Abstract
We generated mice expressing a COX-2 transgene in colon epithelium and found that they did not develop spontaneous colon tumors. But when treated with azoxymethane, a colon carcinogen, COX-2 mice had a higher tumor load compared to wild type mice. There was no change in the number of pre-neoplastic lesions, indicating that COX-2 does not affect tumor initiation. Tumors in the COX-2 transgenic mice had higher levels of phosphorylated epidermal growth factor receptor and Akt compared to wild type mice. Collectively, our data indicate that COX-2 promotes colon tumor progression, but not initiation, and it does so, in part, by activating EGFR and Akt signaling pathways.
Indexing Terms: cyclooxygenase, prostaglandin, colon cancer, epidermal growth factor receptor, azoxymethane
Introduction
Elevated levels of COX-2 are often found in human colorectal adenomas and adenocarcinomas. COX-2 over-expression in colorectal cancer indicates a poor clinical prognosis and a generally marginal response to conventional therapy [1–3]. While not normally detected in most tissues, COX-2 is induced at sites of inflammation and neoplastic growth, and its expression levels often surpass the levels of the other COX isoform, COX-1, leading to profound increases in prostanoid secretion, particularly prostaglandin E2 (PGE2) [3]. By binding to its receptors, PGE2 promotes cell proliferation and angiogenesis, indicating that its overproduction supports tumor development [4,5]. As a major provider of PGE2, COX-2 over-expression likely contributes to tumorigenesis. Indeed, reducing COX-2 activity with selective inhibitors, or deleting COX-2 or PGE2 receptors, attenuated colon tumor formation in experimental mouse models [5–9]. Collectively, these data indicate an important role for COX-2 in colorectal tumorigenesis and have stimulated interest in COX-2 as a therapeutic target. Several retrospective studies have shown that chronic administration of aspirin and other non-steroidal anti-inflammatory drugs (NSAID) confers protection from polyp formation and colorectal cancer in some populations [10–14]. However, NSAIDs that target both COX enzymes have side effects that limit their potential as anti-tumor agents; specific COX-2 inhibitors also appear to have limiting toxicities [15].
Given the importance of COX-2 in colorectal tumorigenesis, it is crucial to determine the contribution of COX-2 to the various stages of tumor development and to understand the signaling mechanisms that underlie its tumor promoting effects. We and others have shown in vitro that PGE2 transactivates EGFR [16–18], which is known to promote proliferation [18–21], survival [22,23], migration [17,19,24], and angiogenesis [25]. Collectively, these data led us to hypothesize that COX-2 might promote colorectal tumorigenesis by activating EGFR signaling. To understand how COX-2 affects colon tumor development we generated mice that expressed a human COX-2 transgene in colon epithelium. The mice did not spontaneously develop colon tumors, even when fed a high fat diet, indicating that COX-2 is not sufficient to initiate colon tumorigenesis. But when colon tumors were induced with azoxymethane (AOM), COX-2 transgenic mice developed higher tumor loads. However, there was no change in the number of pre-neoplastic aberrant crypt foci (ACF) indicating that transgenic expression of COX-2 did not affect tumor initiation. Both EGFR and Akt were excessively phosphorylated in the COX-2 transgenic mice compared to their wild-type littermates, indicating that EGFR signaling contributes to tumor formation. Collectively, these data demonstrate that in the colon, COX-2 promotes tumor progression, but not initiation, and that its activation of EGFR and Akt signaling pathways likely contributes to its tumorigenic effects.
Materials and methods
Materials
AOM was purchased from Midwest Research Institute. The PGE2 assay kit was from Cayman Chemical (#514010). Antibodies to detect COX-2, EGFR, pEGFR Tyr1068, pEGFR Tyr992, Akt, pAkt Ser473, and pAkt Thr308 were purchased from Cellular Signaling (#4842, #2232, #2234,#2235, #4057, #3787, and #9266 respectively). COX-2 antibodies were also purchased from Santa Cruz Biotechnologies (sc-1475). A third custom COX-2 antibody was kindly provided by J. Maclouf, Lariboisiere Hospital, Federated Institute of Circulation-Lariboisiere, Paris, France [26]. NeoMarkers provided anti-Ki67 antibodies (#RM-9106). MP Biomedicals provided anti-actin antibodies (#691001).
Mice
All animal experiments were reviewed and approved by the University of Utah Institutional Review Board. Equal numbers of male and female mice were used throughout the study; there was no gender difference noted in collected data. Founder C57Bl/6, A/J, and AKR/J were purchased from Jackson Laboratories. To generate COX-2 transgenic mice, the cDNA encoding human COX-2 (lacking expression of 3′UTR AU-rich elements to increase its levels of expression [27]), were ligated to the FABPL promoter (released from pEPLFABP, a gift from Jeff Gordon, Washington University, St Louis [28]) and to the SV40 intron/pA tail from pcDNA1 (Invitrogen). The product was inserted into pBluescript, and propagated in E. coli. The resulting constructs were linearized and then injected into 129Sv/J blastocysts. Two COX-2 founders were generated and then crossed to C57Bl/6 mice. Of the two COX-2 founder lines, we used the one expressing higher levels of COX-2 in the intestine epithelium. Progeny were then backcrossed six generations into AKR/J or A/J strains, which were then used for the AOM studies.
Assay for COX-2 expression
The presence of the COX-2 transgene was determined by PCR amplification of genomic DNA isolated from mouse tails using salt precipitation. Mice were sacrificed using CO2 asphyxiation. Two different tissue sample preparations were used for COX-2 immunoblot analyses of age-matched mice. Whole tissue from mouse colons, kidneys, and livers were mechanically homogenized in cell lysis buffer. Alternatively, longitudinally dissected colons were incubated in PBS containing 3mM EDTA and 50μM DTT for 90 minutes at 4°C. The colons were then washed once and vigorously shaken in ice-cold PBS 3–4 times to release intact crypts. Crypts were centrifuged at 40xG for 10 minutes at 4°C. The pellets were reconstituted in ice-cold cell lysis buffer and frozen (adapted from Whitehead et al. [29]). Protein content for both samples was determined using the Pierce-BCA protein assay kit. Lysates (50μg for the liver and kidney tissue homogenates, 25μg for the colon tissue homogenate, and 15μg for the crypt isolates ) were separated by electrophoresis on 10% SDS-PAGE, transferred to PVDF membranes, and immunoblotted for COX-2, according to the manufacturer’s instructions (Cellular Signaling).
PGE2 assay
Crypts were isolated as described above, except that 90% of each sample was reconstituted in 37°C PBS instead of cell lysis buffer, and then incubated for 10 minutes in the absence and presence of exogenous arachidonic acid (20 μM). After centrifugation (15,000×G, 10 min) we quantified the levels of PGE2 in the supernatants using an ELISA (Cayman Chemical). We determined protein levels in the remaining 10% of each sample.
Tumor studies
Mice were divided into 4 groups according to treatment and genotype. Two groups, transgenic and non-transgenic, were treated with AOM (10mg/kg), while two additional groups were treated with saline as controls. Injections were administered once a week intraperitoneally, starting at 6 weeks of age, for 6 weeks. Mice were allowed to reach 25 weeks of age before being sacrificed [30].
Tissue harvesting and tumor assessment
When the animals reached 25 weeks of age, they were sacrificed using CO2 asphyxiation. The colon was dissected longitudinally and washed with ice-cold PBS. Colons were then fixed for 4 hours in 10% formalin in PBS, and then stored in 70% ethanol at 4°C. We washed the colons with PBS, stained them using methylene blue, and then assessed ACF, number of crypts per ACF, polyps, and number of tumors, with the aide of a dissecting microscope. The studies were independently performed by two laboratory members who had no knowledge of the genotype and/or type of treatment (saline or AOM) to which each mouse had been subjected. Each tumor was photographed, the largest and smallest diameters were recorded, and tumor volumes were calculated according to the equation (Volume (mm3) = π/6 × Largest diameter × Smallest diameter2). Tumor load was calculated by summing all measured tumor volumes in a mouse.
Statistical Analysis
Data was analyzed using the Microsoft Excel statistical package. A two tail homoscedastic or heteroscedastic unpaired Student’s t-Test was used, p-value of less than 0.05 was considered statistically significant.
Immunohistochemistry
Tumor and surrounding normal tissue from AOM-treated 25 week-old matched mice were fixed and paraffin-embedded. Five-micrometer-thick paraffin sections were cut and applied to Superfrost Plus slides (VWR). Sections were then processed in pairs of transgenic and non-transgenic littermates according to manufacturer’s instructions. Antibodies to detect COX-2 (1:50), EGFR (1:100), pEGFR Tyr1068 (1:200), pEGFR Tyr992 (1:100), Akt (1:150), pAkt Ser473 (1:50), and pAkt Thr308 (1:50) were purchased from Cellular Signaling (#4842, #2232, #2234,#2235, #4057, #3787, and #9266 respectively). NeoMarkers provided anti-Ki67 (1:200) antibodies (#RM-9106). All antibodies were incubated over night at 4°C and a secondary 1:1000 Biotin-SP-conjugated antibody for 30 minutes, goat anti rabbit for the rabbit primary antibodies and Fab fragment goat anti-mouse for the mouse primary antibodies (Jackson Immunoresearch 111-065-003, and 111-067-003 respectively). ABC Vetcastain (avidin/biotin) with Vector NovaRED was used to develop color. Slides were then counterstained with hematoxylin, dehydrated, and then cover slips were added. Images were acquired using an Olympus IX70 microscope equipped with a Microfire/Qcam CCD camera. To quantify the level of immunostaining, the integrated density of red was calculated from the digital images by multiplying the mean intensity of red determined using Adobe Photoshop software by the area of red staining measured using N.I.H. ImageJ software.
Results
Transgenic expression of COX-2 in the intestine
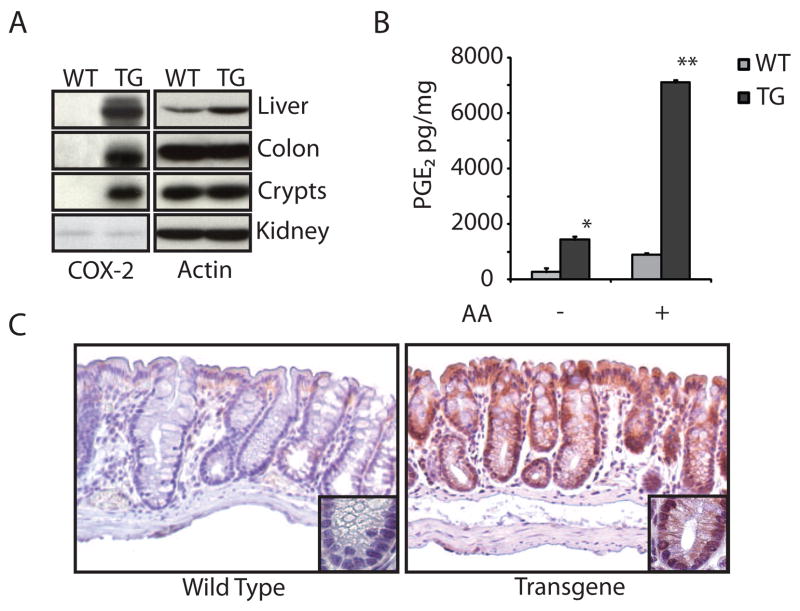
COX-2 is often over-expressed in both the stromal and epithelial components of human colorectal tumors and the resulting prostaglandin products promote tumorigenesis by a variety of proposed mechanisms. Its over-expression occurs early in tumorigenesis, but it is not clear if COX-2 participates in tumor initiation, tumor progression, or both. To understand the effects of COX-2 over-expression in colorectal carcinogenesis, we generated transgenic mice that over-expressed COX-2 in the colon using a human COX-2 transgene under control of the liver fatty acid binding protein (LFABP) promoter, which is active in colon epithelium [31,32]. We confirmed integration of the COX-2 transgene using RT-PCR of genomic DNA (not shown). As expected, we found using immunoblotting and immunohistochemical analyses that COX-2 was highly expressed in colon tissue and crypts (Figs. 1A,C). Expression of COX-2 in the colon was most prominent in the proximal half of the colon and decreased distally (not shown). We also found that COX-2 was expressed in liver, a tissue where the LFABP promoter is also active, but its expression was not enhanced in kidney tissue (Figure1A). To confirm higher levels of COX activity in the transgenic mice, we tested colon crypt isolates from a representative selection of age-matched littermates for their ability to make PGE2. We found that crypts from COX-2 transgenic mice produced significantly more PGE2 both in the presence and absence of exogenous arachidonic acid (Figure 1B). The patterns for COX-2 expression and activity are the same for both the mixed paternal strain and the A/J and AKR/J strains used for AOM treatment in this study. Collectively, these data indicate that the transgenic mice express active COX-2 in colon epithelium.
Figure 1.
Characterization of transgenic COX-2 expression. A. COX-2 Western blots. Colon, kidney, and liver lysates or isolated colon crypt lysates were probed with a COX-2 antibody that recognizes an epitope that is identical in mouse and human COX-2 (cellular signaling #4842). Immunoblots were reprobed for actin. B. PGE2 assay. Isolated colon crypts were reconstituted in warm PBS for 10 minutes with or without 20μM arachidonic acid. PGE2 was assayed in the supernatants by ELISA (the assays were performed in duplicate and each point represents the average of two mice) and then normalized to total protein in the crypt lysates. The * indicates statistically significant increased PGE2 production in COX-2 transgenic mice compared to wild type litter mates (p<0.03); ** Indicates statistically significant increased PGE2 production in COX-2 transgenic mice compared to wild type littermates when treated with arachadonic acid (p<0.005); error bars indicate standard deviation. C. COX-2 immunohistochemistry. Paraffin-embedded mouse colons were probed with anti-COX-2 antibodies. The insets show a representative crypt.
COX-2 over-expression is not sufficient to initiate colon tumorigenesis
Colon tumor development can be roughly divided into initiation and progression phases. The initiation phase is characterized by the development of ACF. These lesions can progress into polyps with further dysplasia, but these polyps retain characteristic features of crypt architecture. Further growth and loss of recognizable crypt architecture are considered properties of adenomas [33]. To determine if COX-2 over-expression was sufficient to induce tumorigenesis, we crossed the mice into the highly tumor susceptible A/J background, sacrificed them at 25 weeks of age, and then assessed tumor development. We measured the number and size of ACF and found no differences between wild type and COX-2 transgenic mice. In addition, the two groups had comparable numbers of polyps (Table I). We found only one adenoma in the COX-2 transgenic mice, but the difference was not statistically significant. We also fed mice a high fat diet (chow with 5% or 20% corn oil) for twelve weeks and did not detect differences between wild-type and COX-2 transgenic mice (data not shown). Together, these data indicate that when expressed in colon epithelium, COX-2 is not sufficient to initiate colon tumorigenesis.
Table I.
Preneoplastic lesions in wild type and COX-2 transgenic mice (A/J)
| WT n=16 | TG n=16 | |
|---|---|---|
| ACF | 2.1 (±1.0) | 2.5 (±0.9) |
| Crypts/ACF | 1.4 (±0.4) | 1.6 (±0.3) |
| Polyps | 2.1 (±0.2) | 1.7 (±0.4) |
These results were consistent with several studies in mice demonstrating that the tumorigenic effects of COX-2 depend on the tissue in which it is expressed. For example, COX-2 over-expression is sufficient for carcinoma formation in breast [34] but not in skin [35]. To further study the role of COX-2 on colorectal tumorigenesis we crossed the mice six generations into an AKR/J background. We used the AKR/J background because these mice form few adenomas when treated with AOM, but develop a moderate number of ACF [36]. These characteristics of AKR/J mice allowed us to study both tumor initiation and progression in response to COX-2 over-expression. To induce tumors in the mice, we injected 6 week-old littermates weekly with either AOM or saline for 6 weeks, and then sacrificed the mice 13 weeks after the final treatment (25 weeks of age). We found no significant differences in the number of ACF and polyps between wild type and COX-2 transgenic mice (Table II), consistent with data obtained on the A/J background (Table I). Together, these data indicate that COX-2 over-expression does not affect early stages of colon tumor development, including tumor initiation.
Table II.
Preneoplastic lesions in wild type and COX-2 transgenic mice (AKR/J)
| Saline | AOM | |||
|---|---|---|---|---|
| WT n=16 | TG n=10 | WT n=20 | TG n=12 | |
| ACF | 5.4 (±0.7) | 6.5 (±1.6) | 39.5 (±5.4) | 38.8 (± 7.5) |
| Crypts/ACF | 2.3 (±0.2) | 1.9 (±0.3) | 2.3 (±0.2) | 2.5 (±0.1) |
| Polyps | 1.1 (±0.3) | 0.7 (±0.2) | 2.1 (±0.4) | 1.7 (±0.9) |
AOM is thought to induce COX-2 expression [37–38], so the lack of change that we observed in the previous experiments might have been due to induction of endogenous COX-2 to levels comparable to those of transgenic COX-2. By immunoblotting, we determined the levels of COX-2 in the colons of six wild-type mice treated with AOM and found that AOM caused only a 10–20% increase in COX-2 protein at one or twelve weeks after the final AOM injection. The COX-2 transgene was still expressed at 30-fold higher levels (data not shown). Thus, the lack of change in ACF was not caused by induction of endogenous COX-2 in wild-type mice.
COX-2 affects tumor progression
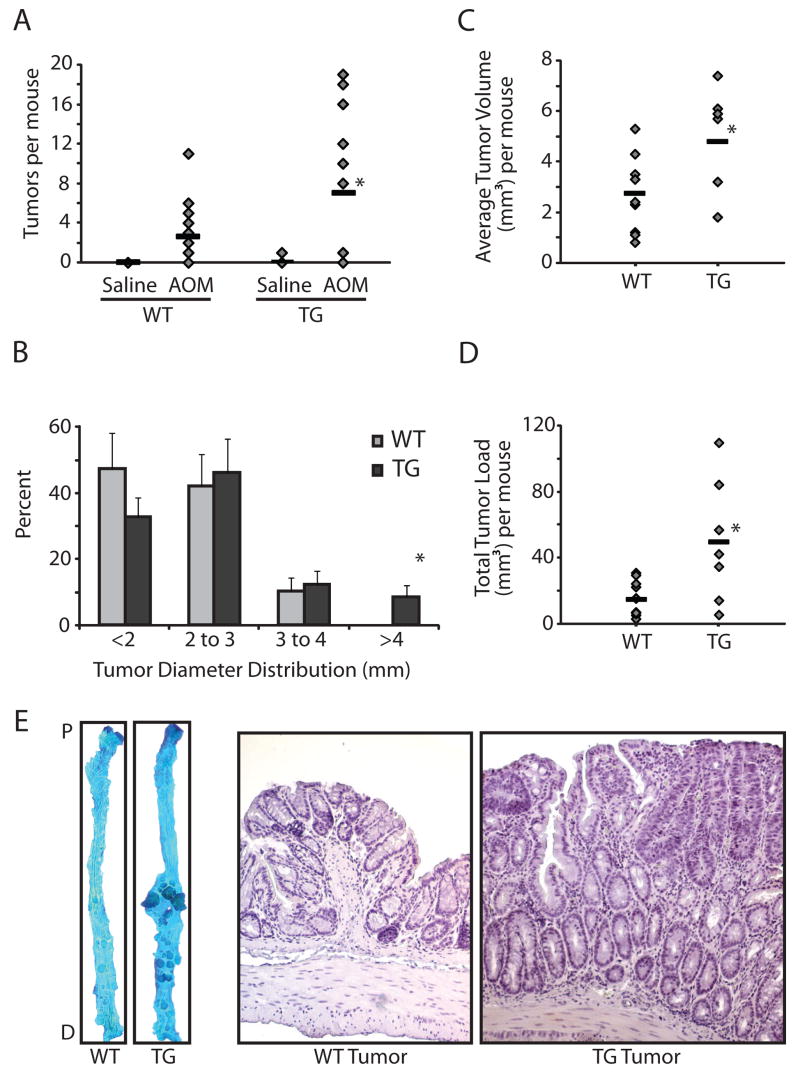
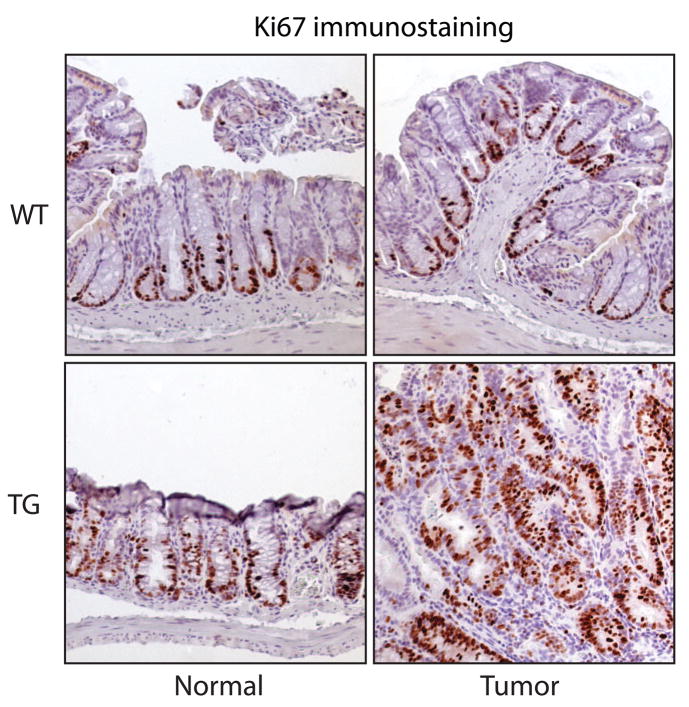
To study the effects of COX-2 on colon tumor progression, we counted and measured colon adenomas from AOM-treated wild type and COX-2 transgenic mice in the AKR/J background (Figure 2A). While AOM-induced tumor incidence was similar between wild-type mice (75%) and COX-2 transgenic mice (67%), we found significantly more tumors in the COX-2 transgenic mice that had been exposed to AOM (7.0 ± 2.1 tumors per mouse, n=12) compared to their wild type littermates (2.6 ± 0.7 tumors per mouse, n=20). The average diameter of colon tumors showed a statistically significant shift toward larger tumors in the transgenic mice compared to their non-transgenic littermates, with four of the twelve transgenic mice having multiple tumors with a diameter larger than 4mm compared to none of their twenty non-transgenic littermates (Figure 2B). The average tumor volume per mouse was also higher in the COX-2 transgenic mice compared to wild type littermates [(4.8 ± 0.8 mm3) versus (2.7 ± 0.4 mm3), respectively (Figure 2C)]. The increase in average tumor volume in COX-2 transgenic mice was statistically significant. In addition, the tumor load per mouse—a summation of tumor volumes which reflects both size and number of tumors—was significantly higher in the COX-2 transgenic mice compared to wild type littermates [(50.2 ± 14.2 mm3) versus (15.4 ± 3.2 mm3), respectively (Figure 2D)]. Morphological analyses revealed that, in addition to the size differences described above, tumors from the COX-2 transgenic mice were less organized and displayed dysmorphic crypt architecture compared to tumors from wild type littermates (Figure 2E). In addition, we detected higher expression of the proliferation marker Ki67 in tumors from COX-2 transgenic mice compared to wild type littermates (Figure 3). Moreover, the proliferation zone in the normal surrounding crypts was larger in the COX-2 transgenic mice (Fig. 3). Collectively, our data indicate that in the colon, COX-2 over-expression promotes tumor progression.
Figure 2.
Increased tumor multiplicity and size in COX-2 transgenic mice. A. Tumor count. Mice treated with AOM or saline were sacrificed 12 weeks after the last injection and tumors were counted (30). COX-2 transgenic mice treated with AOM (n=12) developed more tumors compared to wild type littermates (n=20, p<0.05). There was no difference in the saline-treated mice. B. Tumor size distribution. There was a statistically significant shift (p<0.05) towards larger diameter tumors in the AOM-treated transgenic mice compared to AOM-treated wild type littermates. *Indicates statistical significance in the >4mm category: 4 out of the 12 transgenic mice developed tumors larger than 4mm in diameter compared to none in the wild type group (p<0.03). Error bars are standard error of the mean. C. Average tumor volume per mouse was calculated. * Indicates a statistically significant increase in average tumor volume in the AOM-treated transgenic mice compared to AOM-treated wild type littermates (p<0.03). D. Total tumor load per mouse. Tumor sizes for each mouse were summed to reflect both tumor number and average tumor size. *Indicates a statistically significant higher tumor load in the AOM-treated transgenic mice compared to AOM-treated wild type littermates (p<0.03). E. Representative methylene blue-stained colons (left) and hematoxylin-stained tissue sections (right) from AOM-treated wild type and COX-2 transgenic mice.
Figure 3.
Increased proliferation COX-2 transgenic mice. Tumor and surrounding normal tissue samples from age-matched, transgenic and non-transgenic mice were fixed, paraffin-embedded, and then immunostained to detect Ki67. Wild-type and transgenic sections were processed together.
Activation of EGFR signaling in COX-2 transgenic mice
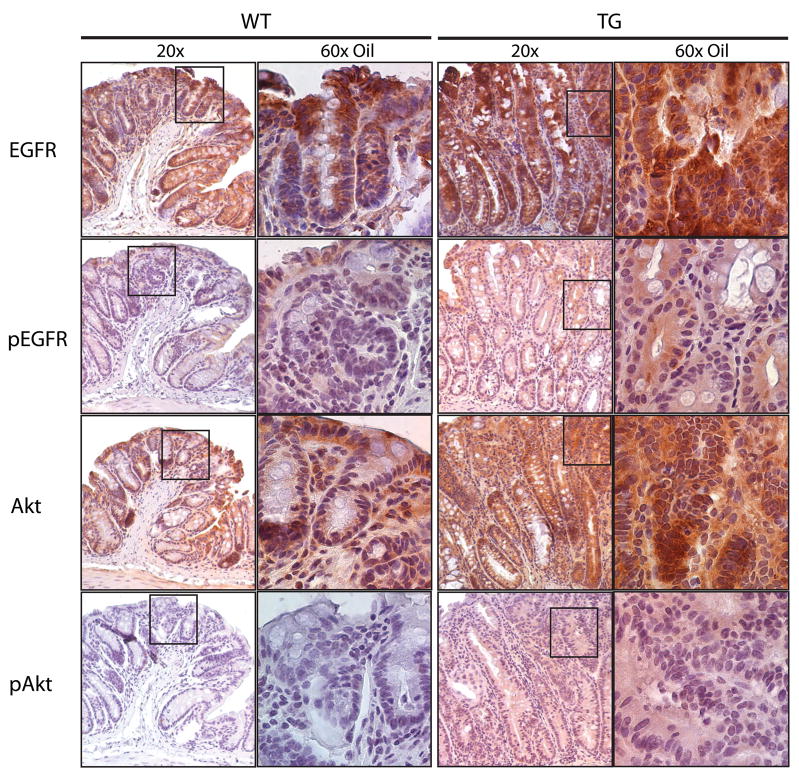
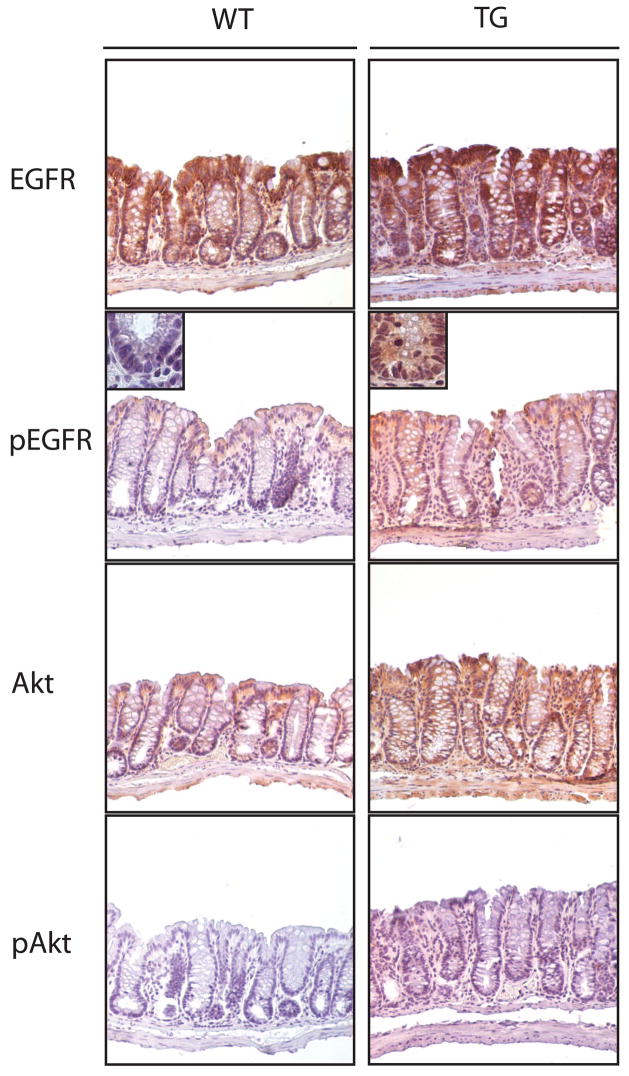
We and others have demonstrated that PGE2 activates EGFR signaling in cultured cells [16,17,24]. To determine if this pathway was activated in the COX-2 transgenic mice, we used immunohistochemistry to examine EGFR signaling in AOM-treated mice in the AKR/J background. To assess EGFR signaling, we determined total EGFR, tyrosine phosphorylated EGFR (pEGFR), total Akt, and phosphorylated Akt (pAkt). We found that COX-2 transgenic mice expressed 3–5 fold higher levels of total EGFR, total Akt, pAkt, and pEGFR compared to wild type littermates in tumor tissue (Figure 4) and 1.5–3 fold higher levels of these proteins in normal surrounding tissue (Figure 5). The differences in pEGFR in normal-appearing tissue were most apparent at the base of the crypts (Figure 5). These data indicate that over-expression of COX-2 activates EGFR and Akt signaling that likely contributed to colorectal tumor progression in the mice.
Figure 4.
Downstream signaling pathways in tumors from COX-2 transgenic and wild type mice. Paraffin-embedded tumors from the mid-colon of age-matched COX-2 transgenic and wild type mice were immunostained to detect EGFR, pEGFR, Akt, and pAkt. Wild type and transgenic tissue was processed together. We found similar results with antibodies specific for phospho-Tyr992-EGFR and phospho-Thr308-Akt.
Figure 5.
Assessment of EGFR and Akt signaling pathways in normal appearing tissue from COX-2 transgenic and wild type mice. Paraffin-embedded tissue samples from the mid-colon of age-matched COX-2 transgenic and wild type mice were immunostained to detect EGFR, pEGFR, Akt, and pAkt. Wild type and transgenic tissue was processed together. Similar results were obtained with antibodies to detect phospho-Tyr992-EGFR and phospho-Thr308-Akt.
Discussion
COX-2 over-expression is an early event in colorectal cancer, and several lines of evidence support a role for COX-2 in this disease. But whether COX-2 contributes to tumor initiation or progression is not yet clear. In this study, we developed an in vivo model system that allowed us to investigate the role of COX-2 in colon tumor initiation and progression and that permitted identification of key signaling pathways activated by COX-2 during these processes. We generated mice over-expressing COX-2 in colon epithelium and found that this was not sufficient to cause colon tumor initiation. Additionally, over-expression of COX-2 did not affect the development of pre-neoplastic lesions in mice treated with AOM. Collectively, these data indicate that in this model, COX-2 over-expression did not affect early events in colon tumorigenesis. AOM treatment is a model of non-familial colorectal cancer, the most common form of this disease in humans. As such, our data indicate that inhibitors of COX-2 might not be useful chemoprevention agents in the general population, a possibility that is reinforced by several recent clinical trials testing routine use of NSAIDs to prevent colorectal cancer in the general population [39].
It is important to note that our results in the colon might not reflect the tumorigenic properties of COX-2 in other organs. For example, contrary to our results in colon, over-expression of COX-2 in mouse skin, pancreas, and bladder, induced pre-neoplastic lesions [40], and when over-expressed in breast tissue, COX-2 was sufficient to cause carcinomas [34]. These data suggest that in other organs, NSAIDs might be useful chemoprevention agents.
Although COX-2 did not affect tumor initiation, its over-expression dramatically hastened tumor progression, causing more and larger tumors to develop in vivo. These data indicate that in this model, COX-2 plays a role in later stages of tumor development and they suggest that COX-2 inhibitors might be useful chemotherapy agents. There are several ongoing clinical trials to test this possibility, but we anticipate that only those patients expressing high levels of COX-2 might benefit from treatment.
It is tempting to speculate that most if not all of our observations in COX-2 over-expressing mice were mediated by changes in PGE2 concentrations. PGE2 has been shown to activate EGFR and Akt signaling pathways [16], both of which contribute to colon carcinogenesis. Indeed, we found evidence that EGFR and Akt were more extensively activated in COX-2 over-expressing mice. These data suggest that EGFR and Akt signaling participate in COX-2-mediated events. But since PGE2 can activate Akt independently of EGFR [16], the extent to which these individual pathways contribute to tumorigenesis requires further study. We also found elevated levels of cytoplasmic β-catenin in tumors from COX-2 transgenic mice compared to wild-type mice, but we did not detect more nuclear β-catenin (not shown). These data suggest that COX-2 might promote tumorigenesis by activating several independent signaling pathways. Since tumors from COX-2 over-expressing mice displayed evidence of activation of at least three signaling axes, our data suggest that inhibiting only one of these signaling pathways, such as that of EGFR, will likely not be a sufficient anti-tumor strategy. Instead, therapeutic interventions should be aimed at COX-2 or enzymes involved in the synthesis of PGE2.
However, it is also possible that COX-2 promoted colorectal tumorigenesis by functions other than, or in addition to, PGE2 biosynthesis, including the ability of COX-2 to utilize arachidonic acid and thus prevent its metabolism to other products (e.g., leukotrienes, lipoxins, HETEs, etc.) that may play important roles in colon carcinogenesis. Since COX-2 can alter numerous signaling pathways, it will be important to determine the mechanisms by which it promotes colorectal tumorigenesis so that we can target the affected pathways using less toxic therapies.
In conclusion we have shown that COX-2 potentiates colon tumor progression but not its initiation. We also found that COX-2 expression activates EGFR and Akt signaling pathways in colon tumors, paving the way for future studies to determine the most effective combination and timing of inhibitors to treat colon cancers where COX-2 is expressed.
Acknowledgments
We are indebted to Kate Lund and Jared Higbee for providing excellent technical assistance. This work was supported by the Huntsman Cancer Foundation, the R. Harold Burton Foundation, the National Institutes of Health Grants R01-CA95463 (to M.K.T.), and P01-CA73992 (to D.M.S.), and in part by the Cancer Center Support Grant P30 CA042014-20. M.A. Al-Salihi was supported by a Pre-doctoral Fulbright Award (2003–05). None of these granting agencies were involved in the study design and implementation, manuscript preparation, or decision to submit the manuscript.
Abbreviations
- COX-2
cyclooxygenase-2
- AOM
azoxymethane
- EGFR
epidermal growth factor receptor
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
- ACF
aberrant crypt foci
- LFABP
liver fatty acid binding protein
- pEGFR
phosphorylated EGFR
- pAkt
phosphorylated Akt
Conflict of Interest Statement
The authors report no financial or personal relationships that could have influenced this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100:1325–1329. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor, pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 5.Rao CV, Reddy BS, Steele VE, Wang C, Liu X, Ouyang N, Patlolla JMR, Simi B, Kopelovich L, Rigas B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats, effects on molecular targets. Mol Cancer Ther. 2006;5:1530–1538. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Huang Y, Porta R, Yanagisawa K, Gonzalez A, Segi E, Johnson DH, Narumiya S, Carbone DP. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006;66:9665–9672. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]
- 7.Howe L, Chang S, Tolle KC, Dillon R, Young LJT, Cardiff RD, Newman RA, Yang P, Thaler HT, Muller WJ, Hudis C, Brown AMC, Hla T, Subbaramaiah K, Dannenberg AJ. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 8.Chang S, Ai Y, Breyer RM, Lane TF, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496–4499. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- 9.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 10.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 11.Imperiale TF. Aspirin and the prevention of colorectal cancer. N Engl J Med. 2003;348:879–880. doi: 10.1056/NEJMp030005. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Thun MJ, Namboodiri MM, Heath CW. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald GA. COX-2 in play at the AHA and the FDA. Trends Pharmacol Sci. 2007;28:303–307. doi: 10.1016/j.tips.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Al-Salihi MA, Ulmer SC, Doan T, Nelson CD, Crotty T, Prescott SM, Stafforini DM, Topham MK. Cyclooxygenase-2 transactivates the epidermal growth factor receptor through specific E-prostanoid receptors and tumor necrosis factor-alpha converting enzym. Cell Signal. 2007;19:1956–1963. doi: 10.1016/j.cellsig.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 18.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates the EGF receptor, a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 19.Shao J, Evers BM, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem. 2004;279:14287–14293. doi: 10.1074/jbc.M313276200. [DOI] [PubMed] [Google Scholar]
- 20.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Re. 2003;63:5218–5223. [PubMed] [Google Scholar]
- 21.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casado M, Mollá B, Roy R, Fernández-Martínez A, Cucarella C, Mayoral R, Boscá L, Martín-Sanz P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 23.Tessne TG, Muhale RF, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J. 2003;17:1640–1647. doi: 10.1096/fj.02-1011com. [DOI] [PubMed] [Google Scholar]
- 25.Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- 26.Maloney CG, Kutchera WA, Albertine KH, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory agonists induce cyclooxygenase type 2 expression by human neutrophils. J Immunol. 1998;160:1402–1410. [PubMed] [Google Scholar]
- 27.Dixon DA. Dysregulated post-transcriptional control of COX-2 gene expression in cancer. Curr Pharm Des. 2004;10:635–646. doi: 10.2174/1381612043453171. [DOI] [PubMed] [Google Scholar]
- 28.Sweetser DA, Birkenmeier EH, Hoppe PC, McKeel DW, Gordon JI. Mechanisms underlying generation of gradients in gene expression within the intestine, an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988;2:1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead RH, Demmler K, Rockman SP, Watson NK. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–865. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]
- 30.Bissahoyo A, Pearsall RS, Hanlon K, Amann V, Hicks D, Godfrey VL, Threadgill DW. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice, effects of dose, route, and diet. Toxicol Sci. 2005;88:340–345. doi: 10.1093/toxsci/kfi313. [DOI] [PubMed] [Google Scholar]
- 31.Simon TC, Roth KA, Gordon JI. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem. 1993;268:18345–18358. [PubMed] [Google Scholar]
- 32.Sweetser DA, Birkenmeier EH, Klisak IJ, Zollman S, Sparkes RS, Mohandas T, Lusis AJ, Gordon JI. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987;262:16060–16071. [PubMed] [Google Scholar]
- 33.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer, consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 34.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 35.Müller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hata K, Yamada Y, Kuno T, Hirose Y, Hara A, Qiang SH, Mori H. Tumor formation is correlated with expression of beta-catenin-accumulated crypts in azoxymethane-induced colon carcinogenesis in mice. Cancer Sci. 2004;95:316–320. doi: 10.1111/j.1349-7006.2004.tb03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh J, Hamid R, Reddy BS. Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of dietary fat during the postinitiation stage of colon carcinogenesis. Cancer Res. 1997;57:3645–3470. [PubMed] [Google Scholar]
- 38.Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, Giardina C, Rosenberg DW. inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–315. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 39.Dube C, Rostrom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. preventive services task force recommendation statement. Ann Int Med. 2007;146:361–375. [PubMed] [Google Scholar]
- 40.Marks F, Fürstenberger G, Müller-Decker K. Tumor promotion as a target of cancer prevention. Recent Results Cancer Res. 2007;174:37–47. doi: 10.1007/978-3-540-37696-5_3. [DOI] [PubMed] [Google Scholar]