Abstract
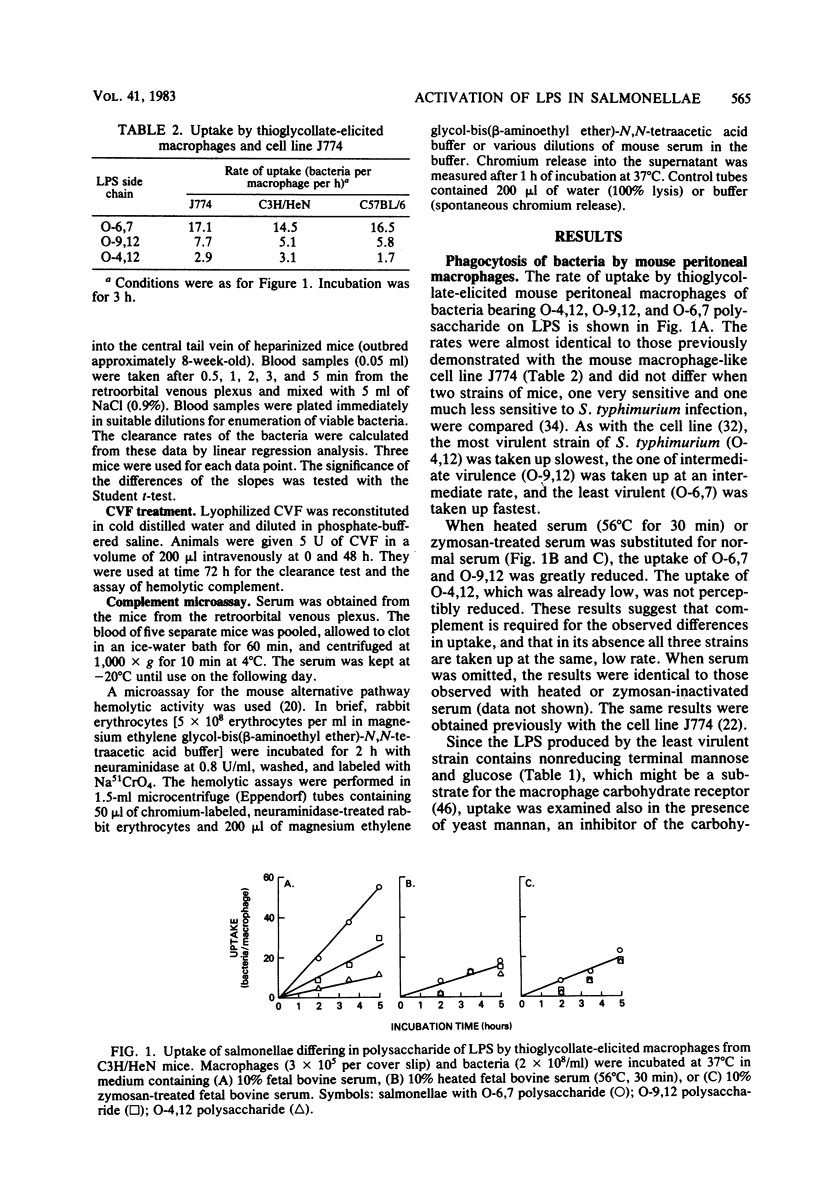
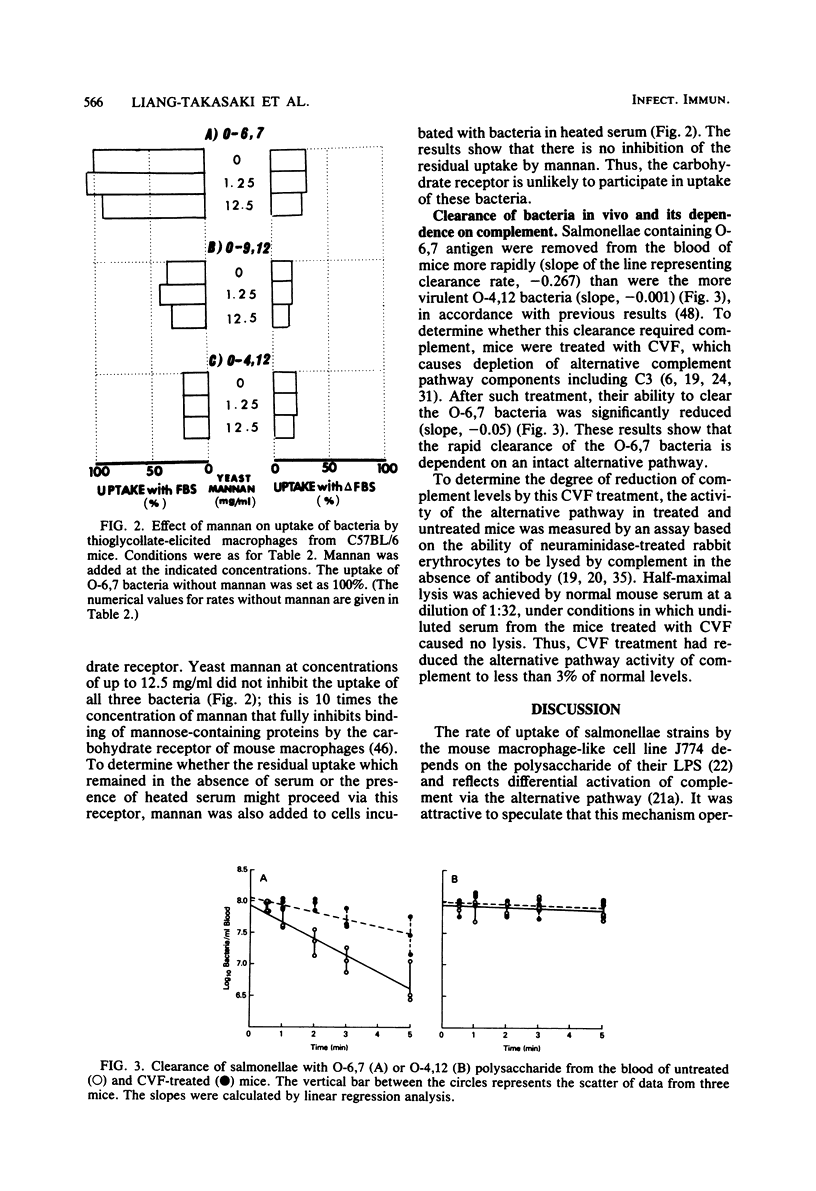
Salmonellae with differences only in the O-antigenic polysaccharide of their lipopolysaccharide were previously shown to differentially activate complement via the alternative pathway, causing them to be ingested at different rates by the mouse macrophage-like cell line J774. We now show that this mechanism could explain the different virulence of these strains in vivo. Mouse peritoneal macrophages (thioglycolate induced) ingest these salmonellae at rates that are inversely proportional to the known virulence of the organisms and virtually identical to the rates observed with J774. As with J774, complement is required for this differential uptake, since serum was required and heating (56 degrees C for 30 min) or zymosan treatment of the serum destroyed activity. The known receptor for nonreducing terminal mannose-, fucose-, N-acetylglucosamine, and glucose-containing glyco-proteins did not participate, since uptake was not inhibited by high concentrations of mannan. When clearance of bacteria from the bloodstream of mice was measured, the least virulent organism was cleared very much faster than the most virulent organism, in confirmation of earlier data. When complement in the mice was destroyed by pretreatment with cobra venom factor, the clearance of the least virulent strain was greatly reduced, whereas the very slow clearance of the most virulent strain was unaffected. These data strongly support the hypothesis that when bacteria have polysaccharide in lipopolysaccharide that activates complement efficiently, the bacteria will be phagocytosed, whereas if the polysaccharide activates complement poorly, the bacteria escape ingestion and may cause disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., HOWARD J. G., HALPERN B. N., STIFFEL C., MOUTON D. The kinetics of blood clearance o isotopically labelled Salmonella entertidis by the reticulo-endothelial system in mice. Immunology. 1960 Jan;3:74–89. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., STIFFEL C., LEMINOR L., MOUTON D., BOUTHILLIER Y. ETUDE QUANTITATIVE DE L'EFFET OPSONISANT DES IMMUNS'ERUMS SUR LA PHAGOCYTOSE DES SALMONELLA PAR LES CELLULES DU SYST'EME R'ETICULO-ENDOTH'ELIAL IN VIVO. Ann Inst Pasteur (Paris) 1963 Oct;105:635–666. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of complement in the localization of pneumococci in the splanchnic reticuloendothelial system during experimental bacteremia. J Immunol. 1981 Jun;126(6):2230–2235. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller N. A., Staub A. M. Immunochemical studies on Salmonella. 13. Chemical changes appearing on the specific polysaccharide of S. cholerae suis (6-2,7) after its conversion by phage 14(6,7). Eur J Biochem. 1968 Apr;4(3):286–300. doi: 10.1111/j.1432-1033.1968.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Gigli I., Nelson R. A., Jr Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp Cell Res. 1968 Jul;51(1):45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Bianco C., Silverstein S. C. Characterization of the macrophage receptro for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J Exp Med. 1975 Jun 1;141(6):1269–1277. doi: 10.1084/jem.141.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. The in vivo division and death rates of Salmonella typhimurium in the spleens of naturally resistant and susceptible mice measured by the superinfecting phage technique of Meynell. Immunology. 1980 Dec;41(4):973–979. [PMC free article] [PubMed] [Google Scholar]
- Hosea S. W., Brown E. J., Frank M. M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980 Dec;142(6):903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hawiger A., Gelfand J. A. The effect of cobra venom factor on alternative pathway hemolytic activity in mice. Immunol Commun. 1980;9(3):277–281. doi: 10.3109/08820138009066000. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Shahon R., Gelfand J. A. A sensitive microassay for the murine alternative complement pathway. J Immunol Methods. 1979;31(3-4):283–290. doi: 10.1016/0022-1759(79)90141-8. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Karthigasu K. Salmonella abony-Salmonella typhimurium recombinant nonvirulent for the mouse. J Bacteriol. 1969 Mar;97(3):1343–1351. doi: 10.1128/jb.97.3.1343-1351.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Mäkelä P. H., Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J Immunol. 1982 Mar;128(3):1229–1235. [PubMed] [Google Scholar]
- Lindberg A. A. Bacterial virulence factors--with particular reference to Salmonella bacteria. Scand J Infect Dis Suppl. 1980;Suppl 24:86–92. [PubMed] [Google Scholar]
- Maillard J. L., Zarco R. M. Décomplémentation par un facteur extrait du venin de cobra. Effet sur plusieurs réactions immunes du cobaye et du rat. Ann Inst Pasteur (Paris) 1968 Jun;114(6):756–774. [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Hellerqvist C. G., Valtonen V. V., Mäkelä P. H. The smooth lipopolysaccharide character of 1,4,(5),12 and 1,9,12 transductants formed as hybrids between groups B and D of salmonella. Eur J Biochem. 1971 Oct 26;22(4):500–505. doi: 10.1111/j.1432-1033.1971.tb01569.x. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., WURZ L., TODD E. W. The properdin system and immunity. III. The zymosan assay of properdin. J Exp Med. 1956 Jan 1;103(1):1–13. doi: 10.1084/jem.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Studies in vivo of cobra factor and murine C3. Immunology. 1975 Feb;28(2):369–377. [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- ROANTREE R. J., PAPPAS N. C. The survival of strains of enteric bacilli in the blood stream as related to their sensitivity to the bactericidal effect of serum. J Clin Invest. 1960 Jan;39:82–88. doi: 10.1172/JCI104031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975 Oct 2;257(5525):393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rother U. A., Rother K. O. Interaction of complement components with a serum-resistant strain of Salmonella typhimurium. Infect Immun. 1975 May;11(5):944–948. doi: 10.1128/iai.11.5.944-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rowley D. Sensitization of complement resistant bacterial strains. Nature. 1969 Mar 29;221(5187):1259–1261. doi: 10.1038/2211259a0. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Weinblatt A. C., O'Brien A. D. Genetic control of resistance to infection in mice. Crit Rev Immunol. 1982 Jun;3(4):263–330. [PubMed] [Google Scholar]
- Rother K., Rother U. Studies on complement defective rabbits. IV. Blood clearance of intravenously injected S. typhi by the reticuloendothelial system. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1055–1059. doi: 10.3181/00379727-119-30375. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Tucker J. F. The virulence of trimethoprim-resistant thymine-requiring strains of Salmonella. J Hyg (Lond) 1976 Feb;76(1):97–108. doi: 10.1017/s0022172400054991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V., Larinkari U. M., Plosila M., Valtonen V. V., Mäkelä P. H. Effect of enterobacterial common antigen on mouse virulence of Salmonella typhimurium. Infect Immun. 1976 Jun;13(6):1601–1605. doi: 10.1128/iai.13.6.1601-1605.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V., Plosila M., Valtonen V. V., Mäkelä P. H. Effect of the quality of the lipopolysaccharide on mouse virulence of Salmonella enteritidis. Infect Immun. 1975 Oct;12(4):828–832. doi: 10.1128/iai.12.4.828-832.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V. Role of phagocytosis in mouse virulence of Salmonella typhimurium recombinants with O antigen 6,7 or 4,12. Infect Immun. 1977 Dec;18(3):574–582. doi: 10.1128/iai.18.3.574-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Valtonen V. V., Mäkelä P. H. The effect of lipopolysaccharide modification--antigenic factors 1,5,12 2 and 27--on the virulence of salmonella strains for mice. J Gen Microbiol. 1971 Nov;69(1):107–115. doi: 10.1099/00221287-69-1-107. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



