Abstract
In the present study, the authors reported on a case in neuropsychiatric syndromes of systemic lupus erythematosus (NPSLE) with irreversible organic brain changes. The authors also longitudinally investigated serum brain-derived neurotrophic factor (BDNF) levels in the patient. We found that serum BDNF levels in the NPSLE patient with irreversible organic brain change were consistently low, independent of the severity of psychiatric symptoms. Thus, the longitudinal measurement of serum BDNF levels might be useful in predicting the prognosis of NPSLE.
Keywords: brain-derived neurotrophic factor, neuropsychiatric syndrome of systemic lupus erythematosus, organic brain change
Introduction
Brain-derived neurotrophic factor (BDNF) is a critical mediator of neuronal development, survival, and function (Lewin and Barde 1996). In addition, BDNF is currently considered to modulate and regulate immune functions (Vega et al 2003; Nockher and Renz 2003). Recently, it has been reported that BDNF is associated with the pathogenesis of several neuropsychiatric diseases such as schizophrenia (Hori et al 2007), depression (Yoshimura et al 2007), multiple sclerosis (Weinstock-Guttman et al 2007), cerebral ischemia (Berger et al 2004), encephalomyelitis (Chiaretti et al 2004), Alzheimer’s disease (Laske et al 2006), Huntington’s chorea (Ciammola et al 2007), and Parkinson’s disease (Howells et al 2000).
Systemic lupus erythematosus (SLE) is a multisystem immune disease characterized by several abnormalities of the cellular immune system, including a loss of B cell tolerance and the production of pathogenic autoantibodies (Shlomchik et al 1994). Between 18% and 67% of patients with SLE exhibit central nervous system involvement, which is associated with inflammatory features in the brain. Neuropsychiatric syndromes of SLE (NPSLE) patients often reveal various psychiatric features such as delirium, cognitive blunting, psychosis, and affective disorder (ACR 1999). Recently, we had reported that serum BDNF levels were increased in the presence of severe psychiatric symptoms and organic changes in the brain (Ikenouchi et al 2006). In that case, serum BDNF levels were normalized in parallel with recovering organic brain changes and improving their associated psychotic symptoms. Here we report a case of NPSLE with irreversible organic brain changes. Interestingly, the serum BDNF levels were continuously low in spite of the severity of psychiatric symptoms.
Case report
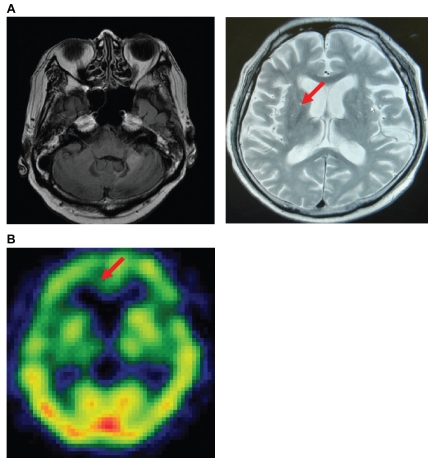
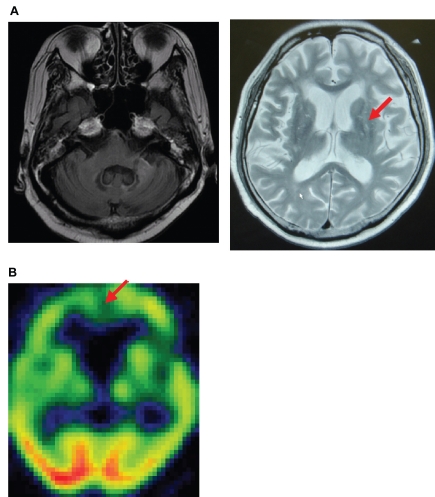
A 58-year-old man in a delirious state was admitted to the psychiatric ward at our university hospital. The patient had been diagnosed with SLE 11 years before, based on the presence of polyarthritis, photosensitivity, discoid, antinuclear antibodies, anti-Sm antibodies, pleuritis, and leucopenia. Six years later, he was codiagnosed with antiphospholipid syndrome. The patient had been treated with predonisolone and cyclophosphamide pulse therapy. On admission to the psychiatric ward (Day 0), he had insomnia, mood swings, agitation, paranoia, memory dysfunction, cognitive impairments and attention deficit. His magnetic resonance imaging (MRI) scan showed old left cerebeller infarctions and lacunar infarctions in the bilateral basal ganglia (Figure 1A). Furthermore the patient’s single photon emission computed tomography (SPECT) images showed an area of hypoperfusion in the bilateral frontal region (Figure 1B). The immunoglobulin G (IgG) index and the levels of interleukin-6 (IL-6) in his level of cerebrospinal fluid (CSF) were 0.6 and 28 pg/mL, respectively (Day 0). In addition, the results of patient’s CSF were cell count 3/μL, protein 93 mg/dL, and glucose 55 mg/dL. In addition, findings in blood cell count and electrolysis were not particular. Thus, the possibility for encephalitis was ruled out. Based on the results of these clinical examinations and imaging studies, the patient was diagnosed with delirium due to NPSLE and/or treatment with prednisolone. His IgG index and CSF IL-6 level were 0.6 and 28 pg/mL, respectively. When the patient experienced a delirious episode; his scores on the Delirium Rating Scale Revised 98 (DRS-R-98) (Trzepacz et al 2001) and the mini-mental state examination (MMSE) were 39 and 16, respectively. His serum BDNF level was 4.5 ng/mL (Day 1 after admission). Although the dose of prednisolone had decreased from 55 to 20 mg/day and cyclophosphamide pulse therapy had been completed because the activity of NPSLE was subsided, his delirious episode persisted. Treatment with quetiapine was initiated, and the dose was gradually increased up to 750 mg/day to treat his psychotic symptoms including the delirious episode. His delirium and paranoia subsided with quetiapine treatment. His DRS-R-98 scores were 9, 8, and 2 at 1, 2, and 4 weeks, respectively after quetiapine administration. However, mood swing was persisted. Thus, valproic acid was added to quetiapine and the dosage was increased to 900 mg/day (plasma valproic acid level: 56.8 mEq/L). Insomnia, mood swing, agitation, and attention deficits were gradually improved. However, cognitive impairments (memory disturbance, visuospatial skill and learning) persisted after recovery of the delirium and psychosis. His MMSE score remained at 16 points. His MRI scan and SPECT did not improve (Day 92 after admission) (Figure 2A and 2B). His serum BDNF levels were 4.6 ng/mL (Day 14 after admission) 1.0 ng/mL (Day 38 after admission), 1.5 ng/mL (Day 70 after adminission). The IgG index and CSF IL-6 level of the patients were 0.51 and 1.9 pg/mL, respectively (Day 38 after admission). One month later, his mental condition was stable with valproic acid (900 mg/day) and quetiapine (750 mg/day), his cognitive impairment (particularly memory impairment and apraxia) gradually had decreased (his serum BDNF level was 0.8 ng/mL).
Figure 1.
MRI (A) and SPECT (B) findings in day 0 after administration demonstrating old left cerebeller infarctions and lacunar infarctions exist in the bilateral basal ganglia in MRI and an area of hypoperfusion in bilateral frontal region was found in SPECT.
Figure 2.
MRI (A) and SPECT (B) findings in day 92 after administration demonstrating not significant changes in MRI and SPECT.
Discussion
We presented a case of NPSLE with predominant symptoms of delirium and psychosis, which responded well to treatment with quetiapine and valproic acid. However, memory disturbance and cognitive impairment persisted. The interesting finding was that the patient with NPSLE and irreversible organic brain changes demonstrated consistently low serum BDNF levels in spite of the severity of psychiatric symptoms. We have recently reported that serum BDNF levels in healthy volunteers and patients with major depression were 23.4 ± 10.1 ng/mL, and 9.6 ± 7.7 ng/mL, respectively. Therefore, serum BDNF levels in the present case were lower compared with those in normal volunteers and even in depressed patients. In contrast, in a case of NPSLE with reversible organic brain changes, we reported that the plasma BDNF levels were raised during the period when the patient’s psychotic symptoms deteriorated, and the plasma BDNF levels were normalized in parallel with the recovery of her organic brain changes and improvement in her psychotic symptoms (Ikenouchi et al 2006). In addition, Frey and colleagues (2006) reported that long term treatment with valproic acid increased hippocampal BDNF levels in rats, and Park and colleagues (2006) demonstrated that long term quetiapine administration attenuated the decreased BDNF mRNA expression in both the hippocampal and cortical regions of rats caused by immobilization stress. In short, we consider that treatment with valproic acid and quetiapine generally promotes BDNF synthesis in the brain. These findings show that a transient raise in serum/plasma BDNF levels during psychotic symptoms might indicate the possibility of nerve recovery, in other words, reversal of brain damage. In contrast, consistently low serum/plasma levels in spite of psychotic symptoms, while a patient is undergoing treatment with valproic acid and quetiapine might reflect neuronal loss of NPSLE. Furthermore, we cannot completely rule out the possibility that prednisolone treatment decreased serum BDNF levels in this case. Indeed, chronic stress decreased BDNF expression in the brain via enhanced corticosteroid release (Jaconsen and Mork 2006). However, serum BDNF levels were at consistently low levels after prednisolone was decreased. Taken together, it is plausible that the BDNF dynamics may differ between NPSLE patients with irreversible organic brain changes and those with reversible ones. Since the patient did not gain significant amounts of weight over the course of treatment, it is unlikely that decreased serum BDNF levels might due to hyperphagia (Hashimoto et al 2005).
Experimental evidences in rodents suggest that the effect of BDNF on psychiatric symptoms differ with respect to brain region. For example, reduced BDNF in the mesolimbic dopamine system is associated with improvement in anxiety and depressive-like behaviors while increased BDNF in the hippocampal formation is associated with antidepressant efficacy and cognitive function (Berton et al 2006; Chhatwal et al 2006; Martinowick et al 2007).
Recently, Oroszi and colleagues (2006) have reported that the association between of BDNF Met66 confers protection against the decline of motor and psychomotor cognitive function in SLE patients suggesting that carriers of the Met66 polymorphism mat be protected from NPSLE. On the other hand, this allele shows a decrease in activity-dependent secretion and its associated with poorer cognitive function and smaller hippocampal volume (Egan et al 2003). The discrepancy between these two findings remains unknown. It is plausible that the role of BDNF in the pathophysiology of SLE might be complicated.
The source of circulating BDNF remains unknown. Platelet, brain neurons, and vascular endothelial cells are currently considered to be putative sources. It was demonstrated that BDNF cross the blood–brain barrier (Pan et al 1998) and that BDNF levels in the brain and serum have been shown to undergo similar changes during the maturation and aging process in rats (Karege et al 2002). These results indicate that blood BDNF levels might in part reflect the BDNF levels in the brain. Nonetheless, it remains unclear to what extent peripheral levels reflect brain BDNF levels.
In conclusion, the finding in the present case indicates that keeping low serum BDNF levels may be associated with the poor prognosis of NPSLE. Further research is needed to confirm the present finding.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- [ACR] American College of Rheumatology. ACR ad hoc committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Berger C, Schabitz WR, Wolf M, et al. Hypothermia and brain-derived neurotrophic factor reduce glutamate synergistically in acute stroke. Exp Neurol. 2004;185:305–12. doi: 10.1016/j.expneurol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Krishnan V, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, et al. Amygdala BDNF signaling is required for consolidation but not encoding extinction. Nat Neurosci. 2006;9:870–2. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti A, Antonelli A, Piastra M, et al. Expression of neurotrophic factors in cerebrospinal fluid and plasma of children with viral and bacterial meningoencephalitis. Acta Paediatr. 2004;93:1178–84. doi: 10.1080/08035250410031314. [DOI] [PubMed] [Google Scholar]
- Ciammola A, Sassone J, Cannella M, et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144:574–7. doi: 10.1002/ajmg.b.30501. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicot JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM, et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79:281–6. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Koizumi H, Nakazato M, et al. Role of brain-derived neurotrophic factor in eating disorders: recent findings and its pathophysiological implications. Prog Neuropsychopharmacol Biol Psychiatry. 2005;20:499–504. doi: 10.1016/j.pnpbp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Hori H, Yoshimura R, Yamada Y, et al. Effects of olanzapine on plasma levels of catecholamine metabolites, cytokines, and brain-derived neurotrophic factor in schizophrenic patients. Int Clin Psychopharmacol. 2007;22:21–7. doi: 10.1097/YIC.0b013e3280103593. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Wong JY, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–35. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- Ikenouchi A, Yoshimura R, Ikemura N, et al. Plasma levels of brain derived-neurotrophic factor and catecholamine metabolites are increased during active phase of psychotic symptoms in CNS lupus: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1359–63. doi: 10.1016/j.pnpbp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Jaconsen JPR, Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221–5. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M, et al. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–4. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, et al. Decreased brain-derived neurotrophic factor (BDNF)- and beta-thromboglobulin (beta-TG)- blood levels in Alzheimer’s disease. Thromb Haemost. 2006;96:102–3. doi: 10.1160/TH06-03-0173. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Martinowick K, Manji H, Lu B, et al. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–93. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins in inflammatory lung diseases: modulators of cell differentiation and neuroimmune interactions. Cytokine Growth Factor Rev. 2003;14:559–78. doi: 10.1016/s1359-6101(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Oroszi G, Lapteva L, Davis E, et al. The met66 allele of the functional val66met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Ann Rhenm Dis. 2006;65:1330–5. doi: 10.1136/ard.2006.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–61. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee SK, Kim JM, et al. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci Lett. 2006;402:25–9. doi: 10.1016/j.neulet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Madaio MP, Ni D, et al. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–42. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- Vega JA, García-Suárez O, Hannestad J, et al. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Zivadinov R, Tamaño-Blanco M, et al. Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. J Neuroimmunol. 2007;188:167–74. doi: 10.1016/j.jneuroim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Mitoma M, Sugita A, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1034–7. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]