Abstract
Background
Maternal mortality is a major public-health problem in developing countries. Extreme differences in maternal mortality rates between developed and developing countries indicate that most of these deaths are preventable. Most information on the causes of maternal death in these areas is based on clinical records and verbal autopsies. Clinical diagnostic errors may play a significant role in this problem and might also have major implications for the evaluation of current estimations of causes of maternal death.
Methods and Findings
A retrospective analysis of clinico-pathologic correlation was carried out, using necropsy as the gold standard for diagnosis. All maternal autopsies (n = 139) during the period from October 2002 to December 2004 at the Maputo Central Hospital, Mozambique were included and major diagnostic discrepancies were analyzed (i.e., those involving the cause of death). Major diagnostic errors were detected in 56 (40.3%) maternal deaths. A high rate of false negative diagnoses was observed for infectious diseases, which showed sensitivities under 50%: HIV/AIDS-related conditions (33.3%), pyogenic bronchopneumonia (35.3%), pyogenic meningitis (40.0%), and puerperal septicemia (50.0%). Eclampsia, was the main source of false positive diagnoses, showing a low predictive positive value (42.9%).
Conclusions
Clinico-pathological discrepancies may have a significant impact on maternal mortality in sub-Saharan Africa and question the validity of reports based on clinical data or verbal autopsies. Increasing clinical awareness of the impact of obstetric and nonobstetric infections with their inclusion in the differential diagnosis, together with a thorough evaluation of cases clinically thought to be eclampsia, could have a significant impact on the reduction of maternal mortality.
Jaume Ordi and colleagues examine the discrepancies between clinical diagnoses of causes of maternal deaths and pathological findings by necropsy in Mozambique.
Editors' Summary
Background.
Every year, about half a million women die during pregnancy or childbirth or soon after delivery—so-called “maternal deaths.” Although nearly all these maternal deaths occur in developing countries, the situation is particularly bad in sub-Saharan Africa where more than a quarter of a million maternal deaths occur annually. The number of maternal deaths per 100,000 live births in this region is nearly 1,000, whereas in developed regions it is only nine deaths. A 15-year-old girl living in sub-Saharan Africa has a lifetime risk of dying during pregnancy or childbirth of 1 in 22, but a girl living in the developed regions of the world has a lifetime risk of only 1 in 7,300. Maternal deaths can be caused by obstetric (childbirth-related) complications such as puerperal septicemia (an infection of the blood contracted during delivery) and eclampsia (seizures associated with high blood pressure during pregnancy), and by nonobstetric conditions such as HIV/AIDS-related infections and other infections.
Why Was This Study Done?
In 2000, the United Nations made reduction of the global burden of maternal mortality one of its Millennium Development Goals (a set of targets designed to eradicate poverty by 2015), but little progress has been made toward achieving this goal. One possible explanation for this failure might be that limited access to diagnostic tests in developing countries results in more clinical diagnostic errors than in developed countries and that, consequently, mothers in developing countries don't always get the right treatment when they become ill. Unfortunately, it is difficult to test this hypothesis, because there is very little accurate information on the causes of maternal death in many developing countries. What information there is comes mainly from clinical records and verbal autopsies (asking relatives about the mother's death) rather than from examination of the body after death (a medical autopsy), the only sure way to ascertain the cause of death. In this study, the researchers retrospectively analyze discrepancies between the clinical diagnoses and autopsy diagnoses of 139 mothers who died at the Maputo Central Hospital, Mozambique, a large hospital providing specialized care for women with high-risk pregnancies.
What Did the Researchers Do and Find?
All the organs from the mothers were visually examined by a pathologist and samples of any abnormal tissues and of the internal organs were examined microscopically. Two pathologists independently established the cause of each death by considering both the clinical diagnosis and the autopsy results (the “autopsy diagnosis”). The discrepancies between the clinical and autopsy (“gold standard”) diagnoses were then analyzed. Major diagnostic errors (errors involving the cause of death) occurred in nearly half of the maternal deaths; the clinical and autopsy diagnoses completely agreed in only a third of cases. 80% of the major diagnostic errors were “class I errors.” That is, errors where a correct diagnosis would have changed patient management and prolonged survival or provided a cure. For example, 12 women were given an incorrect diagnosis of eclampsia when they had other conditions that could have been successfully treated if correctly diagnosed. Furthermore, many infections detected in the autopsies were missed in the clinical diagnoses (false-negative diagnoses), some of which could have been treated.
What Do These Findings Mean?
These findings show that clinical and autopsy diagnoses of the causes of maternal death frequently disagree in this hospital. Further studies are needed to see whether similar levels of disagreement exist in other hospitals in sub-Saharan Africa. The discrepancy reported here might, for example, be an overestimate of the general situation, because the high-risk pregnancies referred to this hospital might involve more hard-to-diagnose problems than the routine pregnancies referred to other hospitals. Nevertheless, these findings suggest that misdiagnosed conditions may affect maternal mortality rates in sub-Saharan Africa and that an increased use of autopsy in the region could reduce maternal mortality by providing more accurate information about why mothers die. In particular, these findings suggest that a more thorough evaluation of cases thought to be eclampsia and a better awareness of the involvement of infectious diseases in maternal deaths might reduce diagnostic errors and consequently reduce the incidence of maternal deaths.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1000036.
UNICEF (the United Nations Children's Fund) provides information on maternal mortality and the WHO/UNICEF/UNFPA/The World Bank estimates of maternal mortality for 2005 by country
The WHO/UNICEF/UNFPA/The World Bank estimates of maternal mortality in 2005 also provides full information about global maternal mortality
The UK Department for International Development provides information about Millenium Development Goal 5: the improvement of maternal health
The Partnership for Maternal, Newborn and Child Health provides information on maternal deaths (in several languages), including information on the situation in Mozambique
Introduction
There is a general consensus that maternal mortality is a major health problem worldwide as well as a fundamental public health indicator [1]. The problem of maternal mortality is concentrated in low-income countries, particularly in sub-Saharan Africa, where one in 16 women die of pregnancy-related complications [2,3]. Reduction of this intolerable burden is one of the Millennium Development Goals set up by the United Nations in 2000 [2].
The main source of information on the causes of maternal deaths in developing countries, clinical records and verbal autopsies (VA) [4–13], have serious limitations due to the frequent discrepancies between the clinically presumed and the actual cause of death [14]. It has recently been claimed that there is an urgent need for studies focused on providing an accurate knowledge of the causes of maternal death in developing countries [15]. Autopsy studies may thus improve the knowledge of the causes of maternal death by increasing the accuracy of cause-of-death reports [16].
Postmortem examination is also an essential tool to improve overall clinical diagnostic performance, since clinicians can only diagnose diseases for which they have been looking. Thus, the analysis of false positive and false negative diagnoses is essential to evaluate and improve the diagnostic process [17,18]. Unfortunately, very little information exists on discrepancies between clinical and necropsy data in maternal mortality causes in developing countries [19]. As a consequence, very little data is available on the impact of medical errors on maternal mortality.
In order to evaluate clinical practice and diagnostic performance, which could help to reduce the problem of maternal deaths in developing countries, we conducted a retrospective study on the discrepancies between clinical and postmortem diagnosis in maternal deaths at the Maputo Central Hospital (MCH) in Mozambique.
Material and Methods
Study Area and Design
The characteristics of the study area have been previously described [20]. The study was conducted at the Maputo Central Hospital, a government-funded tertiary health facility that serves as the referral center for other hospitals in Southern Mozambique. All women fulfilling the standard definition of the World Health Organization (WHO) for a pregnancy-related death (i.e., death during pregnancy, delivery, or within 42 d after completion of a pregnancy, irrespective of the cause of death) between October 2002 and December 2004, and for whom the family had given verbal informed consent to perform the autopsy, were included in the study. The study protocol was approved by the National Mozambican Ethics Committee and the Hospital Clinic of Barcelona Ethics Review Committee.
A complete dissection with macroscopic evaluation of each organ was performed by a pathologist using a standardized macroscopic protocol. Samples of all grossly identified lesions and of all viscera were collected in each case for histological study. A blood sample (100 μl) was obtained from the inferior vena cava and stored on filter paper.
Clinical diagnoses were obtained from those listed by the clinician on the clinical process, after a complete revision of the case notes. The final diagnoses were established by two pathologists after reviewing the clinical process, the macroscopic protocols, and the histological slides. Major diagnoses were those involving the principal underlying cause of death [21]. Minor diagnoses were: antecedent disorders, related diagnoses, contributing causes, or other important disorders [21]. Clinical and necropsy diagnoses were grouped into different categories according to the International Classification of Disease, tenth revision (ICD-10). HIV/AIDS-related diseases included all cases with opportunistic infections or other conditions included in the CDC revised criteria [22]. In this category the diagnosis of the opportunistic infection causing death, and not only the diagnosis of HIV/AIDS, were considered in the evaluation of discrepancies. The diagnostic criteria for severe malaria have been described elsewhere [20].
Assessment of Discrepancies between Clinical and Necropsy Diagnoses
Diagnostic discrepancies were classified following the classification of Goldman et al. [23], modified by Battle et al. [21], and as nonclassifiable cases [24]. Major discrepancies were those involving major diagnoses and were classified as class I or class II discrepancies. In class I, the knowledge of the diagnosis before death, would have led to changes in the management that could have prolonged the survival or cured the patient (e.g., pyogenic meningitis treated as eclampsia), while in class II the survival would have not been modified (e.g., fulminant hepatitis treated as septicemia or terminal AIDS with multiple opportunistic infections treated as a bacterial infection). Minor discrepancies were those involving minor diagnoses and were classified as class III (diseases with symptoms that should have been treated or would have eventually affected the prognosis, e.g., mild aspirative pneumonia in a patient with eclampsia) and class IV (nondiagnosed diseases with possible epidemiological or genetic importance, e.g., schistosomal infections). Correctly diagnosed patients were classified as class V. Class VI were nonclassifiable cases (necropsy unsatisfactory or with no clear diagnosis).
A single class of major discrepancy (I and II) was assigned to each maternal death. The discrepancies were independently evaluated by two investigators (CR, JO). When minor disagreements were detected (class I versus class II, or class III versus class IV), a consensus meeting was held involving a third investigator (JAB). If a major disagreement was detected (e.g., class I versus class III), a senior pathologist uninvolved in the study was consulted.
Laboratory Methods
Tissue specimens were fixed in 10% buffered formalin for 2–15 d and embedded into paraffin wax using standard procedures. For each sampled tissue 4-μm sections were stained with haematoxylin and eosin (HE). Ancillary histochemical and immunohistochemical stains were performed to confirm or exclude specific lesions suspected on the HE stains.
As previously reported [20], HIV provirus was determined using the standard Amplicor HIV-1 kit (Roche) on blood collected onto filter paper. In 46 women, HIV status was assessed prior to death using the rapid test Determine HIV (Abbot Laboratories), and positive results were confirmed with Unigold HIV (Trinity Biotech). Malaria parasitaemia was assessed prior to death on thick and thin air-dried blood films, stained with Giemsa. In the autopsy material a histologic evaluation of malarial pigment was done with light microscopy under polarized light [25,26].
Definitions and Statistical Methods
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for the most frequent clinical diagnoses, considering the pathological diagnosis as the gold standard. Sensitivity was calculated as the proportion of true positives divided by the sum of true positives and false negatives. Specificity resulted from the proportion of true negative divided by the sum of true negatives and false positives. PPV was calculated as the number of true positive cases divided by the sum of true and false positives and NPV as the number of true negatives divided by the sum of true and false negatives. Accuracy was calculated as the sum of true positive and true negative diagnoses in each diagnostic category divided by all maternal deaths. False-negative diagnoses were defined as class I and II discrepancies for which the necropsy diagnosis was in the assessed diagnostic category but the clinical diagnosis was in another. False-positive diagnoses were cases with class I and II discrepancies, in which the clinical diagnosis was in the diagnostic category but not the necropsy diagnosis. Data were analyzed with the program STATA (Version 8.0, StataCorp). Differences between groups were analyzed with the χ2 statistical analysis.
Results
During the study period, there were 179 maternal deaths. In 139 women (77.6%) a complete autopsy with adequate histological sampling as well as clinical information was available.
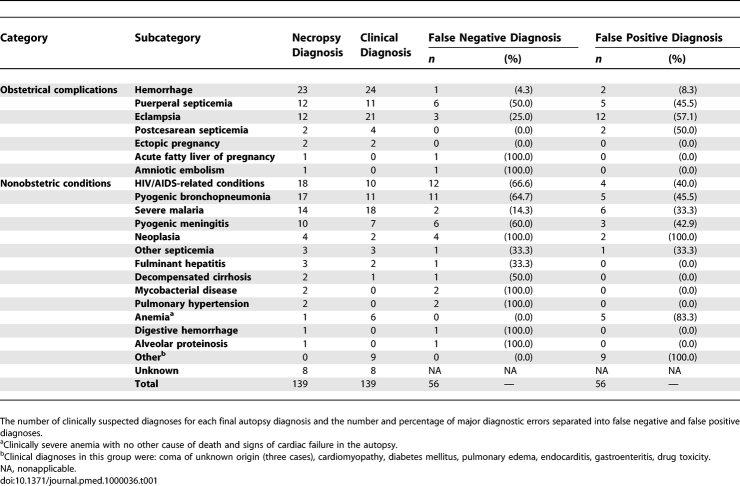
A major diagnostic discrepancy was detected in 56 (40.3%) maternal deaths; 47 (83.9%) of them were classified as class I, and nine (16.1%) as class II. A minor diagnostic discrepancy (class III or IV) was identified in 30 maternal deaths (21.6%). An additional minor discrepancy was identified in 24 maternal deaths with a major error. Thus, an overall number of 54 minor discrepancies (38.8%) were identified. In 45 maternal deaths (32.4%), there was complete agreement between the clinical and the autopsy diagnoses (class V). In eight maternal deaths (5.7%), no diagnosis was reached in the autopsy and the deaths were thus considered as not suitable for evaluation (class VI). Macroscopic examination alone detected 24/56 (42.9%) clinical major errors, while the remaining 32 errors were detected only in the histological study. Table 1 shows the causes of death detected in the autopsies, the number of clinically suspected diagnoses for each final autopsy diagnosis, and the number and percentage of major diagnostic errors.
Table 1.
Causes of Death Detected in the Autopsies
Both evaluations of diagnostic discrepancy were coincident in 117 cases. A discordant evaluation was observed in 19 cases (13.7%), thus requiring a consensus. Sixteen of these disagreements were qualified as minor (eight class I versus class II; eight class III versus class IV). Three cases were qualified as major disagreements and required consultation with a senior pathologist.
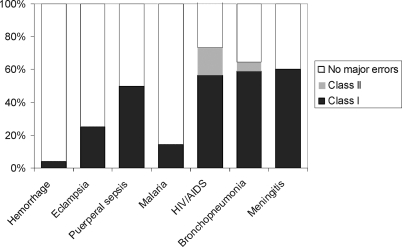
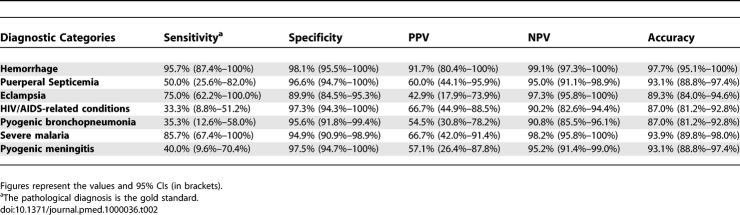
Seven diagnoses (obstetric hemorrhage, eclampsia, puerperal sepsis, HIV/AIDS-related conditions, pyogenic bronchopneumonia, malaria, and pyogenic meningitis), each responsible for ten or more maternal deaths, were further analyzed. Figure 1 shows the percentage of major errors (class I and II) in each of these diagnostic categories, and Table 2 the sensitivity, specificity, PPV, NPV, and accuracy of the clinical diagnosis for these seven categories.
Figure 1. Prevalence of the Major Diagnostic Errors by Pathology at Autopsy.
Table 2.
Sensitivity, Specificity, PPV, NPV, and Accuracy of the Clinical Diagnosis for All Frequent Diagnostic Categories
Disseminated tuberculosis was diagnosed in 12 women, of whom ten were HIV positive (and thus, included as HIV/AIDS-related condition). The diagnosis of tuberculosis had an overall sensitivity of 25.0% (95% confidence interval [CI]: 0.5%–49.5%), a specificity of 98.3% (95% CI: 96.0%–100%), a PPV of 60.0% (95% CI: 32.3%–87.7%), an NPV of 92.9% (95% CI: 88.3%–97.5%), and an accuracy of 91.6% (95% CI: 86.8%–96.4%).
Table 3 shows the reported clinical diagnoses in maternal deaths of obstetric hemorrhage, puerperal septicemia, eclampsia, pyogenic bronchopneumonia, severe malaria, and pyogenic meningitis with false negative diagnoses in the autopsy. The HIV positive status had been confirmed before death in eight out of 18 (44.4%) women with HIV/AIDS-related diseases, but only in six of them (33.3%) had the opportunistic infection directly causing the death been correctly diagnosed. Table 4 shows the false negative clinical diagnoses in maternal deaths with final diagnosis of HIV/AIDS-related diseases.
Table 3.
Clinical Diagnoses in Cases with False Negative Major Errors in Patients with Obstetric Hemorrhage, Puerperal Septicemia, Eclampsia, Pyogenic Bronchopneumonia, Severe Malaria, and Pyogenic Meningitis
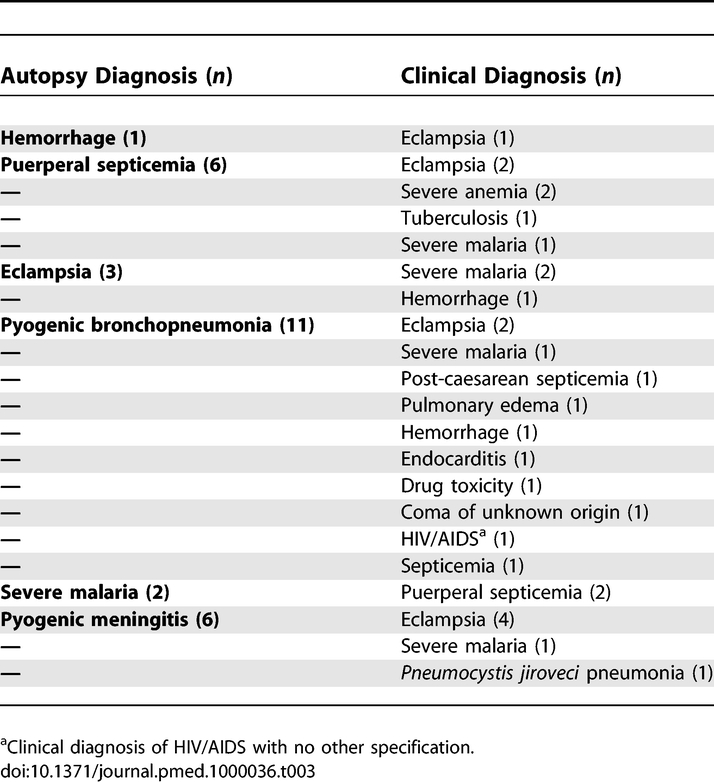
Table 4.
Clinical Diagnoses in Cases with False Negative Major Errors in Patients with HIV/AIDS Conditions as Cause of Death
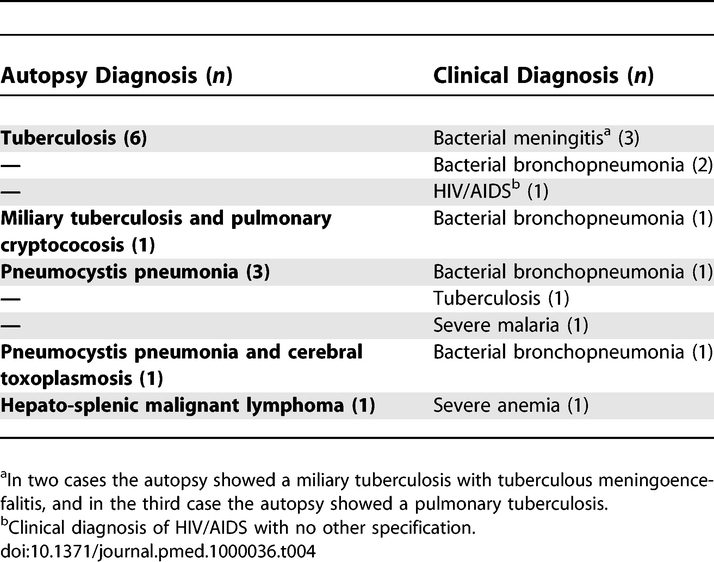
False positive diagnoses were particularly frequent for eclampsia with 12 maternal deaths (Table 1). The autopsy diagnoses in maternal deaths with false positive clinical diagnosis of eclampsia were: pyogenic meningitis (four maternal deaths), meningioma (two maternal deaths), puerperal sepsis (two maternal deaths), pyogenic bronchopneumonia (two maternal deaths), tuberculosis (one maternal death), and postpartum hemorrhage (one maternal death). No pathological lesions related to eclampsia were detected in any of these women.
Discussion
This is, to our knowledge, the first study focused on the evaluation of diagnostic discrepancies in maternal mortality in sub-Saharan Africa and even in developing countries, based on complete autopsies and including a histological study. This study has shown a high frequency of major clinico-pathological discrepancies (40.3%) in maternal deaths in a tertiary-referral hospital in sub-Saharan Africa. In most cases a change in clinical management could have significantly modified the prognosis. The proportion of discrepancies observed in this study is higher than that currently reported in studies based on nonselected hospital patients [17,27]. Remarkably, this study, unlike most previous studies, was based only on maternal deaths. These are extremely infrequent in developed countries [2,28], and tend to show lower discrepancy rates between clinical and autopsy diagnoses [16,21].
A sole description of the correlation between clinical and autopsy diagnosis was done retrospectively in Nigeria including data from 1989 to 1998 [19]. The prevalence of major diagnostic errors in that study was 10.4%, a figure much lower than that reported here. This fact may be explained mainly by methodological differences, since neither histological study nor HIV testing were used in the Nigerian study. Interestingly, in our study only 17.3% clinical major discrepancies were detected in the macroscopic study, whereas 57.1% of the discrepancies were found only in the histological study, as it has been shown in other studies [29,30].
The evaluation of sensitivity, specificity, PPVs, and NPVs may provide some insight into the possible factors behind major diagnostic errors. A high rate of false negative diagnoses was observed for infectious diseases, both obstetrical (puerperal septicemia) and nonobstetric. Other studies have shown that infectious diseases tend to show higher rates of diagnostic errors [21,31]. Interestingly, three frequent nonobstetric infectious categories (HIV/AIDS-related conditions, pyogenic bronchopneumonia, and pyogenic meningitis, as well as tuberculosis) had sensitivities below 40%. This number is very relevant since it indicates that significant reductions in maternal mortality could be reached by decreasing the false negative clinical diagnoses of some frequent infectious diseases, through improvements in their diagnosis. Although false negative diagnoses may occur either by omission, or lack of sensitivity of available diagnostic tests or inadequate synthesis in the diagnostic process [14,32,33], underestimation of prevalence plays an important role in this type of error [14,32,33]. It has been shown that increasing clinical awareness and the correct estimation of the prevalence of infectious diseases may lead to a reduction in the number of diagnostic errors [14,34]. Limited access to necessary diagnostic tests and particularly to microbiological cultures in developing countries, especially in sub-Saharan Africa, represents a major handicap for the diagnosis of many infectious diseases and significantly slows down the provision of drug therapy [35,36].
Eclampsia was the main source of false positive diagnoses (n = 12, 57.1%). It cannot be completely excluded that preeclampsia–eclampsia was a true diagnosis in some of these patients, and lack of an appropriate management of the condition a contributor to maternal death. However, a different cause of death was found in all these women. Moreover, no pathological lesions related to eclampsia were detected in any of them. False-positive diagnoses may occur due to premature closure of the diagnostic process and low specificity of diagnostic tests, but overestimation of prevalence also plays a major role in this type of error [14,32,33]. Generally, eclampsia is more likely to be diagnosed too frequently rather than overlooked. On the other hand, epilepsy, infections or space-occupying lesions of the central nervous system, cerebrovascular accidents, hypertensive diseases, metabolic disorders, and thrombotic thrombocytopenic purpura may all simulate eclampsia, but they are less frequent [37–39]. On the basis of clinical reports and VAs [4–12], eclampsia is widely accepted as one of the major causes of maternal death in sub-Saharan Africa [4,8,12]. The current study suggests that eclampsia remains a significant cause of maternal death in that setting, but its prevalence is probably overestimated.
The main limitation of our study is that it was conducted in a large university hospital located in the capital of Mozambique, which could limit the extrapolation of these findings to other hospitals and health facilities. It has been shown that the frequency of discrepancies between clinical and autopsy diagnoses is lower in larger hospitals [21], and this might suggest that the number of diagnostic errors in other health facilities would be underestimated. On the other hand, complicated or high risk pregnancies are often referred to these larger hospitals resulting in a high number of cases of increased diagnostic difficulty.
Four necessary conditions have been proposed for necropsy to be a valid monitor of clinical diagnosis performance: a high necropsy rate, specified and stable conditions of autopsy procedure (extent of organ assessment and sampling, availability of clinical information), calculation of sensitivity and specificity rather than accuracy, and estimate of the errors in postmortem diagnosis [40]. Our study met three of these conditions, but did not assess the error of autopsy diagnosis itself. The classification into the discrepancy classes was not clear-cut in all cases. A disagreement rate of 13.7% in class assignment was detected in our study, a figure that is similar to the disagreement rate reported in other studies [14]. This result could be seen as a limitation of the discrepancy classification.
The significant reduction of diagnostic errors reported in developed countries during the second half of the 20th century has been mainly attributed to the improvement of clinical skills and to the impact of new diagnostic procedures [14]. Necropsy has had in this regard the dual role of a method to detect diagnostic errors and a source of knowledge to be applied to future cases. This dual role has not only influenced learning but has also added relevant data on local epidemiology of diseases. The scientific, public health, and educational benefits of the autopsy remain difficult to evaluate, but are generally acknowledged [41]. The almost complete absence of studies based on necropsy data and focused on diagnostic error is a severe handicap for medical practice in sub-Saharan Africa. Thus, autopsy could help to reduce maternal mortality by providing information critical to improve diagnostic accuracy and therefore clinical management. In this regard, this study suggests that correct awareness of the prevalence of infectious diseases and the implementation of a few inexpensive diagnostic tools and a thorough evaluation of cases clinically thought to be eclampsia may lead to a significant reduction in the number of diagnostic errors [42]. The implementation of morbidity and mortality conferences may also have an important effect on maternal mortality in sub-Saharan Africa [43]. Additional studies are needed to assess whether analysis of how and when diagnostic errors occur might improve epidemiological data in maternal mortality cases and clinical diagnostic performance.
In addition to their intrinsic clinical relevance, missed diagnoses detected at autopsy may have important implications for research. The main source of information on the causes of maternal deaths in developing countries is VA, an established method of ascertaining the likely causes of death by interpretation of interviews with relatives or carers of the deceased. VAs are not based on clinical or laboratory measures and have been questioned because they are subject to a relatively high degree of misclassification error [44,45]. Our data indicate that not only VAs but also medical records may contain substantial inaccuracies regarding the main diagnoses causing or contributing to death. It is noteworthy that studies based on these methodologies tend to underreport infectious diseases [2,46]. Since diagnoses and causes of death are determined without autopsy in the vast majority of cases, especially in sub-Saharan-Africa, vital statistics, clinical registries, and even randomized trials, may capture incorrect causes of death. These inaccuracies have important policy implications, as major funding and policy decisions are derived in part from vital statistics and other registries of disease burden.
Acknowledgments
We thank all the families of the mothers who were included in the study. We thank Elias Walle and the staff of the Maternity Ward and Pathology Departments of the Maputo Central Hospital, whose support made this study possible. We also thank Josep M. Miro for his help in the evaluation of HIV-related maternal deaths. The authors are grateful to the Centro de Investigaçao de Manhiça for their logistical assistance.
Abbreviations
- CI
confidence interval
- NPV
negative predictive value
- PPV
positive predictive value
- VA
verbal autopsy
Footnotes
Author contributions. JO, MRI, JAB, JB, and CM designed the experiments/the study. MRI, CR, NO, and CM enrolled patients. MRI, CC CR, NO, and FM collected data or did experiments for the study. JO, MRI, CR, NO, FM, JAB, and JB analyzed the data. JO and CM wrote the first draft of the paper. MRI, CC, CR, NO, JAB, JB, PLA, and CM contributed to writing the paper.
Funding: This work was partly supported by grants from the Fondo de Investigaciones Sanitarias (PI060207) and The European Commission Research Directorates General, Fifth Framework (Contract PREMA-EU-ICA 4 CT-2001-1110012). The study was supported by the Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III through a career development fellowship to CR (number CM03/00125). The Centro de Investigaçao em Saude de Manhiça receives major core funding from the Spanish Agency for International Cooperation. The funders had no role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
References
- Rutstein DD, Berenberg W, Chalmers TC, Child CG, III, Fishman AP, et al. Measuring the quality of medical care. A clinical method. N Engl J Med. 1976;294:582–588. doi: 10.1056/NEJM197603112941104. [DOI] [PubMed] [Google Scholar]
- WHO. Maternal mortality in 2005: estimates developed by WHO, UNICEF UNFPA and the world bank. Geneva: WHO; 2004. [Google Scholar]
- AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11. doi: 10.1093/bmb/ldg015. [DOI] [PubMed] [Google Scholar]
- Bartlett LA, Mawji S, Whitehead S, Crouse C, Dalil S, et al. Where giving birth is a forecast of death: maternal mortality in four districts of Afghanistan, 1999–2002. Lancet. 2005;365:864–870. doi: 10.1016/S0140-6736(05)71044-8. [DOI] [PubMed] [Google Scholar]
- Geelhoed DW, Visser LE, Asare K, Schagen van Leeuwen JH, van RJ. Trends in maternal mortality: a 13-year hospital-based study in rural Ghana. Eur J Obstet Gynecol Reprod Biol. 2003;107:135–139. doi: 10.1016/s0301-2115(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Granja AC, Machungo F, Gomes A, Bergstrom S, Brabin B. Malaria-related maternal mortality in urban Mozambique. Ann Trop Med Parasitol. 1998;92:257–263. doi: 10.1080/00034989859816. [DOI] [PubMed] [Google Scholar]
- Hoestermann CF, Ogbaselassie G, Wacker J, Bastert G. Maternal mortality in the main referral hospital in The Gambia, west Africa. Trop Med Int Health. 1996;1:710–717. doi: 10.1111/j.1365-3156.1996.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Kodio B, de Bernis L, Ba M, Ronsmans C, Pison G, et al. Levels and causes of maternal mortality in Senegal. Trop Med Int Health. 2002;7:499–505. doi: 10.1046/j.1365-3156.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Oladapo OT, Sule-Odu AO, Olatunji AO, Daniel OJ. “Near-miss” obstetric events and maternal deaths in Sagamu, Nigeria: a retrospective study. Reprod Health. 2005;2:9. doi: 10.1186/1742-4755-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sule-Odu AO. Maternal deaths in Sagamu, Nigeria. Int J Gynaecol Obstet. 2000;69:47–49. doi: 10.1016/s0020-7292(99)00199-x. [DOI] [PubMed] [Google Scholar]
- Thonneau PF, Matsudai T, Alihonou E, De SJ, Faye O, et al. Distribution of causes of maternal mortality during delivery and post-partum: results of an African multicentre hospital-based study. Eur J Obstet Gynecol Reprod Biol. 2004;114:150–154. doi: 10.1016/j.ejogrb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Toure B, Thonneau P, Cantrelle P, Barry TM, Ngo-Khac T, et al. Level and causes of maternal mortality in Guinea (West Africa) Int J Gynaecol Obstet. 1992;37:89–95. doi: 10.1016/0020-7292(92)90487-4. [DOI] [PubMed] [Google Scholar]
- Bouvier-Colle MH, Ouedraogo C, Dumont A, Vangeenderhuysen C, Salanave B, et al. Maternal mortality in West Africa. Rates, causes and substandard care from a prospective survey. Acta Obstet Gynecol Scand. 2001;80:113–119. [PubMed] [Google Scholar]
- Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: a necropsy study. Lancet. 2000;355:2027–2031. doi: 10.1016/s0140-6736(00)02349-7. [DOI] [PubMed] [Google Scholar]
- Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–1200. doi: 10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- Ornelas-Aguirre JM, Vazquez-Camacho G, Gonzalez-Lopez L, Garcia-Gonzalez A, Gamez-Nava JI. Concordance between premortem and postmortem diagnosis in the autopsy: results of a 10-year study in a tertiary care center. Ann Diagn Pathol. 2003;7:223–230. doi: 10.1016/s1092-9134(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Bombi JA, Ramirez J, Sole M, Grau JM, Chabas E, et al. Clinical and autopsy correlation evaluated in a university hospital in Spain (1991–2000) Pathol Res Pract. 2003;199:9–14. doi: 10.1078/0344-0338-00346. [DOI] [PubMed] [Google Scholar]
- Scottolini AG, Weinstein SR. The autopsy in clinical quality control. JAMA. 1983;250:1192–1194. [PubMed] [Google Scholar]
- Daramola AO, Elesha SO, Banjo AA. Medical audit of maternal deaths in the Lagos University Teaching Hospital, Nigeria. East Afr Med J. 2005;82:285–289. doi: 10.4314/eamj.v82i6.9298. [DOI] [PubMed] [Google Scholar]
- Menendez C, Romagosa C, Ismail MR, Carrilho C, Saute F, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. doi: 10.1371/journal.pmed.0050044. doi: 10.1371/journal.pmed.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle RM, Pathak D, Humble CG, Key CR, Vanatta PR, et al. Factors influencing discrepancies between premortem and postmortem diagnoses. JAMA. 1987;258:339–344. [PubMed] [Google Scholar]
- 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- Goldman L, Sayson R, Robbins S, Cohn LH, Bettmann M, et al. The value of the autopsy in three medical eras. N Engl J Med. 1983;308:1000–1005. doi: 10.1056/NEJM198304283081704. [DOI] [PubMed] [Google Scholar]
- Bellwald M. [Autopsies with unsatisfactory results] Schweiz Med Wochenschr. 1982;112:75–82. [PubMed] [Google Scholar]
- Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- Romagosa C, Menendez C, Ismail MR, Quinto L, Ferrer B, et al. Polarisation microscopy increases the sensitivity of hemozoin and Plasmodium detection in the histological assessment of placental malaria. Acta Trop. 2004;90:277–284. doi: 10.1016/j.actatropica.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47:551–559. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstet Gynecol. 2003;101:289–296. doi: 10.1016/s0029-7844(02)02587-5. [DOI] [PubMed] [Google Scholar]
- Bernardi FD, Saldiva PH, Mauad T. Histological examination has a major impact on macroscopic necropsy diagnoses. J Clin Pathol. 2005;58:1261–1264. doi: 10.1136/jcp.2005.027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitoun AM, Fernandez C. The value of histological examination in the audit of hospital autopsies: a quantitative approach. Pathology. 1998;30:100–104. doi: 10.1080/00313029800169036. [DOI] [PubMed] [Google Scholar]
- Sarode VR, Datta BN, Banerjee AK, Banerjee CK, Joshi K, et al. Autopsy findings and clinical diagnoses: a review of 1,000 cases. Hum Pathol. 1993;24:194–198. doi: 10.1016/0046-8177(93)90300-6. [DOI] [PubMed] [Google Scholar]
- Kassirer JP, Kopelman RI. Cognitive errors in diagnosis: instantiation, classification, and consequences. Am J Med. 1989;86:433–441. doi: 10.1016/0002-9343(89)90342-2. [DOI] [PubMed] [Google Scholar]
- Voytovich AE, Rippey RM, Suffredini A. Premature conclusions in diagnostic reasoning. J Med Educ. 1985;60:302–307. doi: 10.1097/00001888-198504000-00004. [DOI] [PubMed] [Google Scholar]
- Zimmermann-Hosli MB, Stahel RA, Vogt P, Oelz O. Reduction of systemic fungal infections in patients with hematological malignancies, neutropenia, and prolonged fever by early amphotericin B therapy. Klin Wochenschr. 1988;66:1010–1014. doi: 10.1007/BF01733443. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Access to diagnostics in support of HIV/AIDS and tuberculosis treatment in developing countries. AIDS. 2007;21(Suppl 4):S81–S87. doi: 10.1097/01.aids.0000279710.47298.5c. [DOI] [PubMed] [Google Scholar]
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis 196 Suppl. 2007;1:S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap L, et al. Hypertensive disorders in pregnancy. In: Cunningham FG, Williams JW, editors. Williams obstetrics. 22nd edition. New York: McGraw Hill Professional; 2005. [Google Scholar]
- Norwitz ER, Hsu CD, Repke JT. Acute complications of preeclampsia. Clin Obstet Gynecol. 2002;45:308–329. doi: 10.1097/00003081-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Brown RH. Epilepsy and other seizure disorders. In: Ropper AH, Brown RH, editors. Adams and Victor's principles of neurology. New York: McGraw-Hill; 2005. pp. 271–301. [Google Scholar]
- Saracci R. Is necropsy a valid monitor of clinical diagnosis performance. BMJ. 1991;303:898–900. doi: 10.1136/bmj.303.6807.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojania KG, Burton EC. The vanishing nonforensic autopsy. N Engl J Med. 2008;358:873–875. doi: 10.1056/NEJMp0707996. [DOI] [PubMed] [Google Scholar]
- Tsu VD. New and underused technologies to reduce maternal mortality. Lancet. 2004;363:75–76. doi: 10.1016/S0140-6736(03)15180-X. [DOI] [PubMed] [Google Scholar]
- Herridge MS. Autopsy in critical illness: is it obsolete. Crit Care. 2003;7:407–408. doi: 10.1186/cc2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker M. The effect of misclassification error on reported cause-specific mortality fractions from verbal autopsy. Int J Epidemiol. 1997;26:1090–1096. doi: 10.1093/ije/26.5.1090. [DOI] [PubMed] [Google Scholar]
- Chandramohan D, Setel P, Quigley M. Effect of misclassification of causes of death in verbal autopsy: can it be adjusted. Int J Epidemiol. 2001;30:509–514. doi: 10.1093/ije/30.3.509. [DOI] [PubMed] [Google Scholar]
- Fottrell E, Byass P, Ouedraogo TW, Tamini C, Gbangou A, et al. Revealing the burden of maternal mortality: a probabilistic model for determining pregnancy-related causes of death from verbal autopsies. Popul Health Metr. 2007;5:1. doi: 10.1186/1478-7954-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]