Abstract
Neurotensin (NT) is a versatile neuropeptide involved in analgesia, hypothermia, and schizophrenia. Although NT is released from and acts upon brain regions involved in social behaviors, it has not been linked to a social behavior. We previously selected mice for high maternal aggression (maternal defense), an important social behavior that protects offspring, and found significantly lower NT expression in the CNS of highly protective females. Our current study directly tested NT’s role in maternal defense. Intracerebroventricular (icv) injections of NT significantly impaired defense in terms of time aggressive and number of attacks at all doses tested (0.05, 0.1, 1.0, and 3.0 μg). Other maternal behaviors, including pup retrieval, were unaltered following NT injections (0.05 μg) relative to vehicle, suggesting specificity of NT action on defense. Further, icv injections of the NT receptor 1 (NT1) antagonist, SR 48692 (30 μg), significantly elevated maternal aggression in terms of time aggressive and attack number. To understand where NT may regulate aggression, we examined Fos following injection of either 0.1 μg NT or vehicle. 13 of 26 brain regions examined exhibited significant Fos increases with NT, including regions expressing NT1 and previously implicated in maternal aggression, such as lateral septum, bed nucleus of stria terminalis, paraventricular nucleus, and central amygdala. Together, our results indicate that NT inversely regulates maternal aggression and provide the first direct evidence that lowering of NT signaling can be a mechanism for maternal aggression. To our knowledge, this is the first study to directly link NT to a social behavior.
Keywords: fight, flight, maternal aggression, maternal defense, lactation, mice
Neurotensin (NT) is a versatile neuropeptide that plays a role in analgesia (Dubuc et al., 1999, Sarret et al., 2005), hypothermia (Nemeroff et al., 1977, Martin et al., 1980, Remaury et al., 2002), and schizophrenia (Nemeroff, 1986, Kinkead and Nemeroff, 2006). NT acts most commonly via either NT receptor 1 (NT1) or receptor 2 (NT2) (Tanaka et al., 1990, Richard et al., 2001, Sarret et al., 2002), but it can also act via NT receptor 3, a sortilin receptor that internalizes the ligand (Mazella, 2001). NT and its receptors are highly conserved among mammals (Dobner, 2005). Although NT is expressed in and acts upon a number of areas critical for social behavior, including nucleus accumbens, lateral septum (LS), bed nucleus of stria terminalis (BNST), preoptic area, amygdala, and periaqueductal gray (Boudin et al., 1996, Binder et al., 2001a, Sarret et al., 2003), it has received almost no research attention regarding its role in social behaviors. NT has strong interactions with dopamine (Binder et al., 2001a, Dobner, 2005), which itself is an important contributor to social and reward related behaviors (Blackburn et al., 1992, Numan and Insel, 2003), again suggesting a link between NT and social behaviors.
We recently selected for high levels of maternal aggression (maternal defense) in mice (Gammie et al., 2006) and then examined gene expression changes in the CNS of highly protective mice (Gammie et al., 2007). Unexpectedly, gene array and Real-time PCR results indicated NT expression was significantly lower in selected mice. NT and maternal defense had not previously been linked, but these results suggested that NT may be lowered to allow the emergence of high maternal aggression. It has been proposed that a typical default behavioral response to a potentially threatening stimulus is freezing behavior, followed by flight, followed by fight, and then followed by fright (going limp/giving up) (Bracha et al., 2004). We recently described a model for maternal aggression whereby this default pathway is altered such that a female quickly transitions from freeze to fight with flight being superseded (Gammie et al., 2008). Interestingly, antagonizing NT1 decreases the flight response in mice exposed to a hand-held rat (Griebel et al., 2001), which suggests that decreasing NT activity in either selected mice or during lactation could support the fight response by reducing the likelihood of flight in response to an intruder. NT enhances pre-pulse inhibition (PPI) (Caceda et al., 2006) and PPI decreases during lactation (Byrnes et al., 2007), so one possibility is that NT activity is decreased during lactation and this supports both decreased PPI and increased maternal aggression.
In this study, we directly tested the hypothesis that NT inversely regulates maternal aggression. We examined whether centrally injected NT impairs aggression and whether antagonizing NT1 promotes defense. As part of this study, we also examined Fos activity in association with NT injections to gain insights into where NT was acting to modulate aggression. We also monitored other maternal behaviors in association with injections to determine whether effects were specific to maternal aggression. To our knowledge, this is among the first studies to examine a role for NT in a social behavior.
Experimental Procedures
Mice
High maternal aggression mice (originally derived from outbred hsd:ICR mice) that we selectively bred for high maternal aggression (Gammie et al., 2006) were used. These mice exhibit consistently high levels of aggression and thus provided a reliable baseline of aggression for testing. All females were tested with their second litter. Mice were bred with breeder males and following impregnation (~1 week), each female was housed individually for the remainder of the study. Female mice were given ad lib access to Breeder Chow (Harlan) and tap water. Two weeks after pairing with the male, female mice were given precut nesting material. Polypropylene cages were cleaned weekly prior to parturition, but afterwards cages were not changed for the duration of the experiment. Litters were culled to 11 pups on postpartum Day 1 (parturition is postpartum Day 0) and females with less than 9 pups were omitted; previous work indicates that females show maximum aggression with 9-11 pups (Gammie et al., 2003). Sexually naïve male mice of the outbred hsd:ICR strain (Harlan) were used as intruders during aggression tests. Intruder males were group housed (4 mice/cage) and never used more than once per day and not for more than 3 total tests each. Over the course of testing, each female was exposed to different intruder males and due to the design of the study (see below), males with varying experience were equally distributed over the various doses of respective treatments. All mice were housed on a 14:10 light/dark cycle with lights on at 0600 CST. All testing was performed between 1000 and 1500 h. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the University of Wisconsin.
Cannulae surgeries
On postpartum Day 1 each lactating female was fitted with a cannula to the lateral ventricle. Under isoflurane anesthesia, mice were placed in a stereotaxic apparatus (David Kopf Instruments). A small hole was drilled -0.6 mm (posterior) to and 1.6 mm (lateral) to Bregma. In the hole, a 26 gauge stainless-steel indwelling cannula (Plastics One) was implanted to -2.5 mm below the skull surface into the lateral ventricle as previously described (Gammie et al., 2004, D’Anna et al., 2005, D’Anna and Gammie, 2006). Each cannula was secured to the skull using dental cement (Plastics One). A dummy cannula was inserted to maintain patency until injections were made. Injections were made using a 33 gauge stainless-steel injector, which extended slightly beyond the guide, attached to PE-50 tubing (Becton Dickenson) and fitted to a digital single infusion pump (World Precision Instruments, Inc).
Intracerebroventricular (icv) injections of NT and NT1 receptor antagonist, SR 48692
Beginning 3 days after surgery (postpartum Day 4), single injections were delivered each day for up to 3 consecutive days (postpartum Days 4-6) to mice under light isoflurane anesthesia. Anesthesia was only applied for a few minutes during injections. Isoflurane was used because acute stress suppresses maternal aggression (Gammie and Stevenson, 2006) and recent work indicates mice continue to display high levels of maternal behavior, including aggression, following brief anesthetization and injections (Gammie et al., 2004, D’Anna et al., 2005, D’Anna and Gammie, 2006). All mice were awake and moving in their home cage within 1-2 minutes following removal of anesthesia. All injections were made using a 1 μl volume over a 60 sec time span and verified by following movement of an air bubble in the tubing; the injector remained in place for 60 sec following each injection. Thirty min after injection, all females were tested for maternal aggression for 5 min and pup retrieval for 2 min. For the NT studies, but not the antagonist studies, other maternal behaviors were monitored for 30 min as described below. After the completion of the testing series and just prior to fixation (described below), a 1 μl volume of 0.01 % Chicago sky blue (Sigma) in saline was injected to verify cannulae placement and only correctly placed injections were used. The following doses were injected: NT (Phoenix Pharmaceuticals); 0.05 μg, 0.1 μg, 1.0 μg, and 3.0 μg dissolved in saline. Saline (pH = 7.0) was used as the vehicle solution and control for each mouse. Although pH of vehicle was slightly lower than found for cerebrospinal fluid (pH = 7.2), it not thought that vehicle resulted in any non-specific neuronal firing because of the small volume injected (1 μl) and the buffering of cerebrospinal fluid. If some non-specific changes in neuronal activity occurred, though, this would be consistent across all treatments.
The four doses of NT were tested across two separate groups of mice (vehicle and 2 doses per group) and each mouse underwent a maximum of three tests. For low-dose group A (0.05 NT, 0.1 μg NT, and vehicle), 20 mice were used. For high dose group B (1.0 μg NT, 3.0 μg NT, and vehicle), 11 mice were used. Each mouse was tested as part of a group and received each treatment within that group over 3 consecutive days (e.g. 0.05 μg NT, 0.1 μg NT, and vehicle) employing a within animal repeated measures experimental design. The orders of all injections of NT (or vehicle) were counterbalanced such that a given treatment was presented equally across the three test days. Doses were chosen based on previous i.c.v. studies of the behavioral effects of NT in mice and rats (Levine et al., 1983, Meisenberg and Simmons, 1985, Castel et al., 1989, Rompre, 1995, Lambert et al., 1996).
For icv injections of the NT1 specific receptor antagonist, SR 48692 (a kind gift of Sanofi Recherche), a repeated measures design was also used (N = 12). Doses of SR 48692 used were: 10 and 30 μg (dissolved in saline). Saline was used as a control and each animal received each of the two doses and saline on different test days as for injection of NT above. Order of injection was counterbalanced. Doses were based on previously published studies (Tirado-Santiago et al., 2006). For the antagonist studies, pup retrieval was examined for 2 min immediately following the aggression test, but no other maternal behaviors were recorded.
Because the half-life of NT in the CNS is less than 15 min (Checler et al., 1986), it is expected that almost no injected NT would remain when testing occurred on the following day. Although SR48692 can be effective for hours (Gully et al., 1993), it crosses the blood brain barrier and small icv injections (1 μl) would expected to be diluted throughout the body within hours. With dilution and degradation, it is expected that injected SR48692 would have almost no effect when testing occurred on the following day. However, we cannot exclude the possibility that there was small residual effect of previous injections on testing the following day.
Maternal aggression and behavior testing
After each injection, females were promptly returned to their home cages and after 30 min elapsed, females were moved into the testing room, where they were tested in their home cages. Pups were removed from the cage just prior to testing as this does not diminish aggression in mice (Svare et al., 1981). Females were not tested with pups in the home cage to reduce any interaction between intruder and pups (including pup killing). It is possible that the acute removal of pups had some overall effect on aggression, but previous work suggests aggression levels stay high even one hour after pups have been removed; longer separation times reduce aggression (Gandelman, 1972). Also, in rats pup removal for 2.5 h does not alter baseline corticosterone or adrenocorticotropic hormone (ACTH) levels, but does dampen ACTH increases in response to a male intruder (Deschamps et al., 2003). We do not think that removal of pups just before testing had a strong stress effect on mothers that altered aggression, but even if it did, mild stressors do not impair aggression (Gammie and Stevenson, 2006, D’Anna et al., 2009) and pup removal was consistent for all testing. The maternal aggression test began by introducing a male intruder for 5 min. After the intruder male was removed, the female remained in the home cage and the pups were scattered evenly away from the nest and the female allowing the female to retrieve and/or interact with her pups and perform maternal behavior for 30 min. For the antagonist studies, only pup retrieval was monitored for 2 min. Each test session was recorded on videotape and subsequently analyzed off-line to quantify behaviors by individuals blind to testing conditions. For quantification of maternal aggression the following features were measured: latency to first attack, number of attacks, and total duration of attacks (Gammie et al., 2004, D’Anna and Gammie, 2006). Pup retrieval was quantified by measuring the time elapsed to retrieval of first and fourth pup (D’Anna et al., 2005, D’Anna and Gammie, 2006). The rate of other maternal behaviors was determined by noting the behavior being performed every 30 sec for the duration of the test. Behaviors examined included on nest, off nest, eating or drinking, pup grooming, self-grooming, nursing, and nest building.
Immunohistochemistry and analysis of Fos
On postpartum Day 7 (the day following the last behavioral test), group A mice tested with the lower doses of NT and vehicle were randomly assigned to one of two groups and then were injected with either 0.1 μg NT (N = 9) or saline (N = 10), returned to their home cage with their pups, and their brains were collected 90 min after injection (±5 min). No behavioral tests were performed. 0.1 μg NT was chosen as the dose for examining NT action because at the time of the collection, the behavioral analysis was not complete and therefore it was not clear whether both doses would impair maternal aggression. Following isoflurane anesthesia, mice were decapitated and the brains removed. Brains were post-fixed overnight in 6% acrolein in phosphate buffered saline (PBS) and cryoprotected. Brains were frozen and cut into 40 micron thick sections using a sliding microtome (Leica, Microsystems) and stored in a cryoprotectant solution at −20 degrees C. The sections then underwent previously described immunohistochemical processes, which included incubating the sections for two days at 4 degrees C with rabbit anti-Fos antibodies (1:15,000; Calbiochem, catalog # PC38) (Gammie et al., 2004, D’Anna et al., 2005, D’Anna and Gammie, 2006). The sections were then mounted, dehydrated, and coverslipped.
Bright field microscopy was employed for counting Fos-positive cells using an Axioskop Zeiss light microscope (Zeiss) and an Axiocam Zeiss high resolution digital camera attached to the microscope and interfaced with a computer. Counting was based on a previously used paradigm (Gammie and Nelson, 2001, Gammie et al., 2004). Using boxes, cells in a specific region within a specific section were automatically counted as previously described (Gammie et al., 2004, D’Anna et al., 2005, D’Anna and Gammie, 2006, Gammie and Stevenson, 2006). One section per brain region was used to quantify Fos immunoreactivity in each animal. To ensure Fos was measured consistently between samples: 1) all sections were exposed to diaminobenzidine for 10 min; 2) the backgrounds were normalized by adjusting light levels; 3) a threshold of staining levels was used to automatically identify Fos-positive cells; 4) all slides were coded and the counting for each region was performed by one individual, blind to the experimental conditions; 5) only Fos-positive nuclei within a specified size range were counted; and 6) all sections were run in one batch.
Data analysis
Data were analyzed with SigmaStat 3.0 (SPSS Inc). Maternal aggression and other maternal behavior testing variables were analyzed using a repeated measures (RM) ANOVA. In the cases where the data were not normally distributed, either data were transformed to achieve normality, or when the transformation was not effective, a non-parametric Friedman RM ANOVA on Ranks test was performed. In the case of latency to first attack, if an animal was non-aggressive, a time of 300 s (the maximum time of the aggression test) was assigned. Likewise, if an animal did not retrieve the first or fourth pup within a time of 120 seconds, then a time of 120 s was assigned as performed previously (D’Anna et al., 2005). The standard p-value cutoff of 0.05 was used to evaluate the significance of the behavioral data.
For Fos analysis, a one-way ANOVA was used to test the effect of NT versus saline. In cases where the data were not normally distributed, then a Mann-Whitney U test was used. To correct for multiple comparisons (26 one-way ANOVAs), the open source software Qvalue (Storey, 2002) was used to estimate the p-value cutoff that will yield a global, experiment-wide, false discovery rate of 5% as previously performed (Gammie et al., 2004, D’Anna et al., 2005, D’Anna and Gammie, 2006). For our data set, the standard p value of 0.05 was appropriate because it would only yield a false discovery rate of 0.6% (e.g. 0.6 out of 100 positive results will be false positives).
Results
Effects of icv NT on maternal aggression
The effect of NT on three measures of maternal aggression was examined in two separate groups of mice. For Group A (lower doses), the effect of 0.05 μg NT and 0.1 μg NT versus vehicle was examined and for Group B (higher doses), the effect of 1.0 μg NT and 3.0 μg NT versus vehicle was examined. When non-parametric tests were used, medians and quartiles are presented in the text. For figures, all results are shown as means and standard errors to allow for comparisons across figures and studies.
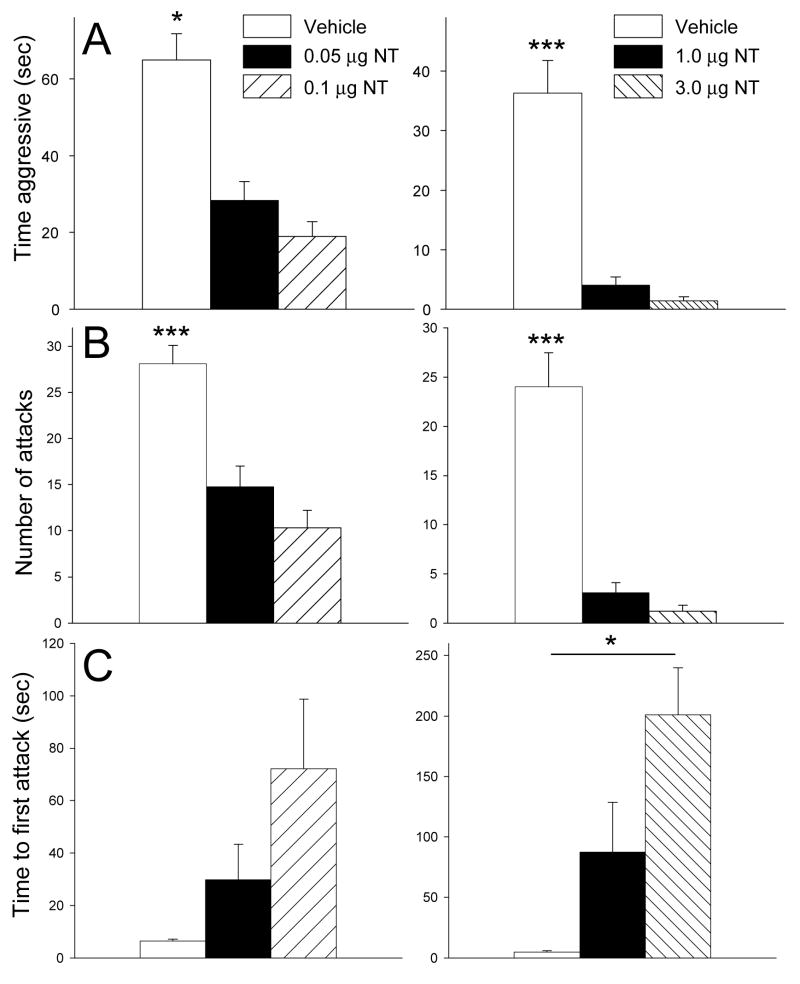
The lower doses administered to Group A significantly affected time aggressive (X2 (2) = 31.6, p < 0.001; Friedman RM ANOVA on Ranks) (vehicle, median= 60.0, 25%=39.5, 75%=89.0; 0.05 μg NT, median = 24.0, 25%=9.5, 75%=37.5; 0.1 μg NT, median= 14.0, 25%=4.0, 75%=35.5) (Fig. 1A). Dunn’s post-hoc tests revealed that both 0.05 μg NT (Q = 5.2, p<0.05) and 0.1 μg NT (Q = 4.1, p<0.05) significantly decreased time aggressive relative to vehicle injections (Fig. 1A). The higher doses administered to Group B also significantly affected time aggressive (F (2,31) = 37.34, p < 0.001; one-way RM ANOVA) (Fig. 1A). Holm-Sidak post-hoc tests revealed that both 1.0 μg NT (t = 7.1, p<0.001) and 3.0 μg NT (t = 7.7, p<0.001) significantly decreased time aggressive relative to vehicle injections (Fig. 1A).
Fig. 1.
Effects of icv NT on measures of maternal aggression in mice. A) Mean time aggressive is significantly reduced by all doses of NT relative to vehicle. B) Mean number of attacks is also significantly decreased by all doses of NT relative to vehicle. C) Mean latency to first attack is significantly elevated by 3.0 μg NT relative to vehicle. Left and right grouping of bars represent separate sets of mice. All studies were performed using a within subjects repeated measures design. For all tests, order of injections was counterbalanced. For left group, N = 20. For right group, N = 11. Bars represent means + SE. * = p<0.05; *** = p<0.001.
The lower doses in Group A significantly altered number of attacks (F (2,58) = 47.1, p < 0.001; one-way RM ANOVA) (Fig. 2A). Holm-Sidak post-hoc tests revealed that both 0.05 μg NT (t = 6.9, p<0.001) and 0.1 μg NT (t = 9.3, p<0.001) significantly decreased number of attacks relative to vehicle injections (Fig. 1B). The higher doses in Group B also significantly affected number of attacks (F (2,31) = 40.86, p < 0.001; one-way RM ANOVA) (Fig. 1A). Holm-Sidak post-hoc tests revealed that both 1.0 μg NT (t = 7.4, p<0.001) and 3.0 μg NT (t = 8.1, p<0.001) significantly decreased number of attacks relative to vehicle injections (Fig. 1B).
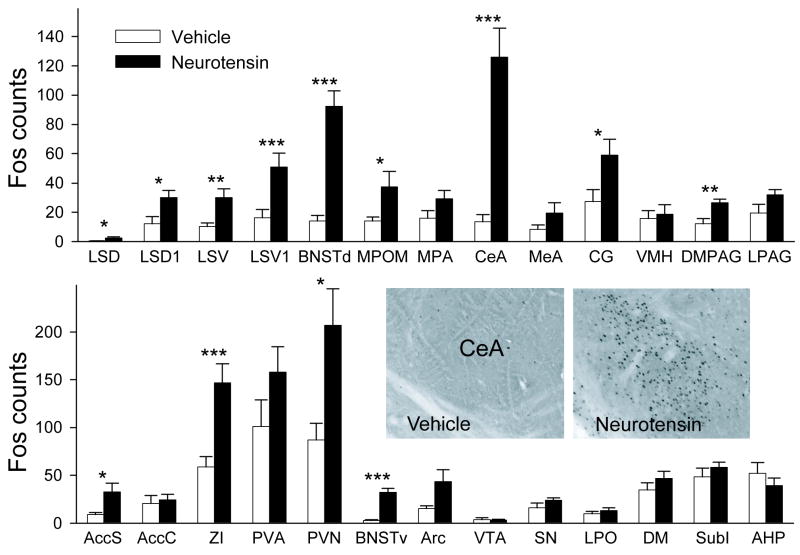
Fig. 2.
Effect of icv NT on Fos expression in the CNS. Injections were given 90 min (±5 min) before brain fixation. Bars represent means + SE. White bars indicate vehicle injections (N = 10) and black bars indicate 0.1 μg NT (N = 9). Top row includes regions previously linked to maternal aggression regulation. For bottom row, only paraventricular nucleus (PVN) has been strongly linked with maternal defense. An example of Fos labeling in CeA is shown. * = p<0.05; ** = p<0.001; *** = p<0.001. Abbreviations: AccC, nucleus accumbens core; AccS, nucleus accumbens shell; AHP, anterior hypothalamic area posterior; Arc, arcuate nucleus; BNSTd, BNST dorsal; BNSTv, BNST ventral; CG, cingulate cortex; CeA; DM, dorsomedial nucleus; DMPAG, dorsomedial PAG; LPAG, lateral PAG; LPO, lateral preoptic area; LSD, LS dorsal (Bregma 0.38 mm); LSD1, LSD (Bregma 0.14 mm) LSV, LS ventral (Bregma 0.38 mm); LSV1, LSV (Bregma 0.14 mm); MeA, medial amygdala; MPA, medial preoptic area; MPOM, medial preoptic nucleus; PVA, paraventricular nucleus of the thalamus; SN, substantia nigra; SubI, subincertal nucleus; VMH, ventromedial hypothalamus; VTA, ventral tegmental area; ZI, zona incerta.
Group A injections did not significantly affect the mean latency to first attack (X2 (2) = 0.53, p = 0.767; Friedman RM ANOVA on Ranks) (Fig. 1C). Group B injections significantly affected latency to first attack (X2 (2) = 11.2, p =.004; Friedman RM ANOVA on Ranks) (vehicle, median= 5.0, 25%=1.5, 75%=6.7; 1.0 μg NT, median = 9.0, 25%=3.5, 75%=232.0; 3.0 μg NT, median= 288.0, 25%=61.25, 75%=300.0) (Fig. 1C). Dunn’s post-hoc tests revealed that only 3.0 μg NT (Q = 1.4, p<0.05) significantly decreased latency to first attack relative to vehicle injections (Fig. 1C).
Effects of icv NT on other maternal behaviors
Group A injections significantly affected the latency to retrieve the first pup (F (2,58) = 4.1, p = 0.024; one-way RM ANOVA). Holm-Sidak post-hoc tests revealed that only 0.1 μg NT (t = 2.8, p=0.007) significantly increased latency to retrieve first pup relative to saline injections. Group B injections also significantly affected latency to retrieve first pup (X2 (2) = 10.0, p =.007; Friedman RM ANOVA on Ranks) (vehicle, median= 120.0, 25%=13.2, 75%=120.0; 1.0 μg NT, median = 120.0, 25%=120.0, 75%=120.0; 3.0 μg NT, median= 120.0, 25%=120.0, 75%=120.0). However, Dunn’s post-hoc tests revealed no significant differences among groups.
Group A injections did not affect the total mean latency to retrieve the fourth pup (F (2,58) = 1.5, p = 0.228; one-way RM ANOVA). Group B injections significantly affected latency to retrieve fourth pup (X2 (2) = 8.0, p =.018; Friedman RM ANOVA on Ranks) (vehicle, median= 120.0, 25%=37.5, 75%=120.0; 1.0 μg NT, median = 120.0, 25%=120.0, 75%=120.0; 3.0 μg NT, median= 120.0, 25%=120.0, 75%=120.0). However, Dunn’s post-hoc tests again revealed no significant differences among groups.
A number of other maternal behaviors were observed following treatment for group A mice. However, for no measures did either 0.05 μg NT or 0.1 μg NT have any effect relative to vehicle injections. For the 30 min behavioral test, the following rates were found for vehicle, 0.05 μg NT, and 0.1 μg NT, respectively: off nest (.45, .44, .50); on nest (.54, .56, .49); nursing (.40, .36, .40); pup licking and grooming (.006, .005, .005); self-grooming (.09, .07, .03); and nest building (.018, .005, .005).
Effects of icv injections of NT on Fos immunoreactivity
Injections of 0.1 μg NT and vehicle were made in the absence of behavioral testing to identify sites of action of NT within the CNS using Fos immunoreactivity. Thirteen out of the 26 brain regions examined showed a significant increase in Fos expression in response to NT relative to vehicle injections (Fig. 2). For the two brain regions with significant differences between groups in which non-parametric tests were performed due to non-normal data distribution, the following features were found: Central amygdala (CeA) (vehicle, median= 4, 25%=3, 75%=18; 0.1 μg NT, median= 115, 25%= 102, 75%= 165.7); CG (vehicle, median= 25, 25%=12.2, 75%=29; 0.1 μg NT, median= 54, 25%= 37, 75%= 72.5).
Effects of icv NT1 receptor antagonist, SR 48692, on maternal aggression and pup retrieval
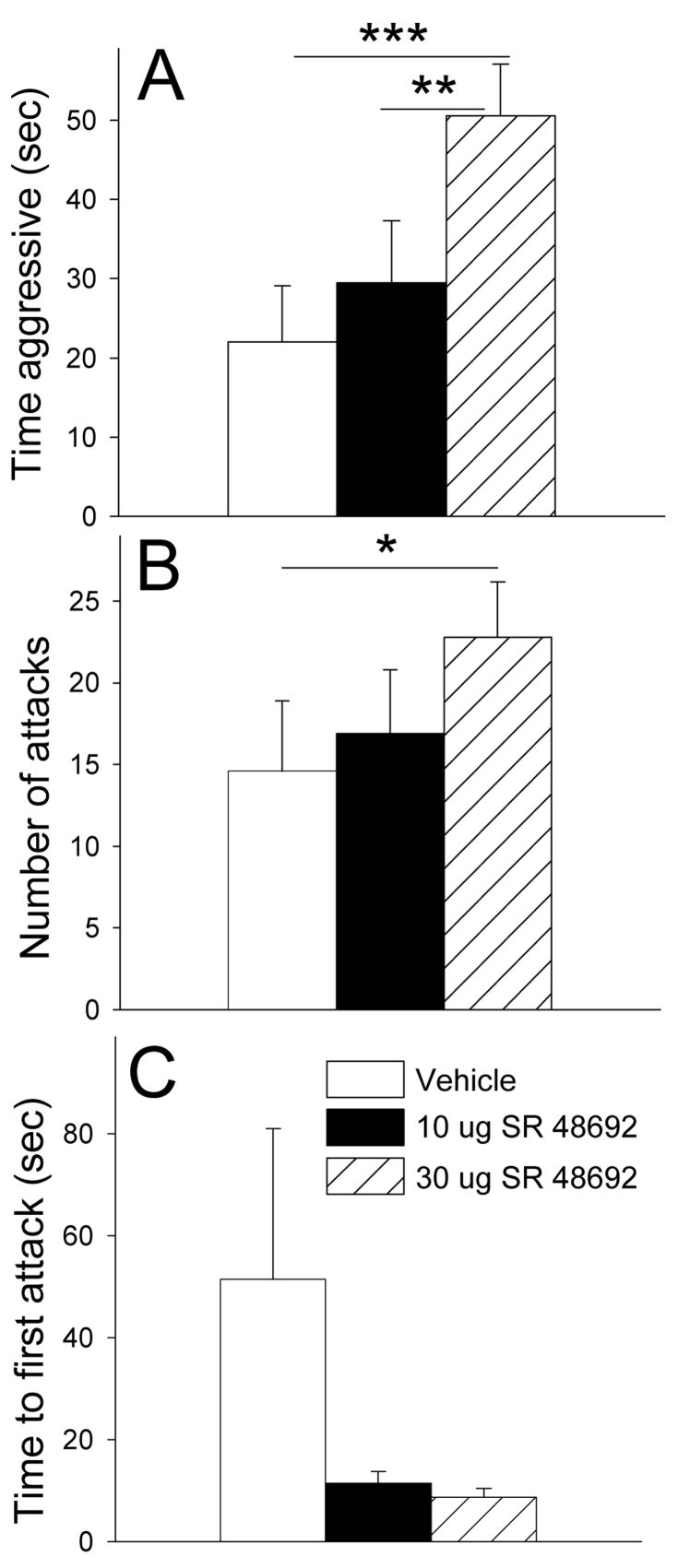
The NT1 antagonist, SR48692, significantly affected time aggressive (F (2,34) = 11.0, p < 0.001; one-way RM ANOVA) (Fig. 3A). Holm-Sidak post-hoc tests revealed that 30.0 μg SR 48692 significantly elevated aggression relative to both vehicle (t = 5.4, p<0.001) and 10.0 μg SR 48692 (t = 3.3, p=0.003) (Fig. 3A). The NT1 receptor antagonist also significantly affected number of attacks (F (2,34) = 3.4, p < 0.05; one-way RM ANOVA; power 0.4 transform used to achieve normality) (Fig. 3B). Holm-Sidak post-hoc tests revealed that 30.0 μg SR 48692 significantly elevated number of attacks relative to vehicle (t = 2.6, p=0.015) (Fig. 3B). No differences were found with treatment in terms of latency to first attack (X2 (2) = 1.5, p =.455; Friedman RM ANOVA on Ranks). SR 48692 had no effect on pup retrieval in terms of time to retrieve either first pup (X2 (2) = 1.5, p =.472; Friedman RM ANOVA on Ranks) or fourth pup (X2 (2) = 4.5, p =.105; Friedman RM ANOVA on Ranks).
Fig. 3.
Effects of icv NT1 antagonist, SR 48692, on measures of maternal aggression in mice. A) Mean time aggressive is significantly elevated by 30 μg SR 48692 relative to vehicle and to 30 μg SR 48692. B) Mean number of attacks is also significantly increased by 30 μg SR 48692 relative to vehicle. C) Mean latency to first attack was not significantly altered by treatment. All studies were performed using a within subjects repeated measures design. For all tests, order of injections was counterbalanced. N = 12. Bars represent means + SE. * = p<0.05; ** = p<0.01; *** = p<0.001. See Results for additional statistical information.
Discussion
In the current study, we demonstrate that NT inversely regulates maternal aggression and highlight for the first time an important role for NT in regulating a social behavior. Our findings indicate that NT can impair protection of offspring without altering other aspects of maternal care. Importantly, antagonizing NT1 receptor significantly elevated aggression, indicating that down regulation of NT signaling can be a mechanism for promoting maternal defense. These findings are consistent with our original observation of lower NT expression in the hypothalamus/preoptic region (determined via gene array and real-time PCR) in mice that we had selectively bred for high maternal aggression (Gammie et al., 2007). One explanation is that the lowering of NT that occurred with selection is causally linked to the elevation of maternal aggression. A related and interesting possibility is that during lactation, NT signaling decreases and facilitates offspring protection, but this has not been examined. There is indirect evidence that NT receptor function or expression is altered during lactation as firing rate of oxytocin neurons in response to NT changes significantly with lactation (Johnstone et al., 2004). Also, NT enhances PPI (Binder et al., 2001b, Caceda et al., 2006) and a decrease in PPI occurs during lactation (Byrnes et al., 2007), suggesting the possibility that NT neurotransmission is decreased during lactation.
We find that antagonizing NT1 promotes aggression and previous work found that antagonizing NT1 significantly decreases avoidance distance and number of escape attempts by mice exposed to a hand held rat (Griebel et al., 2001). If expression of maternal aggression involves a change in the default behavioral response to a potentially threatening stimulus, then decreasing activation of NT1 may be a means for decreasing the likelihood that an animal flees from a threat and elevating the chance that the animal expresses aggression. Protective behavior by females is critical for the survival of most mammalian offspring and females that remain with and defend offspring when faced with an attacker do not have the same options (fight or flight) as males (Taylor et al., 2000). A lowering of NT activity in either selected mice or during lactation could support the fight response by reducing the likelihood of flight. However, these possibilities still need to be tested. A highly specific NT2 antagonist does not exist and currently we do not know the contribution of NT2 to maternal aggression regulation.
To identify possible key brain regions mediating NT’s actions, we examined Fos in the CNS following injection of 0.1 μg NT or vehicle. We identified 13 brain regions exhibiting elevated Fos with icv NT injection (Fig. 2). Some of these regions, such as LS, BNSTd, MPOM, PVN, CeA, and PAG, have previously been implicated in the neural regulation of maternal aggression. In a previous study in male rats, icv NT elevated Fos and Zif268 in PVN and amygdala (Lambert et al., 1996), although much higher doses were used to achieve this effect in rats than in our study in lactating mice. Most of the regions examined in our study were not examined in the previous study in rats. It is possible that the Fos results could represent a state-dependency effect of association of prior treatment with aggression. However, high maternal aggression is associated with Fos increases (Gammie and Nelson, 2001, Hasen and Gammie, 2005) and in this study, vehicle would have been associated with high aggression, yet NT (which produced low aggression) yielded the increased Fos. Thus, it is most likely Fos increases with NT represent direct action on NT receptors and do not reflect state-dependency triggered by previous injections.
NT1 is found in LS, BNST, PVN, cortex, nucleus accumbens, amygdala, substantia nigra, and ventral tegmental area (VTA) (Boudin et al., 1996, Alexander and Leeman, 1998, Binder et al., 2001a, Pickel et al., 2001, Rowe et al., 2006). NT2 is expressed in BNST, nucleus accumbens, preoptic area (including MPOM), amygdala, SN, VTA, PAG, and dorsal raphe (Mazella et al., 1996, Sarret et al., 1998, Walker et al., 1998, Sarret et al., 2003, Caceda et al., 2006). With our current results, we cannot rule out a role for activation of NT2 in the impairment of aggression. However, the behavioral results with the NT1 antagonist highlight regions enriched with NT1 as being interesting candidates for sites where either NT impairs aggression or NT1 antagonists promote aggression. LS and BNSTd are strong candidate regions because they are reciprocally connected (Sheehan et al., 2004) and multiple studies from our lab and others implicate these in maternal aggression (Flannelly et al., 1986, Gammie and Nelson, 2001, Gammie et al., 2004, D’Anna et al., 2005, Lee and Gammie, 2007). LS plays a role in regulating processes related to mood and motivation, including diminishing or enabling fear responding (Sheehan et al., 2004). Because of inputs to LS and BNST from cortex and hippocampus and reciprocal connections with amygdala, hypothalamus, raphe, VTA, and PAG (Sheehan et al., 2004), LS and BNST are well-positioned for gating behavioral responses to a potentially threatening stimulus during lactation. CeA (Bosch et al., 2005), PAG (Lonstein et al., 1998), and PVN (Olazabal and Ferreira, 1997) have directly been linked to maternal defense regulation. PVN connects with LS and PAG, while CeA has reciprocal connections with LS, BNST, and PAG (Paredes et al., 2000, Walker et al., 2003, Sheehan et al., 2004). One interpretation is that increasing or decreasing NT signaling within a few key sites would be a mechanism for inhibiting or promoting maternal aggression. The results from the current study do not allow us to pinpoint which of these regions may be the most important in the modulation of aggression by NT, but site-directed injections of NT and NT1 antagonists into these regions would address this issue.
Recent work indicates excitatory (Bosch et al., 2004, Bosch et al., 2005) and inhibitory (Lubin et al., 2003) roles for oxytocin in maternal aggression. Vasopressin appears to impair maternal aggression because antagonizing the vasopressin 1a receptor enhances aggression (Nephew and Bridges, 2008). We have conducted a number of studies identifying an inhibitory role of corticotropin-releasing factor and related peptides, urocortin 1 and 3, in maternal aggression (Gammie et al., 2004, D’Anna et al., 2005, Gammie et al., 2009). We and others have identified a positive effect of GABA A signaling on maternal defense (Olivier et al., 1985, Palanza et al., 1996, Lee and Gammie, 2007). When developing an understanding of the mechanisms by which NT regulates maternal aggression, it will be important to determine whether or how and where it interacts with other known regulators of this behavior.
We monitored maternal behaviors in association with NT injection only for Group A mice receiving the lowest doses of NT. With the lowest dose of NT to impair aggression (0.05 μg), though, neither pup retrieval nor any other maternal behaviors were affected. This finding is important because it suggests that NT can specifically regulate maternal aggression without altering other maternal behaviors. Because we did not monitor retrieval of all pups, though, we cannot exclude the possibility than an effect would have been found in this measure. Although NT can lower temperature and even activate indirectly corticosterone release, the 0.05 μg dose of NT is well below the levels that trigger these responses in rats (Lambert et al., 1996). Previously, a 0.05 μg dose of NT injected icv in male mice did not affect grooming, rearing, or running, but did increase levels of immobility (Meisenberg and Simmons, 1985). In our study, immobility appeared unaltered by the 0.05 μg NT dose because only maternal aggression was altered and not any other behaviors, including the active motor behavior of pup retrieval. Also, the current study examined NT in a lactating female and it is possible that the physiological reactivity of a lactating female to NT differs from that of a male. Although we did not examine additional maternal behaviors following injection of NT1 antagonist, pup retrieval was not affected. In this study we used mice selected for high maternal defense to provide a baseline of aggression well above zero, so that we could examine increases or decreases in aggression with treatment. However, we often see some variability of levels of aggression when testing different groups of mice at different times and this occurs here as control aggression is highest in Group A mice. We do not think this alters the interpretation because we see a strong inhibitory effect of agonist and a strong excitatory effect of antagonist. We expect the effectiveness of the NT1 antagonist in promoting aggression to be even greater in outbred or inbred mouse strains with lower baseline levels of aggression (and presumably higher baseline levels of NT), but this has not been tested.
NT is a highly conserved neuropeptide, with all 13 amino acids being identical in mice and humans. We do not know the mechanisms by which NT expression was decreased in mice with selection for high maternal defense. The promoter region for NT includes AP-1, cAMP response, and glucocorticoid response elements (Kislauskis and Dobner, 1990). Estrogen can elevate NT expression, but this occurs via the cAMP pathway (Watters and Dorsa, 1998). Selection could have occurred either with changes in transcription factors binding to the NT promoter or with changes to NT promoter itself. Our findings that NT impairs aggression and the NT1 antagonist promotes aggression are exciting because they provide direct evidence for the first time that lowering NT activity can be a mechanism for the emergence of a critical social behavior. Also, they give insights into how selection for a complex behavior can occur. Given that maternal aggression emerges with lactation, it would be interesting to know whether a dynamic down regulation of NT signaling occurs in lactating females as a mechanism for regulating the timing and intensity of maternal aggression. An important point is that we conducted selection only on lactating mice and we examined gene expression only during lactation. Thus, we may have selected for females that strongly down regulate NT during lactation. We cannot rule out a role for NT2 in the regulation of maternal aggression, but our current results suggest brain regions enriched with NT1 as being the likeliest sites for regulating maternal aggression. This work highlights the value of selection studies as a tool for gaining novel insights into the regulation of complex social behaviors and suggests a need to evaluate a possible role for NT in other social behaviors.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH066086 to SCG, the University of Wisconsin Graduate School, and the Wisconsin Alumni Research Foundation. The authors wish to thank Amy Toberman, Kate Lentz, Derek Powell, and Sarang Patel for technical support and Kate Skogen and Jeff Alexander for animal care.
Comprehensive list of abbreviations
- AccC
nucleus accumbens core
- AccS
nucleus accumbens shell
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- Arc
arcuate nucleus
- BNST
bed nucleus of the stria terminalis
- BNSTd
bed nucleus of the stria terminalis dorsal
- BNSTv
bed nucleus of the stria terminalis ventral
- CeA
central amygdala
- CG
cingulate cortex
- cPAG
caudal periaqueductal gray
- DM
dorsomedial nucleus
- DMPAG
dorsomedial periaqueductal gray
- ICV
intracerebroventricular
- LPAG
lateral periaqueductal gray
- LPO
lateral preoptic area
- LS
lateral septum
- LSD
lateral septum dorsal at Bregma 0.38 mm
- LSD1
LSD at Bregma 0.14 mm
- LSV
lateral septum ventral at Bregma 0.38 mm
- LSV1
LSV at Bregma 0.14 mm
- MeA
medial amygdala
- MPA
medial preoptic area
- MPOM
medial preoptic nucleus
- NT
neurotensin
- NT1
neurotensin receptor 1
- NT2
neurotensin receptor 2
- PAG
periaqueductal gray
- PBS
phosphate buffered saline
- PPI
pre-pulse inhibition
- PVA
paraventricular nucleus of the thalamus
- PVN
paraventricular nucleus of the hypothalamus
- RM
repeated measures
- SN
substantia nigra
- SubI
subincertal nucleus
- VMH
ventromedial nucleus of the hypothalamus
- VTA
ventral tegmental area
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. J Comp Neurol. 1998;402:475–500. [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001a;53:453–486. [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. The role of neurotensin in the pathophysiology of schizophrenia and the mechanism of action of antipsychotic drugs. Biol Psychiatry. 2001b;50:856–872. doi: 10.1016/s0006-3223(01)01211-2. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bracha AS. Does “fight or flight” need updating? Psychosomatics. 2004;45:448–449. doi: 10.1176/appi.psy.45.5.448. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS, Scanlan VF, Babb JA, Byrnes JJ. Sensorimotor gating and dopamine function in postpartum rats. Neuropsychopharmacology. 2007;32:1021–1031. doi: 10.1038/sj.npp.1301222. [DOI] [PubMed] [Google Scholar]
- Caceda R, Kinkead B, Nemeroff CB. Neurotensin: Role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Castel MN, Stutzmann JM, Lucas M, Lafforgue J, Blanchard JC. Effects of ICV administration of neurotensin and analogs on EEG in rats. Peptides. 1989;10:95–101. doi: 10.1016/0196-9781(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Checler F, Mazella J, Kitabgi P, Vincent JP. High-affinity receptor sites and rapid proteolytic inactivation of neurotensin in primary cultured neurons. J Neurochem. 1986;47:1742–1748. doi: 10.1111/j.1471-4159.1986.tb13083.x. [DOI] [PubMed] [Google Scholar]
- D’Anna KD, Gammie SC. Hypocretin-1 dose-dependently modulates maternal behaviour in mice. J Neuroendocrinol. 2006;18:553–566. doi: 10.1111/j.1365-2826.2006.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna KD, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:161–171. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Stevenson SA, Gammie SC. Maternal profiling of corticotropin-releasing factor receptor 2 deficient mice in association with restraint stress. Brain Res. 2009 doi: 10.1016/j.brainres.2008.08.071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Dobner PR. Multitasking with neurotensin in the central nervous system. Cell Mol Life Sci. 2005;62:1946–1963. doi: 10.1007/s00018-005-5128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc I, Sarret P, Labbe-Jullie C, Botto JM, Honore E, Bourdel E, Martinez J, Costentin J, Vincent JP, Kitabgi P, Mazella J. Identification of the receptor subtype involved in the analgesic effect of neurotensin. J Neurosci. 1999;19:503–510. doi: 10.1523/JNEUROSCI.19-01-00503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behav Neural Biol. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA. Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav. 2007;6:432–443. doi: 10.1111/j.1601-183X.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, D’Anna KD, Lee G, Stevenson SA. Role of corticotropin releasing factor-related peptides in the neural regulation of maternal defense. In: Bridges RS, editor. Neurobiology of the Parental Brain. San Diego, CA: Elsevier; 2008. [Google Scholar]
- Gammie SC, Garland T, Stevenson SA. Artificial selection for increased maternal defense behavior in mice. Behav Genet. 2006;36:713–722. doi: 10.1007/s10519-006-9071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Rhodes JS, Girard I, Garland T., Jr Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Horm Behav. 2003;44:209–221. doi: 10.1016/s0018-506x(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Seasholtz AF, Stevenson SA. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2008.09.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Stevenson SA. Effects of daily and acute restraint stress during lactation on maternal aggression and behavior in mice. Stress. 2006;9:171–180. doi: 10.1080/10253890600969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R. Mice: postpartum aggression elicited by the presence of an intruder. Horm Behav. 1972;3:23–28. doi: 10.1016/0018-506x(72)90003-7. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moindrot N, Aliaga C, Simiand J, Soubrie P. Characterization of the profile of neurokinin-2 and neurotensin receptor antagonists in the mouse defense test battery. Neurosci Biobehav Rev. 2001;25:619–626. doi: 10.1016/s0149-7634(01)00045-8. [DOI] [PubMed] [Google Scholar]
- Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard JC, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, et al. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci U S A. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84:684–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Leng G, Brown CH. Effects of neurotensin on the organization of activity in supraoptic nucleus cells in virgin and lactating rats. J Neuroendocrinol. 2004;16:605–611. doi: 10.1111/j.1365-2826.2004.01208.x. [DOI] [PubMed] [Google Scholar]
- Kinkead B, Nemeroff CB. Novel treatments of schizophrenia: targeting the neurotensin system. CNS Neurol Disord Drug Targets. 2006;5:205–218. doi: 10.2174/187152706776359655. [DOI] [PubMed] [Google Scholar]
- Kislauskis E, Dobner PR. Mutually dependent response elements in the cis-regulatory region of the neurotensin/neuromedin N gene integrate environmental stimuli in PC12 cells. Neuron. 1990;4:783–795. doi: 10.1016/0896-6273(90)90205-t. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Ely TD, Gross RE, Kilts CD. Neurotensin induces Fos and Zif268 expression in limbic nuclei of the rat brain. Neuroscience. 1996;75:1141–1151. doi: 10.1016/0306-4522(96)00210-2. [DOI] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA enhancement of maternal defense in mice: Possible neural correlates. Pharmacol Biochem Behav. 2007;86:176–187. doi: 10.1016/j.pbb.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Kneip J, Grace M, Morley JE. Effect of centrally administered neurotensin on multiple feeding paradigms. Pharmacol Biochem Behav. 1983;18:19–23. doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci. 1998;112:1502–1518. doi: 10.1037//0735-7044.112.6.1502. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Bacino CB, Papp NL. Hypothermia elicited by the intracerebral microinjection of neurotensin. Peptides. 1980;1:333–339. doi: 10.1016/0196-9781(80)90011-x. [DOI] [PubMed] [Google Scholar]
- Mazella J. Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal. 2001;13:1–6. doi: 10.1016/s0898-6568(00)00130-3. [DOI] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenberg G, Simmons WH. Motor hypoactivity induced by neurotensin and related peptides in mice. Pharmacol Biochem Behav. 1985;22:189–193. doi: 10.1016/0091-3057(85)90376-4. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The interaction of neurotensin with dopaminergic pathways in the central nervous system: basic neurobiology and implications for the pathogenesis and treatment of schizophrenia. Psychoneuroendocrinology. 1986;11:15–37. doi: 10.1016/0306-4530(86)90029-6. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Prange AJ, Jr, Loosen PT, Barlow TS, Lipton MA. Neurotensin: central nervous system effects of a hypothalamic peptide. Brain Res. 1977;128:485–496. doi: 10.1016/0006-8993(77)90173-1. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav. 2008;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Olazabal DE, Ferreira A. Maternal behavior in rats with kainic acid-induced lesions of the hypothalamic paraventricular nucleus. Physiol Behav. 1997;61:779–784. doi: 10.1016/s0031-9384(96)00567-7. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R. Maternal aggression in rats: effects of chlordiazepoxide and fluprazine. Psychopharmacology (Berl) 1985;86:68–76. doi: 10.1007/BF00431686. [DOI] [PubMed] [Google Scholar]
- Palanza P, Rodgers RJ, Ferrari PF, Parmigiani S. Effects of chlordiazepoxide on maternal aggression in mice depend on experience of resident and sex of intruder. Pharmacol Biochem Be. 1996;54:175–182. doi: 10.1016/0091-3057(95)02109-4. [DOI] [PubMed] [Google Scholar]
- Paredes J, Winters RW, Schneiderman N, McCabe PM. Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: neuroanatomical substrates for affective behavior. Brain Res. 2000;887:157–173. doi: 10.1016/s0006-8993(00)02972-3. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Delle Donne KT, Boudin H, Pelaprat D, Rostene W. High-affinity neurotensin receptors in the rat nucleus accumbens: subcellular targeting and relation to endogenous ligand. J Comp Neurol. 2001;435:142–155. doi: 10.1002/cne.1198. [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, Shire D, Kopf M, Ferrara P. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Richard F, Barroso S, Martinez J, Labbe-Jullie C, Kitabgi P. Agonism, inverse agonism, and neutral antagonism at the constitutively active human neurotensin receptor 2. Mol Pharmacol. 2001;60:1392–1398. doi: 10.1124/mol.60.6.1392. [DOI] [PubMed] [Google Scholar]
- Rompre PP. Psychostimulant-like effect of central microinjection of neurotensin on brain stimulation reward. Peptides. 1995;16:1417–1420. doi: 10.1016/0196-9781(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Kar S, Meaney MJ, Quirion R. Neurotensin receptor levels as a function of brain aging and cognitive performance in the Morris water maze task in the rat. Peptides. 2006;27:2415–2423. doi: 10.1016/j.peptides.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Sarret P, Beaudet A, Vincent JP, Mazella J. Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. J Comp Neurol. 1998;394:344–356. [PubMed] [Google Scholar]
- Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T, Beaudet A. Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors. J Neurosci. 2005;25:8188–8196. doi: 10.1523/JNEUROSCI.0810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Gendron L, Kilian P, Nguyen HM, Gallo-Payet N, Payet MD, Beaudet A. Pharmacology and functional properties of NTS2 neurotensin receptors in cerebellar granule cells. J Biol Chem. 2002;277:36233–36243. doi: 10.1074/jbc.M202586200. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol. 2003;461:520–538. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J Royal Stat Soc Ser B. 2002;64:479–498. [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiol Behav. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tirado-Santiago G, Lazaro-Munoz G, Rodriguez-Gonzalez V, Maldonado-Vlaar CS. Microinfusions of neurotensin antagonist SR 48692 within the nucleus accumbens core impair spatial learning in rats. Behav Neurosci. 2006;120:1093–1102. doi: 10.1037/0735-7044.120.5.1093. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker N, Lepee-Lorgeoux I, Fournier J, Betancur C, Rostene W, Ferrara P, Caput D. Tissue distribution and cellular localization of the levocabastine-sensitive neurotensin receptor mRNA in adult rat brain. Brain Res Mol Brain Res. 1998;57:193–200. doi: 10.1016/s0169-328x(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]