Abstract
We recently developed a sensitive assay for 3′,5′-cAMP using high-performance liquid chromatography-tandem mass spectrometry. Using this assay, we investigated the release of 3′,5′-cAMP from isolated, perfused rat kidneys. To our surprise, we observed a dominant chromatographic peak that was because of an endogenous substance that had the same parent ion as 3′,5′-cAMP and that fragmented to the same daughter ion (adenine) as 3′,5′-cAMP. However, the retention time of this unknown was approximately 2.9 min, compared with 6.3 min for authentic 3′,5′-cAMP. We hypothesized that the unknown substance was an isomer of 3′,5′-cAMP. The unknown substance had the same retention time and mass spectral properties as authentic 2′,3′-cAMP. Renal venous secretion of 2′,3′-cAMP was greater in kidneys from 20-week-old genetically hypertensive rats compared with age-matched normotensive rats (12.49 ± 2.14 versus 5.32 ± 1.97 ng/min/g kidney weight, respectively; n = 18). Isoproterenol (1 μM; β-adrenoceptor agonist) increased renal venous 3′,5′-cAMP secretion (approximately 690% of control) but had no effect on 2′,3′-cAMP production. In contrast, rapamycin (0.2 μM; activator of mRNA turnover) and iodoacetate + 2,4-dinitrophenol (50 μM; metabolic inhibitors) increased the renal venous secretion of 2′,3′-cAMP (approximately 1000 and 4100% of control, respectively) while simultaneously decreasing the renal venous secretion of 3′,5′-cAMP. In conclusion, 2′,3′-cAMP is a naturally occurring isomer of 3′,5′-cAMP that is: 1) not made by adenylyl cyclase; 2) released from kidneys into the extracellular compartment; 3) released more by kidneys from rats with long-standing hypertension; 4) derived from mRNA turnover; and 5) increased by energy depletion.
Because 3′,5′-cAMP is an important second messenger in most cells comprising organ systems, it is desirable to be able to investigate, with a high level of precision, the production of 3′,5′-cAMP in intact organs. In this regard, it is fortunate that intracellular 3′,5′-cAMP is robustly transported to the extracellular compartment by various active transporters, including multidrug resistance protein (MRP) 4 and MRP5 (Kruh et al., 2001; Deeley et al., 2006). As a consequence, increases in intracellular levels of 3′,5′-cAMP can be detected by measuring 3′,5′-cAMP in the venous effluent of organ systems, such as the kidney (Vyas et al., 1996).
With regard to the kidney, 3′,5′-cAMP is importantly involved in regulation of renal vascular resistance, glomerular filtration rate, renin release, tubular epithelial transport, and the actions of hormones such as antidiuretic hormone (Cheng and Grande, 2007). Moreover, many of these physiological parameters are altered in kidneys from chronically hypertensive animals, compared with normotensive animals, in part because of target organ damage induced by the chronic elevation of arterial blood pressure.
To study the role of 3′,5′-cAMP in the hypertensive kidney, we recently developed an assay to measure 3′,5′-cAMP in the renal venous effluent from isolated, perfused kidneys (Ren et al., 2008). Unlike the commercially available kits for 3′,5′-cAMP that rely upon the selectivity of antibody recognition, our assay was based on the platform technology of high-performance liquid chromatography-tandem mass spectrometry (Ren et al., 2008).
While investigating the production of 3′,5′-cAMP from isolated, perfused kidneys obtained from normotensive and hypertensive rats, we observed the release of a substance with mass spectral characteristics similar to 3′,5′-cAMP, but that was clearly not 3′,5′-cAMP. The purpose of this report is to describe the identification and quantification of this substance and to describe its origin and regulation.
Materials and Methods
Animals. Studies used adult (20 weeks of age) male spontaneously hypertensive rats (SHRs) and male normotensive Wistar-Kyoto (WKY) rats obtained from Taconic Farms (Germantown, NY). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Isolated, Perfused Kidney Preparation. Rats were anesthetized with Inactin (90 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO), and the left kidney was isolated and perfused with Tyrode's solution containing 3-isobutyl-1-methylxanthine (10 μM; an inhibitor of phosphodiesterases; Sigma-Aldrich) using a Hugo Sachs Elektronik-Harvard Apparatus GmbH (March-Hugstetten, Germany) kidney perfusion system as described previously (Gao et al., 2003). In brief, all branches of the left renal artery and vein were ligated. A polyethylene 50 cannula was placed into the left renal artery, and a polyethylene 90 cannula was placed into the left renal vein. The left kidney was removed, attached to the perfusion system, kidneys were perfused (single-pass mode) at a constant flow (5 ml/min), and perfusion pressure was monitored with a pressure transducer.
Protocols. Kidneys were isolated from adult SHRs and WKY rats and perfused in vitro as described above. After a 30- to 60-min stabilization period, perfusate exiting the renal vein was collected for 1 min and immediately placed on ice. Next, in some experiments, isoproterenol (a β-adrenoceptor agonist; 1 μM; Sigma-Aldrich) was added to the arterial perfusate, and 5 min later, another 1-min renal venous sample was collected. In other experiments, after the basal sample of venous perfusate was collected, the kidney was treated with either rapamycin (0.2 μM; Sigma-Aldrich) or iodoacetate + 2,4-dinitrophenol (50 μM each; Sigma-Aldrich), and venous sampling was repeated at the indicated times.
High-Performance Liquid Chromatography-Tandem Mass Spectrometry. 3′,5′-cAMP and 2′,3′-cAMP were purchased from Sigma-Aldrich. The internal standard (13C10-adenosine) was from Medical Isotopes, Inc. (Pelham, NH). All standards were stored at -20°C, and the internal standard was stored at -80°C. Solutions of standards (50 ng/μl) and internal standard (1 ng/μl) were prepared in ultrapure water and stored at -20°C. The mixed solution of cAMPs (1 ng/μl) was prepared each day by dilution in ultrapure water and kept at 4°C. Additional dilutions of standards were prepared from this solution by serial dilution. Methanol [for liquid chromatography (LC)-tandem mass spectrometry MS/MS] was from Riedel-Dehae (Seelze, Germany), and analytical grade formic acid was from Fluka (Buchs, Switzerland).
3′,5′-cAMP and 2′,3′-cAMP were resolved by reversed-phase LC (Agilent Zorbax eclipse XDB-C-18 column, 3.5-μm beads; 2.1 × 100 mm; Agilent Technologies, Santa Clara, CA) and quantified using a triple quadrupole MS (TSQ Quantum-Ultra; Thermo Fisher Scientific, Waltham, MA) operating in the selective reaction monitoring (SRM) mode with a heated electrospray ionization source. The mobile phases were delivered by an ultrapressure LC system (Accela; Thermo Fisher Scientific) and consisted of linear gradient changes involving two buffers; buffer A was 0.1% formic acid in water, and buffer B was 0.1% formic acid in methanol. The mobile phase flow rate was 300 μl/min. The gradient (A/B) was as follows: 0 to 2 min, 98.5/1.5%; 2 to 4 min, to 98/2%; 5 to 6 min, to 92/8%; 7 to 8 min, to 85/15%; and 9 to 11.5 min, to 98.5/1.5%. Sample tray temperature was set at 4°C, and the column temperature was kept at 20°C.
For maximum sensitivities, instrument parameters were optimized as follows: ion spray voltage, 3.8 kV; ion transfer capillary temperature, 270°C; source vaporization temperature, 220°C; Q2 collision-induced dissociation gas, argon at 1.5 mTorr; sheath gas, nitrogen at 50 arbitrary units; auxiliary gas, nitrogen at 40 arbitrary units; Q1 and Q3 resolution, 0.70 U full-width half-maximum; source collision-induced dissociation, off; scan width, 0.2 U; scan time, 0.05 s; and tube lens offset, 123 V. Two SRM transitions were monitored: 278 → 141 m/z for 13C10-adenosine as internal standard, with a collision energy of 19 V; and 330 → 136 m/z for 3′,5′-cAMP and 2′,3′-cAMP with a collision energy of 28 V. Calibration standard curves were constructed at concentrations of 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, and 10 pg/μl in ultrapure water.
Statistics. Data were analyzed by two-factor analysis of variance. The criterion of significance was p < 0.05. All values in text and figures are means ± S.E.M.
Results
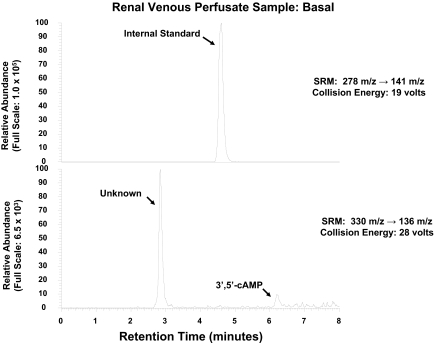
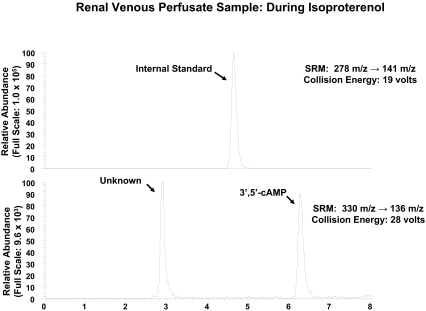
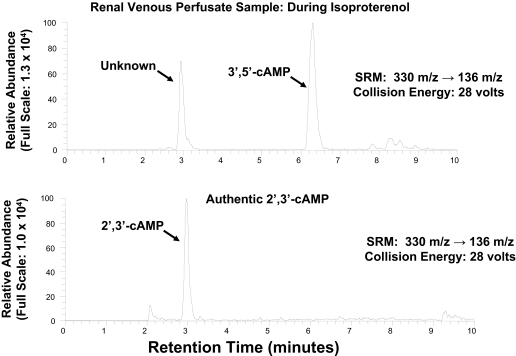
As shown in Fig. 1, with SRM (330 → 136 m/z), injection of a sample of renal venous perfusate into the LC-MS/MS system gave rise to two prominent peaks in the chromatogram, one with a retention time similar to that of authentic 3′,5′-cAMP (approximately 6.3 min) and the other with a retention time of approximately 2.9 min. When a renal venous perfusate sample was obtained from the same kidney as shown in Fig. 1 but during administration of isoproterenol, an agonist that stimulates adenylyl cyclase via β-adrenoceptors, the 3′,5′-cAMP peak was greatly increased, whereas the unknown peak was not (Fig. 2). Thus, it was unlikely that the unknown peak was endogenous 3′,5′-cAMP. For the unknown substance giving rise to the peak at 2.9 min to be observed, the substance would have to have a parent ion of 330 m/z (mol. wt. of 3′,5′-cAMP plus 1) with a daughter fragment of 136 m/z (mol. wt. of adenine fragment plus 1). We hypothesized, therefore, that the unknown substance was a positional isomer of 3′,5′-cAMP, possibly 2′,3′-cAMP. Because, at least to our knowledge, 2′,3′-cAMP has not been reported as an endogenous product released from intact cells, tissues, or organs, we thought it necessary to secure the identity of the putative 2′,3′-cAMP peak. Therefore, we obtained authentic 2′,3′-cAMP and determined its retention time relative to the unknown substance using our LC-MS/MS system, again with SRM (330 → 136 m/z). As shown in Fig. 3, authentic 2′,3′-cAMP and the unknown substance had the same retention time (approximately 2.9 min). To further confirm the identity of the putative 2′,3′-cAMP peak, we obtained another renal venous perfusate sample, split the sample, added authentic 2′,3′-cAMP to one part, and injected both samples. The area under the putative 2′,3′-cAMP peak increased from 545,047 arbitrary units to 1,763,662 arbitrary units with the addition of authentic 2′,3′-cAMP, and the ratio of the area under the putative 2′,3′-cAMP peak to the area under the 3′,5′-cAMP peak increased from 1.69 to 3.24. Thus, addition of authentic 2′,3′-cAMP increased the signal of the putative 2′,3′-cAMP peak without introducing any new peaks in the chromatogram. This indicated that the substance giving rise to the putative 2′,3′-cAMP peak had the precise retention time, parent ion, and daughter ion of authentic 2′,3′-cAMP.
Fig. 1.
LC-MS/MS SRM chromatogram of renal venous perfusate obtained from untreated, isolated, and perfused SHR kidney. Two transitions were monitored: 278 → 141 m/z for the internal standard (top), which was 13C10-adenosine; and 330 → 136 m/z for endogenous 3′,5′-cAMP (bottom). Note the prominent peak with a retention time of approximately 2.9 min (bottom), which was much too short to be 3′,5′-cAMP, which has a retention time of approximately 6.3 min.
Fig. 2.
Figure illustrates a chromatogram of renal venous perfusate obtained from the same kidney as in Fig. 1 but during the administration of isoproterenol (1 μM). Two transitions were monitored: 278 → 141 m/z for the internal standard (top), which was 13C10-adenosine; and 330 → 136 m/z for endogenous 3′,5′-cAMP (bottom). Comparing with Fig. 1, note the marked increase in the area of the peak corresponding to 3′,5′-cAMP (6.3 min), whereas the area of the unknown peak (2.9 min) was little changed.
Fig. 3.
Figure illustrates a chromatogram of a renal venous sample (top) versus authentic 2′,3′-cAMP (bottom). The same transition was monitored in each panel: 330 → 136 m/z for endogenous 3′,5′-cAMP. Note that authentic 2′,3′-cAMP had a retention time precisely that of the unknown substance.
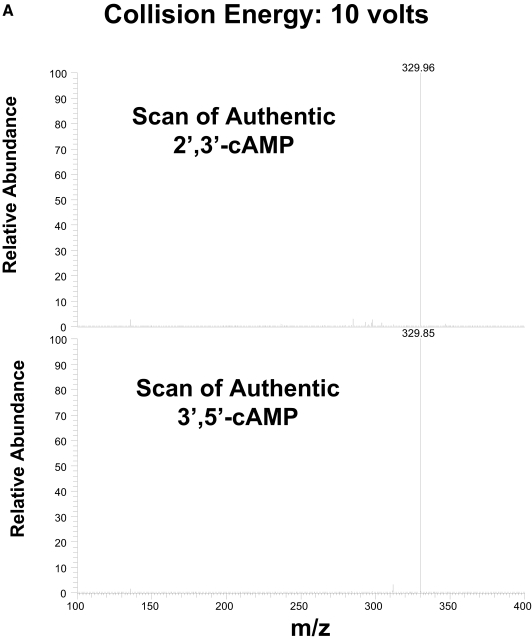
To further secure the identity of the substance giving rise to the putative 2′,3′-cAMP peak, we injected authentic 2′,3′-cAMP and authentic 3′,5′-cAMP into the LC-MS/MS system and then acquired mass spectral scans from 100 to 400 m/z at different collision energies to fragment the parent ion. As shown in Fig. 4A, at a collision energy of 10 V, both authentic 2′,3′-cAMP and authentic 3,′5′-cAMP gave rise to a mass spectrum consisting of mainly the parent ion (330 m/z). Thus, 10 V was insufficient to fragment the parent ion of either form of cAMP. However, when the collision energy was increased to 18 V (Fig. 4B), there was substantial fragmentation of authentic 2′,3′-cAMP to the 136 m/z fragment; in fact, the 136 m/z fragment became the most abundant ion. In contrast, although authentic 3′,5′-cAMP showed some fragmentation at 18 V of collision energy, the 330 m/z remained the most abundant ion. For both forms of cAMP, at a collision energy of 23 V, mainly the 136 m/z daughter ion was observed (Fig. 4C). These data indicate that 2′,3′-cAMP can be further identified and differentiated from 3′,5′-cAMP by using the collision energy necessary to fragment the parent ion.
Fig. 4.
A to C, mass spectrum of authentic 2′,3′-cAMP and 3′,5′-cAMP at different levels of collision energy (10, 18, and 23 V, respectively).
As shown in Fig. 5A, when renal venous perfusate was injected into the LC-MS/MS, and a mass spectral scan was obtained at the center of the putative 2′,3′-cAMP peak and the 3′,5′-cAMP peak, at 10 V of collision energy, only a single mass was observed at 330 m/z for both peaks. It is important that when the collision energy was increased to 18 V (Fig. 5B), for the putative 2′,3′-cAMP peak, the major fragment was 136 m/z, whereas for the 3′,5′-cAMP peak, the major fragment was still 330 m/z. At 23 V of collision energy (Fig. 5C), both the putative 2′,3′-cAMP and 3′,5′-cAMP peaks gave rise mainly to a daughter ion of 136 m/z. Thus, the susceptibility of the putative 2′,3′-cAMP peak to fragmentation by collision energy was very similar to that observed for authentic 2′,3′-cAMP and was different from that observed for 3′,5′-cAMP.
Fig. 5.
A to C, mass spectrum of the putative 2′,3′-cAMP peak and 3′,5′-cAMP peak at different levels of collision energy (10, 18, and 23 V, respectively).
Taken together, the above results indicate that the putative 2′,3′-cAMP peak was, without any reasonable doubt, 2′,3′-cAMP. Therefore, we turned our attention to quantifying the amounts of 2′,3′-cAMP versus 3′,5′-cAMP release from isolated, perfused kidneys obtained from 20-week-old SHRs and WKY rats. As shown in Fig. 6, the basal renal venous secretion of 3′,5′-cAMP was similar in SHR versus WKY kidneys and was significantly (p < 0.0001) and similarly increased by isoproterenol (7.7- and 6.2-fold in SHR and WKY kidneys, respectively). In contrast, the renal venous 2′,3′-cAMP secretion was 2.4-fold greater (p = 0.0052) in SHRs compared with WKY kidneys, and isoproterenol had no effect on the renal venous secretion of 2′,3′-cAMP (Fig. 7).
Fig. 6.
Bar graph depicts the renal venous secretion rate of 3′,5′-cAMP in kidneys obtained from SHRs and WKY rats both under basal conditions and with the addition of isoproterenol (1 μM) to stimulate adenylyl cyclase via β-adrenoceptors. Statistical analysis is from two-factor analysis of variance.
Fig. 7.
Bar graph depicts the renal venous secretion rate of 2′,3′-cAMP in kidneys obtained from SHRs and WKY rats both under basal conditions and with the addition of isoproterenol (1 μM) to stimulate adenylyl cyclase via β-adrenoceptors. Statistical analysis is from two-factor analysis of variance.
Biochemical studies suggest that 2′,3′-cAMP can be produced by RNases (Thompson et al., 1994), and recent evidence supports enhanced mRNA turnover in SHR tissues (Klöss et al., 2005). Therefore, we hypothesized that 2′,3′-cAMP may derive from degradation of mRNA. To test this hypothesis, we used two approaches to accelerate mRNA turnover. One approach was infusion of rapamycin, a drug that activates mRNA turnover via the mammalian target of rapamycin pathway (Banholzer et al., 1997; Hashemolhosseini et al., 1998; Albig and Decker, 2001), into the kidney. In this experimental series, four kidneys were from SHRs, and four were from WKY rats; however, the results were similar so the data were combined. As shown in Fig. 8, rapamycin caused a time-related and large (approximately 1000% of basal; p = 0.0381) increase in renal venous 2′,3′-cAMP secretion while inhibiting the renal secretion of 3′,5′-cAMP (approximately 50% of basal; p = 0.0007).
Fig. 8.
Line graph depicts the time-related effects of rapamycin (0.2 μM) on renal venous secretion rate of 2′,3′-cAMP and 3′,5′-cAMP in kidneys obtained from four SHRs and four WKY rats. The effects of rapamycin were independent of strain, so the results from all eight kidneys were combined. Data are shown as percentage of time 0 (basal) levels of 2′,3′-cAMP and 3′,5′-cAMP at the indicated time after administration of rapamycin. Statistical analysis is from two-factor analysis of variance.
Because previously published studies show that tissue ischemia triggers mRNA degradation (Akahane et al., 2001a,b; Almeida et al., 2004), in a second approach to stimulate mRNA degradation, we treated kidneys with a combination of metabolic inhibitors (iodoacetate, inhibitor of glycolysis; 2,4-dinitrophenol, inhibitor of oxidative phosphorylation). In this experimental series, four kidneys were from SHRs, and two were from WKY rats; however, as with the rapamycin study, the results were similar, so the data were combined. As shown in Fig. 9, the combination of iodoacetate + 2,4-dinitrophenol caused a time-related and massive (approximately 4100% of basal; p < 0.0001) increase in renal venous 2′,3′-cAMP secretion while inhibiting the renal secretion of 3′,5′-cAMP (approximately 40% of basal; p = 0.0002).
Fig. 9.
Line graph depicts the time-related effects of iodoacetate + 2,4-dinitrophenol (50 μM) on renal venous secretion rate of 2′,3′-cAMP and 3′,5′-cAMP in kidneys obtained from four SHRs and two WKY rats. The effects of iodoacetate + 2,4-dinitrophenol were independent of strain, so the results from all six kidneys were combined. Data are shown as percentage of time 0 (basal) levels of 2′,3′-cAMP and 3′,5′-cAMP at the indicated time after administration of iodoacetate + 2,4-dinitrophenol. Statistical analysis is from two-factor analysis of variance.
Discussion
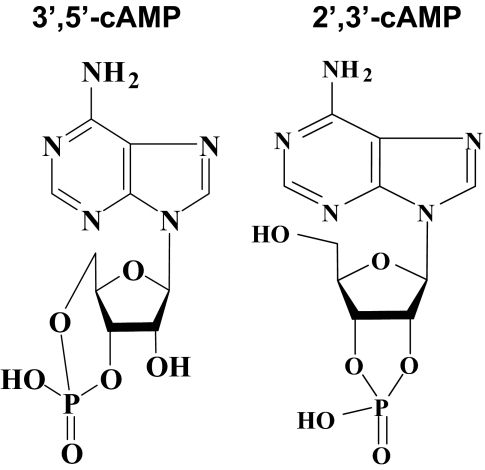
The present study confirms that 2′,3′-cAMP is released from the kidney. What is the source of 2′,3′-cAMP? It is well known that mRNA is degraded by the action of multiple RNases and that RNases catalyze the hydrolysis of the P-O5′ bond of mRNA (Wilusz et al., 2001). What is not widely appreciated is that this hydrolysis reaction, at least with isolated RNases, proceeds via two steps: transphosphorylation to form 2′,3′-cyclic nucleotides (such as 2′,3′-cAMP; Fig. 10, right), which differs from 3′,5′-cAMP (Fig. 10, left) and hydrolysis of these cyclic intermediates to form 3′-nucleotides (Markham and Smith, 1952). Studies by Thompson et al. (1994) using 31P NMR spectroscopy to monitor the accumulation of the 2′,3′-cyclic nucleotides during the transphosphorylation and hydrolysis reactions catalyzed by various RNases and by small molecules confirm this conclusion. In this regard, the experiments by Thompson et al. show that 2′,3′-cyclic nucleotides accumulate during catalysis by monomeric RNase A, a dimer and a trimer of RNase A, bovine seminal RNase, RNase TI, barnase, and RNase I. These enzymes, which are of widely disparate phylogenetic origin, release, rather than hydrolyze, most of the intermediate 2′,3′-cyclic nucleotides formed by transphosphorylation of mRNA. In contrast, 2′,3′-cyclic nucleotide intermediates do not accumulate during catalysis of mRNA by hydroxide ion or imidazole buffer (Thompson et al., 1994). Moreover, trapping experiments to assess the throughput of the reaction catalyzed by RNase A show that only 0.1% of the mRNA substrate is found to be both transphosphorylated and hydrolyzed without dissociating from the enzyme (Thompson et al., 1994).
Fig. 10.
Comparison of chemical structures for 3′,5′-cAMP (left) versus 2′,3′-cAMP (right).
The biochemical findings in isolated systems described above suggest, but do not prove, that mRNA degradation is the source of 2′,3′-cAMP from intact tissues. It is important that the present study demonstrates that both rapamycin and metabolic inhibitors cause a massive increase in the renal venous secretion of 2′,3′-cAMP while simultaneously decreasing the renal production of 3′,5′-cAMP. These findings add support to the conclusion that mRNA is the source of 2′,3′-cAMP released from the isolated, perfused kidney because both rapamycin (Banholzer et al., 1997; Hashemolhosseini et al., 1998; Albig and Decker, 2001) via the mTOR pathway and energy depletion via tissue ischemia (Akahane et al., 2001a,b; Almeida et al., 2004) are general activators of mRNA degradation.
Based on the aforementioned considerations, we conclude that the most likely source of 2′,3′-cAMP in the kidney is mRNA. Because most eukaryotic mRNAs contain a polyadenine tail, eukaryotic mRNA contains on average greater than 25% adenine nucleotides; therefore, mRNA turnover would be an extremely efficient mechanism for generating intracellular 2′,3′-cAMP. Moreover, because mRNA turnover is initiated by hydrolysis of the polyadenine tail (Wilusz et al., 2001), release of 2′,3′-cAMP would be a leading event in the degradation of mRNA.
Would 2′,3′-cAMP be expected to egress to the extracellular compartment? Research by us (Jackson and Mi, 2000; Dubey et al., 2001; Jackson and Dubey, 2001; Jackson et al., 2003, 2006, 2007) and by others (Davoren et al., 1963; Broadus et al., 1970; Kuster et al., 1973; Cramer and Lindl, 1974; King and Mayer, 1974; Doore et al., 1975; O'Brien and Strange, 1975; Brunton and Mayer, 1979; Barber and Butcher, 1983; Fehr et al., 1990; Kather, 1990; Li et al., 2007; Chiavegatti et al., 2008; Giron et al., 2008) demonstrate that 3′,5′-cAMP (the product of adenylyl cyclase) is robustly and actively transported to the extracellular compartment. In this regard, our unpublished studies show that the active transporter, MRP4, is responsible for most, if not all, of the transport (egress or efflux) of 3′,5′-cAMP from the cytoplasm to the extracellular space in preglomerular vascular smooth muscle cells (unpublished data). MRP4 and the related MRP5 are widely expressed and relatively indiscriminant organic anion transporters (Kruh et al., 2001; Deeley et al., 2006; Borst et al., 2007) that differ from other MRP-like proteins in their ability to transport cyclic nucleotides (Kruh et al., 2001; Deeley et al., 2006; Borst et al., 2007). Thus, MRP4 and MRP5 are probably involved in determining extracellular levels of cyclic nucleotides. MRP4, in particular, is expressed at relatively high levels in kidney cells, where it contributes to urinary excretion of 3′,5′-cAMP and 3′,5′-cGMP (van Aubel et al., 2002). Therefore, it is very likely that 2′,3′-cAMP produced by mRNA turnover would egress to the extracellular space via active transport by MRP4, MRP5, or both, depending on the cell type. The results of the present study clearly support the concept that endogenous 2′,3′-cAMP formed from the metabolism of mRNA actually appears in the extracellular compartment.
Could extracellular levels of 2′,3′-cAMP be used as a convenient and easily accessible biomarker for mRNA turnover/degradation? Because 2′,3′-cAMP is known to be made by RNases and because cyclic nucleotides can be transported out of cells by MRPs, measurement of 2′,3′-cAMP in the extracellular compartment of tissues and organs most likely is a biomarker for mRNA turnover/degradation. The elevated production of 2′,3′-cAMP in SHR kidneys supports this conclusion. Klöss et al. (2005) report a greater than 50% reduction in the expression of human antigen R in the aortas from SHRs with long-standing hypertension but not in aortas from prehypertensive SHRs (Klöss et al., 2005). The elav-like (embryonic-lethal abnormal vision) mRNA-binding protein human antigen R stabilizes many mRNAs by binding to highly conserved AU-rich elements (AREs; AUUUA) in the 3′-untranslated region (Fan and Steitz, 1998). AREs are targeted for rapid mRNA decay; thus, the presence of AREs within the 3′-untranslated region of numerous mRNAs plays a critical role in regulating mRNA stability and degradation (Fan and Steitz, 1998). Therefore, a reduction in human antigen R in organs from animals with long-standing hypertension would be predicted to result in decreased mRNA stability, increased mRNA degradation, and therefore increased extracellular levels of 2′,3′-cAMP.
In the present study, we were able to differentiate 2′,3′-cAMP from 3′,5′-cAMP using LC-MS/MS. Do commercially available assay kits for 3′,5′-cAMP discern between these positional isomers of cAMP? Although we cannot generalize to all commercially available kits, we have examined the popular Amersham cAMP Biotrak Enzymeimmunoassay System (GE Healthcare, Chalfont St. Giles, UK) and find that this kit does not detect 2′,3′-cAMP.
In summary, this study shows for the first time that 2′,3′-cAMP is produced by an intact organ and is secreted into the extracellular compartment. It is noteworthy that polyadenine tails of mRNA would provide a large cellular reservoir of potential 2′,3′-cAMP, that hydrolysis of the polyadenine tail of eukaryotic mRNA is an early event in mRNA turnover/breakdown, and that 2′,3′-cAMP is probably transported to the extracellular compartment. Thus, it is conceivable that an important role of the polyadenine tail of mRNA is to provide, in response to tissue injury, extracellular 2′,3′-cAMP, which might then mediate biological functions, for example tissue protection, and this possibility is under investigation.
This work was supported by the National Institutes of Health [Grants HL69846, DK068575, DK079307].
doi:10.1124/jpet.108.146712.
ABBREVIATIONS: MRP, multidrug resistance protein; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto rat; LC, liquid chromatography; MS/MS, tandem mass spectrometry; SRM, selective reaction monitoring; ARE, AU-rich element.
References
- Akahane M, Ono H, Ohgushi H, and Takakura Y (2001a) Viability of ischemia/reperfused bone determined at the gene expression level. J Reconstr Microsurg 17 203-209. [DOI] [PubMed] [Google Scholar]
- Akahane M, Ono H, Ohgushi H, and Tamai S (2001b) Viability of ischemia/reperfused muscles in rat: a new evaluation method by RNA degradation. J Orthop Res 19 559-564. [DOI] [PubMed] [Google Scholar]
- Albig AR and Decker CJ (2001) The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol Biol Cell 12 3428-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Paul Thiery J, Magdelénat H, and Radvanyi F (2004) Gene expression analysis by real-time reverse transcription polymerase chain reaction: influence of tissue handling. Anal Biochem 328 101-108. [DOI] [PubMed] [Google Scholar]
- Banholzer R, Nair AP, Hirsch HH, Ming XF, and Moroni C (1997) Rapamycin destabilizes interleukin-3 mRNA in autocrine tumor cells by a mechanism requiring an intact 3′ untranslated region. Mol Cell Biol 17 3254-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R and Butcher RW (1983) The egress of cyclic-AMP from metazoan cells. Adv Cyclic Nucleotide Res 15 119-138. [Google Scholar]
- Borst P, de Wolf C, and van de Wetering K (2007) Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch 453 661-673. [DOI] [PubMed] [Google Scholar]
- Broadus AE, Kaminsky NI, Northcutt RC, Hardman JG, Sutherland EW, and Liddle GW (1970) Effects of glucagon on adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in human plasma and urine. J Clin Invest 49 2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton LL and Mayer SE (1979) Extrusion of cAMP from pigeon erythrocytes. J Biol Chem 254 9714-9720. [PubMed] [Google Scholar]
- Cheng J and Grande JP (2007) Cyclic nucleotide phosphodiesterase (PDE) inhibitors: novel therapeutic agents for progressive renal disease. Exp Biol Med 232 38-51. [PubMed] [Google Scholar]
- Chiavegatti T, Costa VL Jr, Araújo MS, and Godinho RO (2008) Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway.[see comment]. Br J Pharmacol 153 1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer H and Lindl T (1974) Release of cyclic AMP from rat superior cervical ganglia after stimulation of synthesis in vitro. Nature 249: 380-382. [DOI] [PubMed] [Google Scholar]
- Davoren PR and Sutherland EW (1963) The effect of l-epinephrine and other agents on the synthesis and release of adenosine 3′,5′-phosphate by whole pigeon erythrocytes. J Biol Chem 238 3009-3015. [PubMed] [Google Scholar]
- Deeley RG, Westlake C, and Cole SPC (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86 849-899. [DOI] [PubMed] [Google Scholar]
- Doore BJ, Bashor MM, Spitzer N, Mawe RC, and Saier MH Jr (1975) Regulation of adenosine 3′:5′-monophosphate efflux from rat glioma cells in culture. J Biol Chem 250 4371-4372. [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, and Jackson EK (2001) Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37 1095-1100. [DOI] [PubMed] [Google Scholar]
- Fan XC and Steitz JA (1998) Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17 3448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr TF, Dickinson ES, Goldman SJ, and Slakey LL (1990) Cyclic AMP efflux is regulated by occupancy of the adenosine receptor in pig aortic smooth muscle cells. J Biol Chem 265 10974-10980. [PubMed] [Google Scholar]
- Gao L, Zhu C, and Jackson EK (2003) α2-Adrenoceptors potentiate angiotensin II- and vasopressin-induced renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther 305 581-586. [DOI] [PubMed] [Google Scholar]
- Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, and Gaion RM (2008) Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134 1116-1126. [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivières S, Mercep L, and Ferrari S (1998) Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem 273 14424-14429. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Jackson EK and Dubey RK (2001) Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol 281 F597-F612. [DOI] [PubMed] [Google Scholar]
- Jackson EK and Mi Z (2000) Preglomerular microcirculation expresses the cyclic AMP-adenosine pathway. J Pharmacol Exp Ther 295 23-28. [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Zacharia LC, Tofovic SP, and Dubey RK (2007) The pancreato-hepatorenal cAMP-adenosine mechanism. J Pharmacol Exp Ther 321 799-809. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Zhu C, and Dubey RK (2003) Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther 307 888-896. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, and Dubey RK (2006) cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther 317 1219-1229. [DOI] [PubMed] [Google Scholar]
- Kather H (1990) Beta-adrenergic stimulation of adenine nucleotide catabolism and purine release in human adipocytes. J Clin Invest 85 106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CD and Mayer SE (1974) Inhibition of egress of adenosine 3′,5′-monophosphate from pigeon erythrocytes. Mol Pharmacol 10 941-953. [Google Scholar]
- Klöss S, Rodenbach D, Bordel R, and Mülsch A (2005) Human-antigen R (HuR) expression in hypertension: downregulation of the mRNA stabilizing protein HuR in genetic hypertension. Hypertension 45 1200-1206. [DOI] [PubMed] [Google Scholar]
- Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, and Belinsky MG (2001) MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr 33 493-501. [DOI] [PubMed] [Google Scholar]
- Kuster J, Zapf J, and Jakob A (1973) Effects of hormones on cyclic AMP release in perfused rat livers. FEBS Lett 32 73-77. [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, et al. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131 940-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R and Smith JD (1952) The structure of ribonucleic acid: I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J 52 552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA and Strange RC (1975) The release of adenosine 3′:5′-cyclic monophosphate from the isolated perfused rat heart. Biochem J 152 429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Mi ZC, and Jackson EK (2008) Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325 920-926. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Venegas FD, and Raines RT (1994) Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry 33 7408-7414. [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, and Russel FG (2002) The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. JAm Soc Nephrol 13 595-603. [DOI] [PubMed] [Google Scholar]
- Vyas SJ, Mi Z, and Jackson EK (1996) The inhibitory effect of angiotensin II on stimulus-induced release of cAMP is augmented in the genetically hypertensive rat kidney. J Pharmacol Exp Ther 279 114-119. [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, and Peltz SW (2001) The cap-to-tail guide to mRNA turnover. Na Rev Mol Cell Biol 2 237-246. [DOI] [PubMed] [Google Scholar]