
| L Superior frontal gyrus | 6 | 2.71 | −8 | 20 | 56 |
| R Frontal pole | 10 | 3.72 | 2 | 62 | 8 |
| R Lateral occipital cortex | 19 | 2.71 | 50 | −18 | 2 |
| R Posterior cerebellum | 2.61 | 26 | −78 | −20 | |
| R Posterior cerebellum | 3.29 | 44 | −58 | −38 | |
| MP (Day 1 > Day 3) | |||||
| No activations | − | − | − | − | − |
| MP (Day 3 > Day1) | |||||
| R Anterior cerebellum | 3.47 | 32 | −56 | −22 | |
| 2. 2-Back>Control Task | |||||
| Placebo (Day 1 > Day 3) | |||||
| L Middle frontal gyrus | 6 | 4.15 | −34 | −4 | −66 |
| R Inferior parietal lobule | 40 | 3.6 | 44 | −50 | 62 |
| R Superior parietal lobule | 7 | 4.02 | 34 | −70 | 56 |
| R Precentral gyrus | 6 | 3.74 | 48 | 0 | 52 |
| L Superior parietal lobule | 7 | 3.24 | −26 | −72 | 50 |
| L Precuneus | 19 | 4.59 | −22 | −84 | 42 |
| R Middle frontal gyrus | 8 | 3.47 | 46 | 20 | 42 |
| R Superior frontal gyrus | 10 | 3.91 | 36 | 62 | −8 |
| Placebo (Day 3 > Day 1) | |||||
| L Medial frontal gyrus | 6 | 3.53 | −10 | −22 | 72 |
| R Medial frontal gyrus | 6 | 3.31 | 16 | 0 | 62 |
| R Middle frontal gyrus | 9 | 3.59 | 30 | 34 | 24 |
| R Superior temporal gyrus | 22 | 3.34 | 62 | −36 | 18 |
| L Insula | 13 | 3.06 | −34 | 6 | 12 |
| R Middle temporal gyrus | 21 | 3.64 | 62 | 4 | −24 |
| R Cerebellum | 3.32 | 16 | −56 | −34 | |
| MP (Day1 > Day 3) | |||||
| L Superior frontal gyrus | 6 | 4.51 | −2 | −6 | 72 |
| R Superior frontal gyrus | 6 | 3.23 | 30 | 4 | 64 |
| R Inferior parietal lobule | 40 | 4.24 | 38 | −52 | 46 |
| R Precuneus | 7 | 4.91 | 12 | −74 | 50 |
| L Inferior parietal lobule | 40 | 3.11 | −32 | −66 | 58 |
| L Cingulate gyrus | 24 | 3.99 | −6 | 6 | 46 |
| L Anterior cingulate | 24 | 4.1 | −2 | 22 | 44 |
| L Precentral gyrus | 6 | 4.75 | −38 | −8 | 42 |
| R Precentral gyrus | 44 | 3.8 | 54 | 8 | 12 |
| L Inferior frontal gyrus | 44 | 3.95 | −58 | 8 | 12 |
| L Thalamus | 4.12 | −46 | 14 | 12 | |
| L Substantia nigra | 4.05 | −12 | −18 | 6 | |
| R Middle temporal gyrus | 22 | 3.74 | −10 | −18 | −8 |
| R Middle temporal gyrus | 21 | 3.99 | 60 | −40 | 6 |
| R Superior temporal gyrus | 22 | 3.61 | −66 | −40 | 4 |
| L Putamen/lentiform nucleus | 3.45 | −26 | −2 | −6 | |
| R Claustrum | 3.3 | 36 | −2 | −6 | |
| R Superior temporal gyrus | 38 | 3.62 | 56 | 14 | −14 |
| R Inferior frontal gyrus | 47 | 3.25 | 36 | 16 | −14 |
| L Amygdala | 3.7 | −26 | −4 | −18 | |
| R Hippocampus | 3.77 | 32 | −10 | −22 | |
| R Parahippocampal gyrus | 28 | 3.96 | 20 | −10 | −22 |
| Brainstem | 3.9 | 6 | −14 | −24 | |
| MP (Day 3 > Day 1) | |||||
| L Lingual gyrus | 19 | 3.26 | −8 | −62 | −10 |
Abbreviations: MP, methylphenidate; HC, health control; R, right; L, left.
Notes: Activations were significant at the threshold Z = 2.3 (P = 0.01 uncorrected for multiple comparison), K = 12 voxels.
Discussion
Our results indicate that in PSD patients, short-term MP treatment increases task relevant activity in several brain regions during the performance of 2-back and mental subtraction tasks. During MP treatment, both cognitive tasks recruited prefrontal, parietal, temporal, and cerebellar regions known to participate in working memory, calculation and attention related information (Paulesu et al 1993; Fiez et al 1996; Rueckert et al 1996; Jonides et al 1998; Burbaud et al 1999; Ranganath et al 2004; Chen and Desmond 2005; Owen et al 2005). Further, healthy subjects activated most of these regions to a greater extent than PSD patients during placebo treatment. When PSD patients were given short-term MP treatment, they recruited these regions to a similar degree as the healthy subjects, especially during the subtraction task. Contrary to our expectations, the enhancement of cognitive neural effects due to MP treatment was not related to improvement in performance or mood symptoms in these PSD patients. Overall, these findings suggest that short-term MP treatment may enhance and normalize activity in cognitive neural networks independent of its effects on performance or mood measures.
MP-modulated neural activity during the cognitive task performance could be mediated predominantly by dopamine and norepinephrine mechanisms, as it enhances dopamine and norepinephrine neurotransmissions by blocking dopamine and norepinephrine transporters, and by increasing dopamine and norepinephrine levels in the brain (Kuczemski and Segal 1997). In support of this notion, there is evidence that MP increases task-dependent dopamine release during cognitive activation (Volkow et al 2001, 2004). Furthermore, dopamine and norepinephrine neurotransmitter systems have been implicated in working memory processing, and arithmetic task performance (Mehta et al 2000; Volkow et al 2004).
MP effects on BOLD responses related to cognitive tasks could be complicated by its effects on blood pressure, and heart rate. MP enhances blood pressure and heart rate by increasing the bioavailability of dopamine (Volkow et al 2002). Our results suggest an increase in pulse rate in MP condition. Further, MP may potentially disrupt neural activity and flow coupling due to its direct global and regional effect on cerebral perfusion. Hence the observed MP-related increases in BOLD responses might be confounded by MP’s direct pharmacological effects on cerebral hemodynamics or cardiovascular system. Although this possibility can not be completely ruled out, previous data suggest that 20 mg oral dose of MP does not alter hemodynamic responses to task activation when task conditions are comparable (Rao et al 2000). Another study investigating the f MRI and EEG measures of the dopaminergic drug effects on brain function showed consistency in both measures implying that BOLD-fMRI responses may not necessarily be influenced by drug effects on cerebral perfusion (Arthurs et al 2004).
The observed MP-induced increases in left premotor (BA6) and left DLPFC (BA9) activity during cognitive task conditions are consistent with their suggested role in information management, executive control of working memory, serial subtraction and 2-back task processing (Burbaud et al 1999; Hugdahl et al 2004; Owen et al 2005). Further, these regions have been shown to have dense dopaminergic and noradrenergic innervation (Gaspar et al 1989). Parietal lobe activity (BA 7,40) seen during subtraction is consistent with its implicated role in storage and retrieval processing of working memory and arithmetic calculation (Jonides et al 1998; Burbaud et al 1999). Our finding of MP-induced activity in cerebellar and temporal regions for both tasks merits further discussion. The task specific activity in the cerebellum during the MP condition is consistent with a large number of studies implicating the role of the cerebellum in working memory task performance and in the modulation of forebrain dopamine blood flow (Paulesu et al 1993; Fiez et al 1996; Chen and Desmond 2005; Udo et al 2007). Fronto-cerebellar networks have been postulated to be involved in the articulatory control system, and parietal-cerebellar networks have been postulated to be involved in the phonological storage system during working memory processing (Chen and Desmond 2005). The increased activity in temporal regions during the MP condition could be explained based on its role in working memory maintenance (Ranganath et al 2004).
The other brain regions that were more highly activated during the MP condition include PCC, hippocampus and parahippocampus. The PCC activity in our study is consistent with previous studies reporting its participation in serial subtraction and working memory performance (Ouchi et al 2004; Hampson et al 2006). PCC plays a role in multimodal associative functioning during serial subtraction and has functional connections with anterior cingulate cortex during working memory tasks (Ouchi et al 2004; Hampson et al 2006). The hippocampal and parahippocampal activity during MP treatment is in accordance with its role in short term memory consolidation and encoding in working memory processing, and in serial subtraction task performance (Harrington et al 2004; Mainy et al 2007; Sammer et al 2007). MP-induced activity was also seen in thalamus, putamen, insula, fusiform gyrus and visual association cortex during subtraction, and their participation in attention, working memory and arithmetic processing has been documented in previous reports (Ojemann 1974; Honey et al 2003; Chang et al 2007; Mayer et al 2007; Sammer et al 2007).
An important finding of our study is that compared to healthy controls, the placebo condition exhibited less activity in frontal, parietal, temporal and cerebellar regions during the 2-back task and in frontal, parietal, temporal occipital and caudate regions during the serial subtraction task, whereas MP treatment showed no reduction in brain activity during the serial subtraction task and improvement in activity during the 2-back task. Considering that placebo related decreases in task-dependent activity are baseline deficits due to the negative effects of depression and anterior stroke lesions on functional cognitive neural networks, MP-related improvement in cognitive brain activity suggests that short term treatment with MP may improve functional cognitive networks, and also seem to normalize the activation within these networks. Further, MP related increases in activity in cuneus, hippocampus, parahippocampus and cerebellum during serial subtraction in depressed stroke patients compared to healthy controls might reflect compensatory mechanisms, especially in memory functions.
Our results showed that the brain adaptive responses to repeat cognitive testing could be influenced by short-term MP treatment. MP treatment seemed to influence the brain adaptive responses to repeat testing, as it showed greater repetition suppression (less activity in the second session than the first session) for the 2-back task and less activation in the second session for serial subtraction than during placebo treatment. Although the practice effect and task difficulty were controlled and performance measures were comparable, the brain adaptive responses for repeat testing were different between treatment conditions. This could be explained partly by the pharmacological effect of repeat dosing of MP on brain responses, which is consistent with the previous observation of decreased brain metabolic responses between single and repeated doses of MP (Volkow et al 1998). Further, the MP treatment-related decreases in brain responses in the second session could be related to the improved adaptation to the task difficulty. This interpretation is congruent with previous studies showing significant attenuation in frontal responses to cognitive load with MP administration (Bullmore et al 2003).
Some methodological issues in relevance to the negative findings of this study need to be addressed. The failure to show enhancement in cognitive performance and improvement in mood during the MP condition could be due to small sample size resulting in type II error and other methodological reasons. We excluded PSD patients with severe cognitive impairment, which might explain the comparable cognitive performance measures between PSD patients during the placebo condition and healthy controls. Since the effect of MP on cognitive performance may be dependent on baseline deficits, we did not find improvement with MP treatment on performance measures. However, our results were consistent with previous studies reporting dissociation between drug related performance measures and brain activity (Shafritz et al 2004), and therefore it is conceivable that functional imaging may be a more sensitive or early marker of drug effects than behavioral measures of cognitive task performance. Contrary to previous reports, we did not find any significant improvement in depression symptoms with 3 days of MP treatment with conventional doses of 20 mg/day (Masand et al 1991). The possible reasons for this discrepancy could be that we used MP as a monotherapy in our study whereas in previous studies, MP was used as an add-on treatment to antidepressants in majority of the patients. The absence of drug effects on clinical measures could also be due to inadequate dosage and duration of treatment in these selected patients.
The other limitations were that the findings of the study should not be generalized beyond the sample analyzed due to small sample size and heterogeneity in lesions locations. Since the study design was complex and involved four imaging sessions, there were significant difficulties in the recruitment of depressed stroke patients. There was also no evaluation of the pharmacokinetics of MP, although the literature suggests considerable inter-subject variability in the pharmacokinetics of MP as well as a linear dose-response relationship between MP plasma concentration and brain activity during cognitive tasks (Muller et al 2005). The MP related increases in brain activity could have been influenced by other medications that the stroke patients were taking. However, within-subject crossover design employed in our study was likely to minimize the above-mentioned shortcomings. In the absence of comparative data from nondepressed stroke patients, it is not possible to interpret whether these results are related to post stroke depression or to stroke in general regardless of depression. The healthy subjects were scanned only one time in an unmedicated condition, which precluded an accurate analysis of drug effects on repeated assessments (such as habituation and learning). Although we used the placebo condition as baseline, fMRI findings during the placebo condition cannot be considered as baseline deficits given the reported placebo related activity in fMRI studies (Benedetti et al 2005). Because of heterogeneity in lesion location in our small sample, we did not assess ipsilateral and contralateral activity separately.
Summary
Despite these methodological limitations, this is the first study to our knowledge to demonstrate the beneficial effects of MP on functional cognitive neural networks in patients with PSD. These brain-based results are important for understanding the beneficial effects of MP in stroke depression, particularly the neural mechanisms of its cognitive effects, which may have important clinical treatment implications in depression associated with cognitive impairment. The observed improvement and normalization in cognitive neuronal network with short term treatment of MP may need further evaluation in a large sample to determine its clinical relevance as well as its potential role in cognitive rehabilitation in depressed and nondepressed stroke patients.
Acknowledgments
This work was supported by a research grant from Medical Services Incorporated (MSI) (Alberta, Canada) and a supplementary grant from the Department of Psychiatry, University of Calgary to R. Ramasubbu. This work was presented in part at the 61st annual scientific meeting of the Society of Biological Psychiatry, Toronto, May 2006 and at the 12th Annual meeting of the Organization for Human Brain Mapping (OHBM), Florence, Italy, June 2006. The authors thank faculty and staff of the stroke program at the University of Calgary and the Calgary Health Region for referring stroke patients for the study, as well as Jodi Edwards and Daniel Pittman of the Seaman Family MR Research Centre for their assistance in fMRI data acquisition and analysis. Special thanks to Richard Frayne and Andrew Demchuk for their contributions to the initial development of the study.
References
- [APA] American Psychiatric Association. Diagnostic and Statistical Manual (4th Edition Test Revised) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arthurs OJ, Stephenson CM, Rice K, et al. Dopaminergic effects on electrophysiological and functional MRI measures of human cortical stimulus-response power laws. Neuroimage. 2004;21:540–6. doi: 10.1016/j.neuroimage.2003.09.067. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE. Working memory. Psychol Sci. 1996;7:125–31. [Google Scholar]
- Barry S, Dinan TG. Alpha-2 adrenergic receptor function in post-stroke depression. Psychol Med. 1990;10:305–9. doi: 10.1017/s003329170001761x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wagner TD, et al. Neurobiological mechanisms of the placebo effect. Neuroscience. 2005;25:10390–402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryer JB, Starkstein SE, Votypka V, et al. Reduction of CSF monoamine metabolites in post-stroke depression. J Neuropsychiatry Clin Neurosci. 1992;4:440–2. doi: 10.1176/jnp.4.4.440. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Suckling J, Zelaya F, et al. Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cereb Cortex. 2003;13:144–54. doi: 10.1093/cercor/13.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbaud P, Camus O, Guehl D, et al. A functional magnetic resonance imaging study in mental subtraction in human subjects. Neurosci Lett. 1999;273:195–9. doi: 10.1016/s0304-3940(99)00641-2. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–69. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory. Neuroimage. 2005;24:332–8. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, et al. A positron emission tomography study of the short term maintenance of verbal information. J Neurosci. 1996;16:808–22. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Metal G. Structured clinical interview for DSM IV axis 1 disorders. New York: New York Biometrics Research Department; 1997. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive sate of patients for the clinician”. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, et al. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hyrdoxylase. J Comp Neurol. 1989;279:249–71. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Torrecillan JL, Mendlewicz J, Lobo A. Effects of early treatment of poststroke depression on neuropsychological rehabitation. Int Psychogeriatr. 1995;78:547–60. doi: 10.1017/s1041610295002286. [DOI] [PubMed] [Google Scholar]
- Grade C, Redford B, Chrostowski J, et al. Methylphenidate in early post stroke recovery: a double-blind, placebo-controlled study. Arch Phys Med Rehabil. 1998;27:1047–50. doi: 10.1016/s0003-9993(98)90169-1. [DOI] [PubMed] [Google Scholar]
- Grunhaus L, Doller OT, Dana P, et al. Monitoring the response to TMS in depression with visual analogue scale. Human Psychopharmacol. 2002;17:349–52. doi: 10.1002/hup.418. [DOI] [PubMed] [Google Scholar]
- Hallman C, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–21. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, et al. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, et al. Neural representation of interval encoding and decision making. Cogn Brain Res. 2004;21:193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya CF, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–81. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Rund BR, Lund A, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161:286–93. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Roberts MD, Ross AR, et al. Methylphenidate in stroke patients with depression. Am J Phys Med Rehabil. 1992;71:239–41. doi: 10.1097/00002060-199208000-00008. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumachere EH, Smith EE, et al. The role of parietal cortex in verbal working memory. Neuroscience. 1998;18:5026–34. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R, Robinson RG, Arndt S, et al. Mortality and post-stroke depression: A placebo controlled trial of antidepressants. Am J Psychiatry. 2003;160:1823–9. doi: 10.1176/appi.ajp.160.10.1823. [DOI] [PubMed] [Google Scholar]
- Kimura M, Robinson RG, Kosia JT. Treatment of cognitive impairment after post-stoke depression: a double-blind treatment trial. Stroke. 2000;31:1482–6. doi: 10.1161/01.str.31.7.1482. [DOI] [PubMed] [Google Scholar]
- Kuczemski R, Segal DS. Effects of methylphenidate on extra cellular dopamine, serotonin, and norepinephrine: compression with amphetamine. J Neurochem. 1997;68:2032–7. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lazarus LW, Moberg PJ, Langlsey PR, et al. Methylphenidate and nortriptyline in the treatment of post-stroke depression: a retrospective comparison. Arch Phys Med Rehabil. 1994;75:403–6. doi: 10.1016/0003-9993(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Lazarus LW, Winemiller DR, Lingam VR, et al. Efficacy and side effects of methylphenidate for post-stroke depression. J Clin Psychiatry. 1992;53:447–9. [PubMed] [Google Scholar]
- Liu F, Muni R, Minami H, et al. Review and compression of the long-acting methylphenidate. Psychiatr Q. 2005;76:259–69. doi: 10.1007/s11126-005-2979-0. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: Barthel Index. Am Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, et al. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–93. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand P, Murray GB, Pickett P. Psychostimulants in post-stroke depression. J Neuropsych Clin Neurosci. 1991;3:23–7. doi: 10.1176/jnp.3.1.23. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikoli D, et al. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–53. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Mehta AM, Owen AM, Sahakian BJ, et al. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Suckling J, Zelaya F, et al. Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacology. 2005;180:624–33. doi: 10.1007/s00213-005-2264-9. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Mental arithmetic during human thalamic stimulation. Neuropsychologia. 1974;12:1–10. doi: 10.1016/0028-3932(74)90021-9. [DOI] [PubMed] [Google Scholar]
- Orr K, Taylor D. Psycho stimulants in the treatment of depression: a review of the evidence. CNS Drugs. 2007;21:239–57. doi: 10.2165/00023210-200721030-00004. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, et al. Activation in the promoter cortex during mental calculation in patients with Alzheimer’s disease: relevance of reduction in posterior cingulate metabolism. Neuroimage. 2004;22:155–63. doi: 10.1016/j.neuroimage.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Lair AR, et al. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons WD, Neims AH. Effects of smoking on caffeine clearance. Clin Pharmacol Ther. 1978;24:40–5. doi: 10.1002/cpt197824140. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, et al. Influence of ethanol and gender on pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2007;81:346–53. doi: 10.1038/sj.clpt.6100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–5. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Ramasubbu R, Robinson RG, Flint A, et al. Functional impairment associated with acute post-stroke depression. J Neuropsych Clin Neurosci. 1998;10:26–33. doi: 10.1176/jnp.10.1.26. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, et al. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 2004;24:3917–25. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Salmeron BJ, Durgerian S, et al. Effects of methylphenidate on functional MRI blood-oxygen-level-dependent contrast. Am J Psychiatry. 2000;157:1697–9. doi: 10.1176/appi.ajp.157.10.1697. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Bloom FE. Pharmacological treatment following experimental cerebral infarction: implications for understanding psychological symptoms of human stroke. Biol Psychiatry. 1977;12:660–80. [PubMed] [Google Scholar]
- Robinson RG. The Clinical Neuropsychiatry of Stroke: Cognitive, Behavioral and Emotional Disorders following Vascular Brain Injury. Cambridge, UK: Cambridge University Press; 1998a. Prevalence of depressive disorders; pp. 535–9. [Google Scholar]
- Robinson RG. The Clinical Neuropsychiatry of Stroke: Cognitive, Behavioral and Emotional Disorders following Vascular Brain Injury. Cambridge, UK: Cambridge University Press; 1998b. Relationship of depression to cognitive impairment; pp. 150–76. [Google Scholar]
- Rueckert L, Lange N, Partiot A, et al. Visualizing cortical activation during mental calculation with functional MRI. Neuroimage. 1996;3:97–103. doi: 10.1006/nimg.1996.0011. [DOI] [PubMed] [Google Scholar]
- Sammer G, Blecker C, Gebhardt H, et al. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum Brain Mapp. 2007;28:793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, et al. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–7. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Maguire RP, Jager PL, et al. Methylphenidate-induced activation of the anterior cingulate but not the striatum: A PET study in healthy volunteers. Hum Brain Mapp. 2007;28:625–35. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Differences in regional brain metabolic responses between single and repeated doses of methylphenidate. Psychiatry Res. 1998;83:29–36. doi: 10.1016/s0925-4927(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, et al. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–66. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–80. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wright FK, White KG. Effects of methylphenidate on working memory in pigeons. Cogn Affect Behav Neurosci. 2003;3:300–8. doi: 10.3758/cabn.3.4.300. [DOI] [PubMed] [Google Scholar]