Abstract
We examined the potential contribution of ventromedial (VM) tissue sparing to respiratory recovery following chronic (1 mo) unilateral C2 spinal cord injury (SCI) in rats. Preserved white matter ipsilateral to the injury was quantitatively expressed relative to contralateral white matter. The ipsilateral-to-contralateral white matter ratio was 0 after complete C2 hemisection (C2HS) and 0.23±0.04 with minimal VM sparing. Inspiratory (breath•min−1) and phrenic frequency (burst•min−1), measured by plethysmography (conscious rats) and phrenic neurograms (anesthetized rats) respectively, were both lower with minimal VM sparing (p<0.05 vs. C2HS). Tidal volume also was greater in minimal VM sparing rats during a hypercapnic challenge (p<0.05 vs. C2HS). In other C2 hemilesioned rats with more extensive VM matter sparing (ipsilateral-to-contralateral white matter ratio = 0.55±0.05), respiratory deficits were indicated at 1 mo post-injury by reduced ventilation during hypercapnic challenge (p<0.05 vs. uninjured). Anterograde (ventral respiratory column-to-spinal cord) neuroanatomical tracing studies showed that descending respiratory projections from the brainstem are present in VM tissue. We conclude that even relatively minimal sparing of VM tissue after C2 hemilesion can alter respiratory outcomes. In addition, respiratory deficits can emerge in the adult rat after high cervical SCI even when relatively extensive VM sparing occurs.
Keywords: plasticity, spinal cord injury, crossed phrenic, breathing, respiratory
1. Introduction
Unilateral spinal lesions have been used extensively to investigate neuroplasticity associated with respiratory motor recovery after spinal cord injury (SCI; reviewed in Lane et al., 2008a; Kastner and Gauthier, 2008, Zimmer et al., 2007a, 2007b; see also Goshgarian, 2003; Teng et al., 2003; Choi et al., 2005). Many studies have focused on anatomical and functional plasticity associated with the “crossed phrenic phenomenon” (CPP; Goshgarian, 2003). In this model, inspiratory phrenic bursting ipsilateral to C2 spinal hemisection (C2HS) can be evoked shortly after injury by contralateral phrenicotomy. Ipsilateral phrenic bursting gradually increases in amplitude over weeks-months post-C2HS (Nantwi et al. 1999) although bursting does not appear to normalize (Fuller et al. 2008) and the underlying mechanisms are not fully defined (Lane et al., 2008a).
There is debate as to how extensive a lateral C2 hemilesion must be for demonstration of the CPP (Duffin and Li, 2006). A portion of the bulbospinal axons innervating ipsilateral phrenic motoneurons are found in the ventral medial (VM) cervical white matter (Lipski et al., 1994). This suggests that hemilesions should extend completely to the spinal midline for unequivocal demonstration of the CPP. Consistent with this, Li et al. (2003) reported that cervical hemilesions sparing the VM spinal cord fail to abolish ipsilateral phrenic bursting. On the other hand, ipsilateral phrenic bursting consistent with the CPP has been observed following anatomically incomplete C2 spinal hemisection with VM sparing (Goshgarian, 1981; Minor et al., 2006; Vinit et al., 2007). The first goal of the present investigation was thus to compare respiratory recovery in hemilesioned rats with anatomically complete C2HS vs. rats with subtotal C2HS involving minimal sparing of VM tissue. Based on the data of Lipski et al. (1994), we hypothesized that minimal sparing would lead to enhanced ventilation. These experiments were coupled with detailed neurophysiological analyses of ipsilateral phrenic motor output.
Baussart and colleagues (2006) recently described a lateralized C2 spinal contusion model in rats in which much of the ventral spinal cord at C2 was spared. As the authors point out, such lesions have experimental advantages vs. C2HS (e.g., quicker recovery time). This lesion reduced, but did not eliminate ipsilateral hemi-diaphragm activity recorded at both 7 days and 1-month post injury. However, despite the reduced diaphragm activity, it has not been determined if such injuries produce an enduring ventilatory deficit as rats generally compensate for unilateral lesions with increased reliance on contralateral motor pathways (Golder et al. 2003; Doperalski and Fuller, 2006). Our second aim was to test the hypothesis that cervical hemilesions with a moderate amount of ventral white matter sparing will still produce significant deficits in ventilation as measured by plethysmography in unanesthetized rats.
The present study also provided an opportunity to critically evaluate several methods for quantifying ipsilateral phrenic burst amplitude following cervical SCI which we reasoned should be correlated with the severity of cervical hemilesions as assessed by morphological evaluation of residual white matter at the lesion site. Prior work demonstrated a correlation between tidal volume and the number of surviving ventral horn neurons after spinal cord contusion injury (Teng et al., 1999). We reasoned that because bulbospinal axons innervating ipsilateral phrenic motoneurons are found throughout the VM and lateral cervical white matter (Lipski et al., 1994), respiratory-related recruitment and discharge of ipsilateral phrenic motoneurons (and therefore ipsilateral phrenic neurogram burst amplitude (Eldrige 1971) should increase as progressively more C2 white matter is spared. Thus, both the raw and normalized value of the rectified and integrated phrenic burst (∫Phr) were compared with lesion volume morphometry to determine which (if any) quantitative neurogram approach revealed a relationship between phrenic output and lesion volume.
2. METHODS
2.1. Animals
Experiments were conducted using adult (4±1 mo-old) male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA, USA; colony Raleigh R04). Plethysmography and phrenic neurophysiology data were obtained from rats with anatomically complete C2HS (n=8) and rats with a lateralized C2 lesions and either “minimal” (n=8) or “moderate” VM tissue sparing (n=9) as described in Results. Injured rats were assigned to experimental groups at the time of surgery. Plethysmography data were also collected from a group of uninjured rats (n=7). The Institutional Animal Care and Use Committee at the University of Florida approved all experimental procedures.
2.2. Spinal cord injury
Surgeries were conducted as previously described (Doperalski and Fuller, 2006; Doperalski et al., 2008; Fuller et al., 2008). After induction of anesthesia with isoflurane, the spinal cord was lesioned on the left side immediately caudal to the C2 dorsal root (i.e. the first visible root since there is no C1 dorsal root in the rat). The spinal cord was initially lesioned on the left side with microscissors. An approximately 1 mm gap was then created at the incision site using a micropipette connected to a suction pump. The overlying muscles and skin were closed with suture and wound clips, respectively. Analgesic (buprenorphine, 0.03 mg/kg, s.q.) and anti-inflammatory drugs (carprofen, 5.0 mg/kg, s.q.) were supplied at 12 hr intervals for 2 days. Lactated ringers solution (18 mL/day) was administered sub-cutaneously until volitional drinking resumed.
2.3. Plethysmography (unanesthetized rats)
Ventilation was quantified using plethysmography (Buxco Inc., Wilmington, NC, USA). Calibration was accomplished by injecting known air volumes into the recording chamber. The chamber pressure, temperature and humidity, rectal temperature of the rat, and atmospheric pressure were measured and the Drorbaugh and Fenn equation (1955) was used to calculate respiratory volumes. Breathing frequency (breath•min−1), inspiratory and expiratory duration, and peak airflow rates were calculated from the airflow traces. Gases flowed through the recording chamber at a rate of 2 L/min. Baseline recordings were made for 60–90 min while the chamber was flushed with 21% O2 (balance N2). Rats were then given a 10 min hypercapnic challenge (7% CO2, 21% O2, balance N2). Rectal temperature was measured before rats were placed in the chamber and again immediately after the hypercapnic exposure. If body temperature had changed the Drorbaugh and Fenn equation was used to recalculate the respiratory volumes measured during hypercapnia using the new temperature.
2.4. Neurophysiology (anesthetized rats)
Phrenic neurogram recordings were obtained from the same rats after plethysmography data were acquired. Our methods have been described recently (Doperalski and Fuller, 2006; Doperalski et al., 2008; Fuller et al., 2008) and are briefly summarized here. The trachea was cannulated under isoflurane anesthesia and rats were bilaterally vagotomized and mechanically ventilated. Catheters were placed in the femoral artery and vein and rats were converted to urethane anesthesia (1.6 g/kg, i.v.; 0.12 g/mL distilled water) and paralyzed with pancuronium bromide (3.5 mg/kg, i.v.). Arterial blood gases were measured periodically (i-Stat, Heska, Fort Collins, CO, USA) and the end-tidal CO2 (PETCO2) was monitored using a neonatal CO2 monitor (Novametrix Medical Systems, Wallingford, CT, USA). Rectal temperature was maintained at 37±1 ºC using a heating pad (model TC-1000, CWE Inc., Ardmore, PA, USA). The phrenic nerves were isolated adjacent to the brachial plexus and are described as “ipsilateral” and “contralateral” to indicate their location relative to the cervical SCI. Electrical activity of the phrenic nerves (silver wire electrodes) was amplified (1000x) and filtered (band pass: 300 – 10,000 Hz; notch: 60 Hz) using a differential A/C amplifier (Model 1700, A-M Systems, Carlsborg, WA, USA). The signal was full-wave rectified, amplified (10x) and moving averaged (time constant 100 ms; MA-1000 moving averager, CWE Inc., Ardmore, PA, USA). All signals were digitized and recorded on a PC using Spike2 software (Cambridge Electronic Design Limited, Cambridge, England).
The PETCO2 was maintained 2 mmHg above the end-tidal CO2 apneic threshold and after a 15-30 min period of stable phrenic bursting (i.e. baseline), rats were exposed to a 5 min period of hypoxia (FIO2 = 0.12 – 0.14). Following the neurophysiology experiments, urethane-anesthetized rats were systemically perfused with heparinized saline followed by 4% paraformaldehyde.
2.5. Spinal cord histology and anterograde tracing
The spinal level of lesion was confirmed upon post-perfusion removal of the spinal cord. The cervical spinal cord was then cryoprotected and sectioned at 50 μm using a cryostat. Tissue sections were mounted on subbed glass slides and stained with luxol fast blue followed by cresyl violet. In a subset of experiments, the animals were perfused with a mixture of paraformaldehyde and glutaraldehyde (4% and 3.5% respectively in 0.1M PBS, pH=7.4) and spinal cord specimens at the lesion site were subsequently processed for epoxy resin (Electron Microscopy Sciences) embedding and semi-thin (2 μm) sectioning followed by staining with toluidine blue as described in Lane et al., 2008b.
For tissue cross-sectional area analyses, sections were examined using a Zeiss Axioplan Microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) at 2.5X magnification coupled with a Hamamatsu Color Chilled 3CCD Camera (Hamamatsu Corp., Bridgewater, NJ). Images were analyzed using Zeiss KS 400 v.3.0 software. For each animal, the lesion epicenter was defined as the tissue cross section demonstrating the least amount of spared ipsilateral tissue. Total cross-sectional white matter at the lesion epicenter (i.e. ipsilateral spared white matter and contralateral white matter) was manually outlined using a software pointer on a computer monitor. The area (μm2) of the white matter was then calculated and expressed as a percentage of the white matter on the contralateral side.
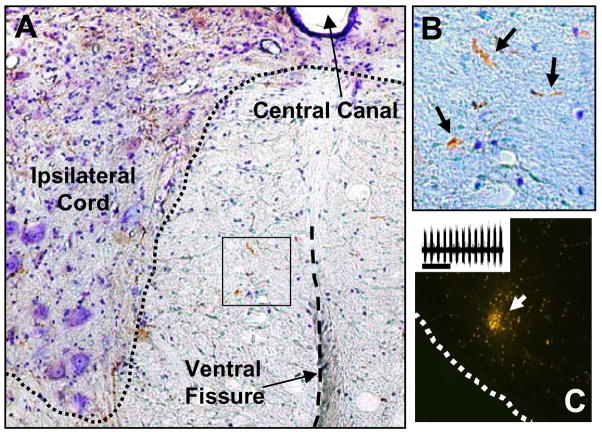
A few (n=2) additional experiments were conducted in which an anterograde tracer was used to label projections from the region of the medullary ventral respiratory column (VRC) to the cervical spinal cord. At one month post-lateralized cervical hemilesion, mini-ruby (biotin dextran amine conjugated with tetramethylrhodamine, Molecular Probes, 10,000 kDa) was injected into the VRC as recently described by Lane et al. (2008b). The VRC was first located using a combination of stereotaxic coordinates and electrophysiological recordings (e.g. Fig. 5). Specifically a microelectrode with a tungsten recording site and micropipette (Carbostar-3, Kation Scientific) was positioned such that robust inspiratory bursting could be observed, and mini-ruby was then delivered by iontophoresis (Lane et al., 2008b). Injections were made on each side of the brainstem. Rats were perfused (4% paraformaldehyde) transcardially 10 days post-injection, and the brainstem and cervical spinal cord were removed and transversely sectioned (40 μm) using a vibrotome. Spinal cord sections were rinsed with PBS and incubated overnight in ABC reagent (Vectastain ABC kit, Vector laboratories, Burlingame, CA, USA) and then developed in freshly prepared diaminobenzidine (DAB) peroxidase substrate solution to detect Miniruby (conjugated with biotin, brown) (Lane et al., 2008b). Tissues were also counter-stained with cresyl violet. Anterogradely labeled processes were then observed using either fluorescence or brightfield microscopy (e.g. Fig. 5).
Figure 5. Example of anterogradely labeled projections in the VM medial cervical spinal cord.
Miniruby conjugated with biotin was delivered via iontophoresis into the brainstem (see methods). Panel A (10x magnification) shows the ventral horn of the cervical spinal cord ipsilateral to the lesion site. This rat had a “moderate VM sparing” lesion. Panel B provides a higher magnification (20x) view of the area indicated by the box in panel A. The arrows indicate processes that are BDA-positive (brown color). Panel C shows an example the medullary injection site. The inset in panel C shows the inspiratory bursting recorded at the injection site and just prior to iontophoresis.
2.6. Data Analyses
Plethysmography data were averaged over a 10 min period prior to hypercapnia (i.e. baseline). It took several minutes for full manifestation of the hypercapnic ventilatory response, and accordingly the hypercapnia data were averaged over the last 5 min of the exposure (Teng et al., 1999; Fuller et al., 2006). Respiratory volume data were expressed per 100 g body mass (Olson et al., 2001). Neurophysiology data were analyzed using Spike2 software (Cambridge Electronic Design Limited, Cambridge, England). During baseline, the raw amplitude (V) of the ∫Phr burst was measured and normalized relative to activity in the nerve during hypoxia (% hypoxia), and relative to burst amplitude in the contralateral nerve (% contralateral). Increases in neurogram burst amplitude during respiratory challenge (i.e. hypoxia) were normalized to values recorded during baseline. The difference between ∫Phr burst amplitude during hypoxia and baseline was also expressed in arbitrary units (i.e. V).
To test the hypothesis that minimal sparing of VM tissue with C2 hemilesion would lead to enhanced respiratory recovery we compared ventilation and phrenic output between rats with complete C2HS to those with minimal VM sparing using a two-way repeated measures analysis of variance (ANOVA) followed by the Student-Neuman-Keuls post-hoc test (SigmaStat v. 2.03). The hypothesis that cervical hemilesions that spare the majority of the ventral spinal cord still produce ventilatory deficits was tested by comparing ventilation in rats classified as having “moderate sparing” (see results section 3.1) with control, uninjured rats using the same approach outlined above. The relationship between the amount of spared tissue in the ipsilateral spinal cord and ∫Phr burst amplitude was examined using linear regression (SigmaStat v. 2.03). The regression analyses included data from all C2HS and both minimal and moderate VM sparing groups. Differences were considered statistically significant when the P-value ≤ 0.05. All data are presented as the mean ± SEM.
3. Results
3.1 Lesion histology
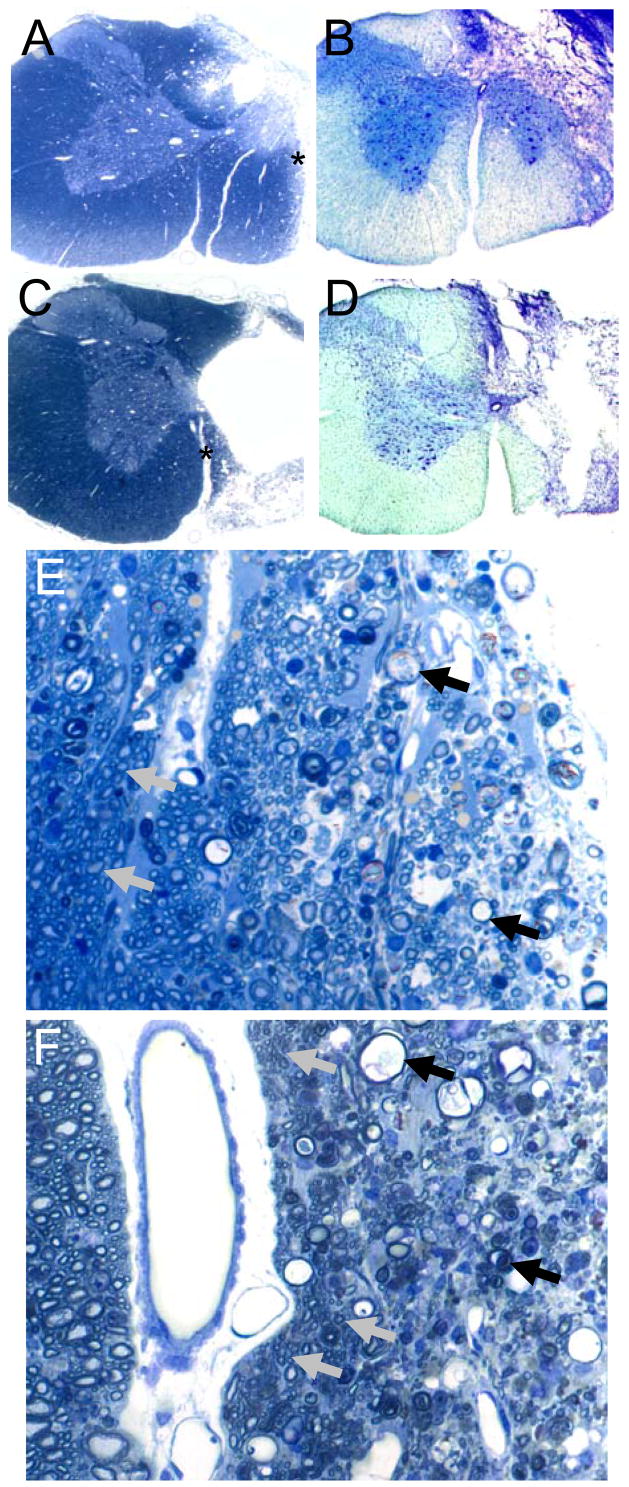
Rats classified as complete C2HS had no evidence of neuronal tissue in the ipsilateral spinal cord at the lesion epicenter as previously described (Golder et al., 2001; Fuller et al., 2008; Doperalski et al., 2008). Lesions classified as anatomically incomplete had residual white matter in the ipsilateral spinal cords at the lesion epicenter. Examples of lesion histology assessed in thin (2 μm) plastic sections and 40 μm cryostat sections are presented in Figure 1. Moderate lesions (Figs. 1A and B) typically spared ventral white matter extending from the ventral median fissure laterally with some ventral gray matter preservation as well. In contrast, hemisections with minimal sparing (Figs. 1C and D) were characterized by preservation of a thin wall of white matter immediately adjacent to the ventral median fissure. In tissue samples from animals with moderate tissue sparing, axonal integrity was largely maintained except along the edges adjacent to the lesion itself (Fig. 1E). On the other hand, examination of spared VM white matter in plastic, semi-thin sections from a minimal VM sparing rat revealed that much of the “spared” tissue consisted of degenerating axonal profiles and reactive astrocytes (Fig. 1F). However, some normal-appearing small axons were present along with occasional larger caliber fibers surrounded by thin myelin sheaths suggestive of remyelination. Rats operationally classified as having minimal VM sparing had a mean ipsilateral:contralateral white matter ratio of 0.23±0.04, whereas those classified as having moderate VM sparing had an ipsilateral:contralateral white matter ratio of 0.55±0.05 (p<0.001 vs. minimal sparing group).
Figure 1. Representative histological examples of cervical hemilesions.
Panel A provides an example of moderate VM white matter sparing in a semi-thin (2 μm) plastic section stained with toluidine blue. Panel B shows an example of moderate VM sparing as assessed in a thicker (40 μm) cryostat section stained with luxol fast blue and cresyl violet. Panels C and D depict minimal VM sparing in a similarly stained semi-thin plastic (C) and 40 μm cryostat section (D). Magnification for panels A-D is 2.5x. Panel E depicts a higher magnification (40x) of the lateral edge of the lesion site as indicated by the asterisk in panel A. The tissue at the right of panel E shows numerous axonal profiles at different stages of Wallerian degeneration (examples indicated by black arrows). Towards the left of panel E (i.e. towards the spinal midline) many apparently healthy axons can be observed (a few are indicated by gray arrows). Panel F is a higher magnification (40x) of the site indicated by the asterisk in panel C. Thus, the injured side is to the right and the intact contralateral side is to the left of the ventromedian fissure (the white area just the left of center). The “spared tissue” at the far right of the figure represents extensively degenerated white matter although a few small axons are present. More medially, the tissue in Panel F consists of numerous axonal profiles at different stages of Wallerian degeneration (black arrows). Some axonal preservation is seen, however, especially along the medial subpial surface adjacent to the ventromedian fissuer as indicated by clusters of small diameter myelinated axons (gray arrows).
3.2. Ventilation and phrenic output in anatomically complete C2HS vs. C2 hemilesion with minimal VM tissue sparing
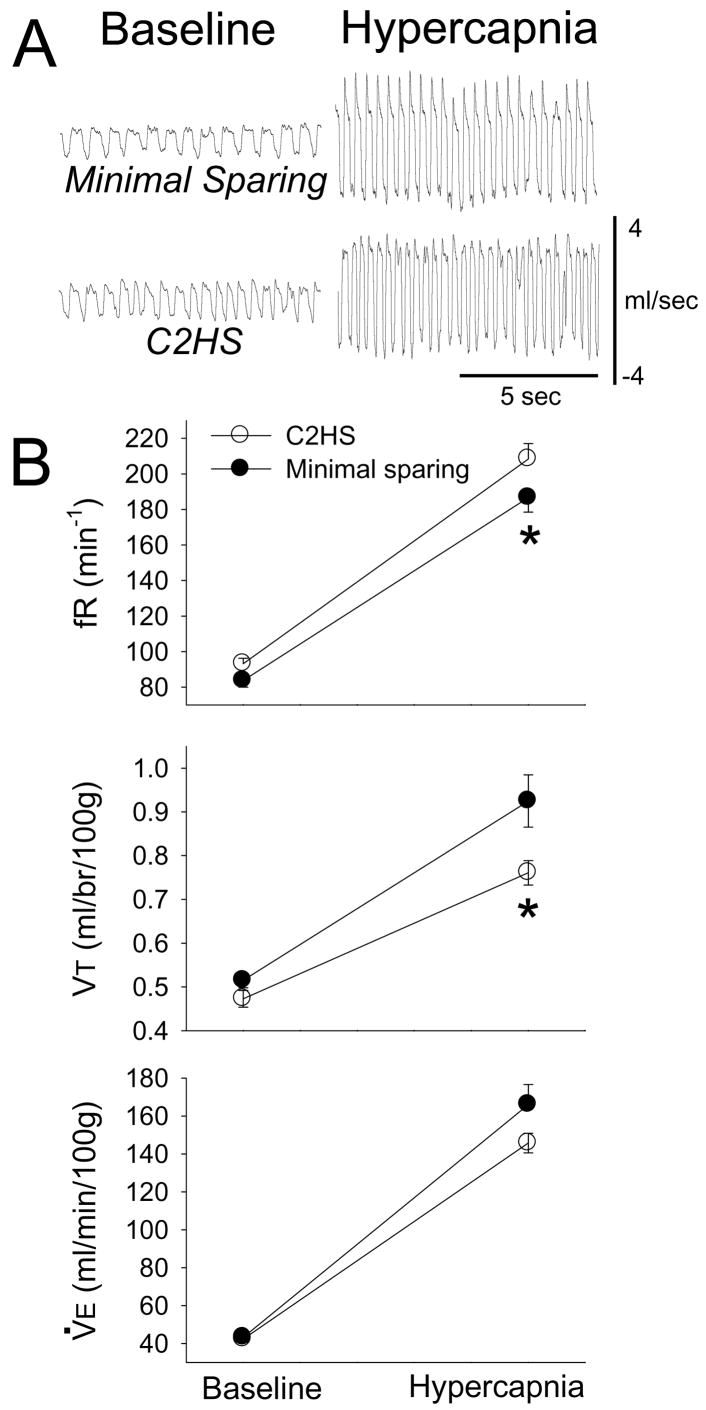
The primary intent of these experiments was to determine if minimal sparing of VM tissue would lead to altered ventilation relative to complete C2HS rats. Examples of plethysmography data used to assess ventilation are presented in Fig. 2A. Body weight (for discussion, see Fuller et al., 2008) was similar between C2HS (370±9 g) and minimal sparing rats (369±6; p=0.99). Breathing frequency (fR, breath•min−1) was greater in C2HS rats vs. those with minimal sparing (Fig. 2B; F1,14=4.780, p=0.046). Post-hoc tests indicated that baseline fR did not differ between groups (p=0.314), whereas fR was greater in C2HS rats during hypercapnia (p=0.014). Analyses of tidal volume (VT) revealed a significant interaction (F1,14=5.908, p=0.029) between group (i.e. C2HS or minimal sparing) and condition (i.e. baseline or hypercapnia) (Fig. 2B). Specifically, differences between groups were not observed during baseline (p=0.408). On the other hand, minimal sparing rats had a greater VT during hypercapnic challenge (p=0.003; Fig. 2B). Baseline minute ventilation (V̇E) was comparable between groups (F1,14=2.235, p=0.149). As expected, hypercapnia caused a significant increase in fR, VT, and V̇E in all rats (Fig. 2B, all p<0.001). The results of additional plethysmography data analysis are presented in Table 1. Of note, inspiratory duration (TI) was shorter in C2HS compared to minimal VM sparing rats.
Figure 2. Ventilation during baseline and hypercapnic challenge (7% CO2) in rats with anatomically complete C2HS injury vs. minimal VM tissue sparing.
Panel A presents representative airflow traces depicting in breathing in unanesthetized rats. Scaling is identical in all panels. Panel B presents mean inspiratory frequency (fR), tidal volume (VT) and minute ventilation (V̇E). See text for description of 2-way repeated measures ANOVA results. *, significantly different than the C2HS group
Table 1. Ventilation data recorded during baseline and hypercapnic challenge in C2 hemilesioned rats with anatomically complete C2HS and minor sparing of VM tissue.
Values are means ± SE. The results of the two-way repeated measures ANOVA are provided for group (C2HS vs. sparing), condition (baseline vs. hypercapnia), and the interaction term. TI, inspiratory duration; TE, expiratory duration; VT/TI, mean inspiratory airflow rate; TI:TTOT, ratio of inspiratory duration to total breath duration; PIF, peak inspiratory airflow rate; PEF, peak expiratory airflow rate
| Condition | Minimal Sparing | C2HS | P-value group | P-value condition | P-value interaction | |
|---|---|---|---|---|---|---|
|
TI
(sec) |
Baseline
Hypercapnia |
0.27±0.01
0.15±0.01 |
0.24±0.01
0.14±0.01 |
0.047 | <0.001 | 0.225 |
|
TE
(sec) |
Baseline
Hypercapnia |
0.47±0.03
0.21±0.01 |
0.43±0.02
0.19±0.01 |
0.172 | <0.001 | 0.729 |
|
VT/TI
(ml/sec) |
Baseline
Hypercapnia |
7.1±0.3
22.4±1.4 |
7.3±0.3
20.7±1.0 |
0.457 | <0.001 | 0.262 |
| TI:TTOT | Baseline
Hypercapnia |
0.37±0.01
0.42±0.00 |
0.36±0.01
0.42±0.01 |
0.732 | <0.001 | 0.323 |
|
PIF
(ml/sec) |
Baseline
Hypercapnia |
11.4±0.1
31.9±1.7 |
11.2±0.5
30.0±1.5 |
0.437 | <0.001 | 0.435 |
|
PEF
(ml/sec) |
Baseline
Hypercapnia |
8.1±0.6
26.0±1.7 |
7.4±0.3
23.0±0.8 |
0.151 | <0.001 | 0.156 |
The plethysmography data were complemented by neurophysiological recordings of phrenic nerve activity in anesthetized rats (e.g. Fig. 3A). Mean arterial blood pressure (MAP) was different between C2HS and minimal sparing rats (Table 2; F1,14=5.385, p=0.036). Consistent with prior reports (Doperalski et al., 2008; Fuller et al., 2008), hypoxia caused a reduction in MAP (F1,14=30.257, p<0.001). Post-hoc tests indicated that MAP was higher in minimal sparing rats during hypoxia (p=0.011) but not during baseline (p=0.189). Blood gases were within physiologically normal ranges during baseline, and arterial O2 (PaO2) and CO2 (PaCO2) partial pressures were not significantly different between groups. However, arterial pH was lower in C2HS rats (p=0.007, see Table 2).
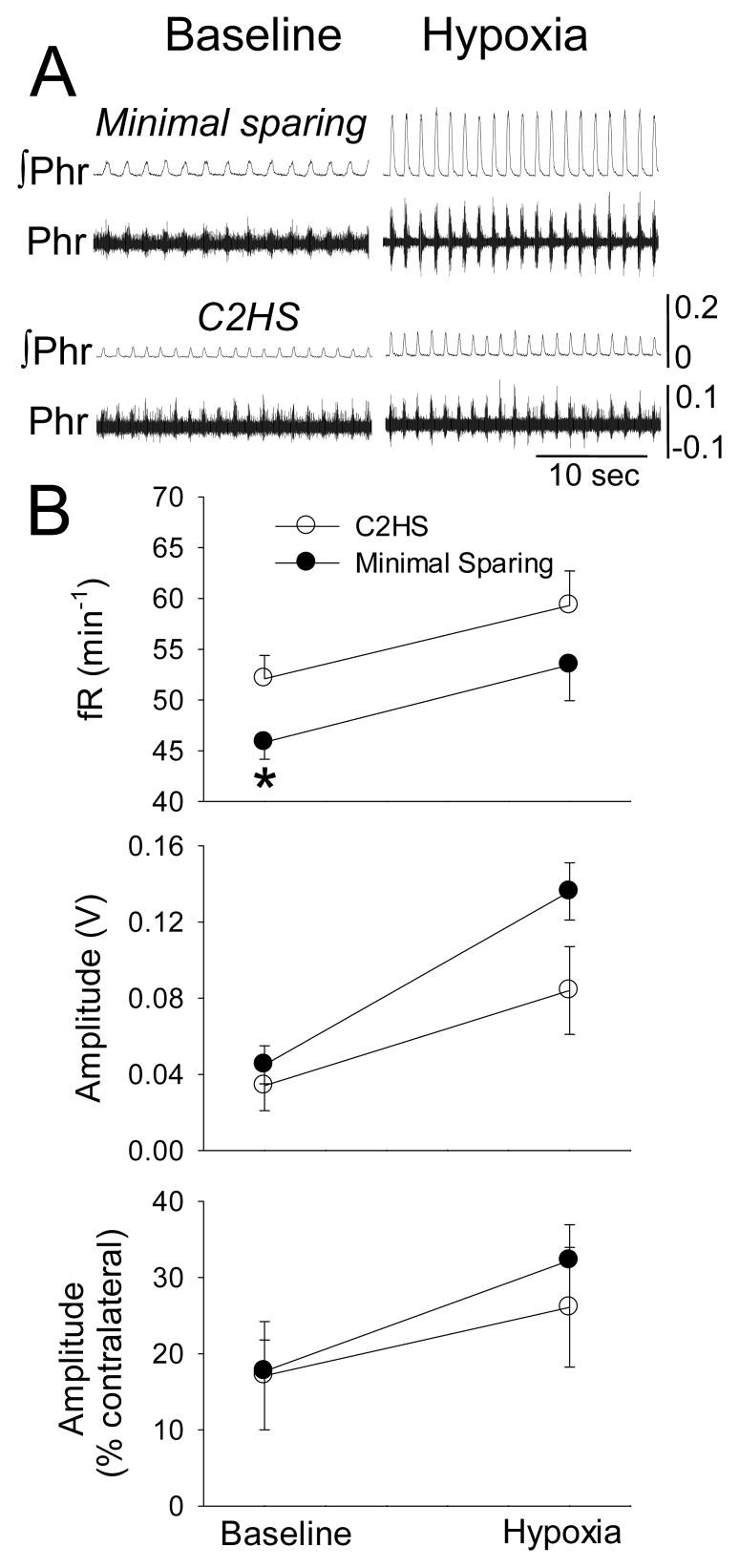
Figure 3. Ipsilateral phrenic bursting during baseline and hypoxic challenge in rats with anatomically complete C2HS injury vs. minimal VM tissue sparing.
Panel A shows representative examples raw (Phr) and integrated (∫Phr) phrenic bursting recorded ipsilateral to C2 lesion. Panel B presents mean burst frequency (fR) and amplitude. Burst amplitude data are presented as raw amplitude (V) or relative to the contralateral burst (%contralateral). See text for description of 2-way repeated measures ANOVA results. *, significantly different than the C2HS group
Table 2. Mean arterial blood pressure (MAP) and arterial blood gases recorded during neurophysiology experiments in anesthetized rats.
Values are means ± SE. Data were obtained during baseline conditions and brief hypoxic challenge. The results of the two-way repeated measures ANOVA are provided for group (C2HS vs. sparing), condition (baseline vs. hypercapnia), and the interaction term.
| Condition | Minimal Sparing | C2HS | P-value group | P-value condition | P-value interaction | |
|---|---|---|---|---|---|---|
|
MAP
(mmHg) |
Baseline
Hypoxia |
119±11
93±13 |
99±9
52±9 |
0.036 | <0.001 | 0.128 |
|
PaO2
(mmHg) |
Baseline
Hypoxia |
119±4
32±2 |
121±10
31±2 |
0.885 | <0.001 | 0.840 |
|
PaCO2
(mmHg) |
Baseline
Hypoxia |
36.4±1.5
33.7±2.0 |
35.3±1.0
34.7±2.7 |
0.709 | 0.342 | 0.969 |
| pH | Baseline
Hypoxia |
7.40±0.02
7.39±0.01 |
7.36±0.01
7.35±0.01 |
0.014 | 0.578 | 0.725 |
Phrenic bursting ipsilateral to hemilesion injury was observed in all rats. The overall phrenic burst frequency was reduced with minimal VM white matter sparing vs. C2HS rats (F1,14=5.180, p=0.039, Fig. 3B). Hypoxia caused an increase in burst frequency (F1,14=11.995, p=0.004) that was similar between groups (C2HS: +7±4 burst•min−1; minimal sparing: +10±3 burst•min−1). The amplitude of inspiratory ∫Phr bursting was quantified with several different approaches (see Methods). During baseline, between-group differences were not observed regardless of quantification approach (Fig. 3B). As expected, hypoxia was associated with increased ∫Phr burst amplitude (F1,14=90.136, p<0.001). However, the increase in ∫Phr burst amplitude (i.e. hypoxia-baseline [Δv]) was greater in rats with minimal sparing (unpaired t-test, p=0.005). In contrast, ipsilateral ∫Phr burst amplitude during hypoxia did not differ between groups when expressed as a percentage of the contralateral burst (p=0.345, Fig. 3) or relative to the baseline burst amplitude (C2HS: 216±39%, minimal sparing: 305±72%; p=0.146).
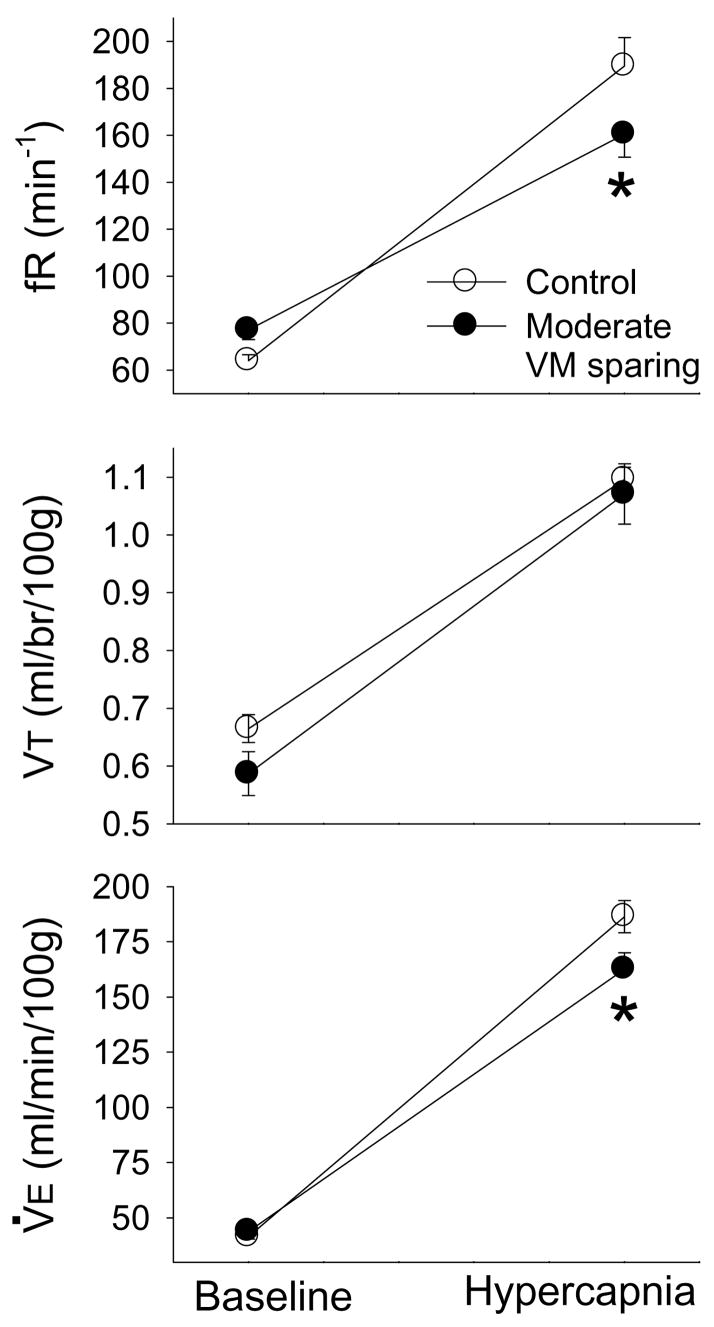
3.3. Ventilation in spinal intact vs. C2 hemilesion with moderate VM tissue sparing
The intent of these experiments was to determine whether deficits in ventilation are also exhibited after cervical hemilesions with more extensive sparing of ventral white matter (Figs. 1A, C) (see introduction). Body weight was not significantly different between the two groups studied: moderate sparing = 376±7 g; uninjured control rats = 359±10 g (p=0.168). There was a significant interaction for fR (breath•min−1) between group (i.e. intact or moderate sparing) and condition (i.e. baseline or hypercapnia) (F1,14=10.02, p=0.007). This interaction is evident in Fig. 4: baseline fR tended to be higher in lesioned rats (p=0.247) whereas hypercapnic fR was greater in uninjured rats (p=0.007). VT was not statistically different between the two groups (F1,14=863, p=0.369) although baseline VT tended to be higher in uninjured vs. moderate sparing rats (Fig. 4, p=0.195). Similar to the fR results, a significant interaction was observed in the V̇E data (F1,14=9.72, p=0.008). This was evident by the similar V̇E during baseline (p=0.713), but a markedly reduced V̇E during hypercapnic conditions in the lesioned rats (p=0.002). Analyses of additional plethysmography variables are shown in Table 3. An interaction was observed for inspiratory duration (TI), whereby TI was similar during baseline (p=0.303) but tended to be greater in moderate VM sparing rats during hypercapnia (p=0.089, Table 3). TE was reduced during baseline in moderate VM sparing rats (p=0<0.001), but was similar during hypercapnia (p=0.527). Accordingly, a significant interaction between group and condition was also observed for TE (Table 3). The ratio of TI to total breath duration (TI:TTOT) was also different between groups (Table 3). Post-hoc tests indicated that TI:TTOT was greater in moderate VM sparing animals during baseline (p=0.005 vs. intact) but not during hypercapnia (p=0.659). An interaction was present in the analyses of peak inspiratory airflow (PIF) data as baseline PIF was similar (p=0.720) but hypercapnic PIF was reduced in lesioned rats (p=0.005). Peak expiratory airflow (PEF) was not different between the two groups (Table 3).
Figure 4. Ventilation during baseline and hypercapnic challenge (7% CO2) in uninjured control rats (○) vs. hemilesioned rats with moderate VM tissue sparing (●).
See text for description of 2-way repeated measures ANOVA results. *, significantly different than the control group
Table 3. Ventilation data recorded during baseline and hypercapnic challenge in C2 hemilesioned rats with moderate sparing of VM white matter and uninjured, control rats.
Values are means ± SE. The results of the two-way repeated measures ANOVA are provided for group (C2HS vs. sparing), condition (baseline vs. hypercapnia), and the interaction term. Ti, inspiratory duration; Te, expiratory duration; VT/TI, mean inspiratory airflow rate; TI:TTOT, ratio of inspiratory duration to total breath duration; PIF, peak inspiratory airflow rate; PEF, peak expiratory airflow rate
| Condition | Control | Moderate Lesion | P-value group | P-value condition | P-value interaction | |
|---|---|---|---|---|---|---|
|
Ti
(sec) |
Baseline
Hypercapnia |
0.28±0.01
0.15±0.01 |
0.27±0.01
0.18±0.01 |
0.673 | <0.001 | 0.022 |
|
Te
(sec) |
Baseline
Hypercapnia |
0.70±0.04
0.22±0.01 |
0.55±0.03
0.24±0.01 |
0.042 | <0.001 | 0.005 |
|
VT/TI
(ml/sec) |
Baseline
Hypercapnia |
8.6±0.5
25.9±1.4 |
8.3±0.4
23.2±1.0 |
0.228 | <0.001 | 0.199 |
| TI:TTOT | Baseline
Hypercapnia |
0.29±0.02
0.41±0.01 |
0.33±0.01
0.42±0.01 |
0.010 | <0.001 | 0.127 |
|
PIF
(ml/sec) |
Baseline
Hypercapnia |
14.0±0.9
37.8±2.2 |
13.3±0.6
31.7±1.5 |
0.050 | <0.001 | 0.036 |
|
PEF
(ml/sec) |
Baseline
Hypercapnia |
8.4±0.3
30.6±1.5 |
9.6±0.5
29.1±1.1 |
0.874 | <0.001 | 0.093 |
3.4. Anterograde neuroanatomical tracing
One interpretation of the plethysmography and phrenic neurophysiology data is that neural projections in the VM portion of the injured cervical spinal cord are functionally important following incomplete lateral cervical hemilesion (Vinit et al., 2008). Accordingly, an additional experiment (n=2) was conducted in which axonal projections originating from neurons in the medullary VRC were anterogradely labeled in C2 hemilesioned rats. Specifically, we wished to determine if VRC projections could be observed in the VM portion of the C2 spinal cord ipsilateral to incomplete hemilesion. Putative BDA-labeled VRC projections were noted in the ipsilateral VM cord at the lesion site in both rats (see also, Lipski et al., 1994). Coupled with evidence of spared fibers seen in plastic sections, the possibility exists that even minimally spared VM white matter may contain a contingent of VRC axons. Selection of operated animals for this experimental subset was done blindly, and subsequent assessment of the lesions showed moderate white matter sparing. We recognize that a more comprehensive series of studies examining similar labeling in rats with minor ventromedial sparing will ultimately be needed. Of note, we also observed labeled axons crossing the spinal midline as originally reported by Goshgarian et al. (1991) (data not shown). These crossed spinal projections are postulated to be part of the anatomical substrate for the CPP (Goshgarian et al., 1991; Lane et al., 2008a, 2008b). Interestingly, those axons were not seen coursing into VM white matter but instead towards the contralateral ventral gray matter.
3.5. Relationship between phrenic burst amplitude and spared tissue at the lesion epicenter
The relationship between ipsilateral ∫Phr burst amplitude and the severity of the cervical hemilesion was examined using linear regression analyses (Table 4). Our goal was to determine the degree to which ipsilateral ∫Phr output (see Methods) was associated with the amount of preserved ipsilateral white matter at the injury site. Thus, the dependent variable for this analysis was ipsilateral ∫Phr bursting (expressed as V, %contralateral, or normalized to baseline values), and the independent variable was the amount of spared ventral white matter at the lesion epicenter. The amount of spared white matter predicted a significant amount of the variance in ∫Phr burst amplitude expressed as raw amplitude (i.e. V) or relative to the contralateral burst (i.e. %contralateral) (Table 4). Similar results were observed regardless of whether the independent variable (spared white matter volume) was expressed relative to the area of the contralateral cord (%contralateral) or in absolute units (i.e. μm2, data not shown).
Table 4. Regression analysis of ipsilateral phrenic nerve burst amplitude and the histological area of cervical spinal cord injury.
Phrenic data were obtained during two conditions: baseline and hypoxic challenge. The area of remaining white matter (see Fig. 1) in the ipsilateral spinal cord at the lesion epicenter was the independent variable and was expressed relative to the area measured in the contralateral spinal cord (see methods). The amplitude of the phrenic burst was expressed as a raw voltage (V), relative to the contralateral burst (%contralateral) or as a percent of the hypoxic response or baseline output.
| Dependent Variable (Y) | Coefficient of Determination (R2) | F-statistic | P-value |
|---|---|---|---|
| BL ∫Phr
(V) |
0.393 | 13.6 | 0.001 |
| BL ∫Phr
(% contralateral) |
0.527 | 23.4 | <0.001 |
| BL ∫Phr
(% hypoxia) |
0.096 | 2.3 | 0.151 |
| Hypoxia ∫Phr
(V) |
0.431 | 15.9 | <0.001 |
| Hypoxia ∫Phr
(% contralateral) |
0.539 | 24.5 | <0.001 |
| Hypoxia ∫Phr
(% baseline) |
0.063 | 1.4 | 0.249 |
4. Discussion
4.1. Medial tissue sparing and respiratory output
The results of the present study show that relatively minimal VM white matter sparing can influence the degree of respiratory deficit induced by a high cervical hemisection in the adult rat. Specifically, we show greater VT in subtotal vs. complete C2HS rats during increased respiratory drive associated with hypercapnia. In addition, fR was reduced with minimal white matter sparing compared to complete C2HS rats as assessed with both plethysmography and neurophysiology. Accordingly, even the presence of some medially located fibers appears sufficient enough to mediate a functionally different outcome after cervical hemilesion, particularly during physiological states leading to elevated respiratory drive. Failure to take this into account could lead to misinterpretation of functional recovery after cervical hemilesion.
These findings, however, do not negate a potential role of the CPP in respiratory function after incomplete C2HS. Indeed the CPP may provide an additive effect after such lesions by enhancing the physiological contribution of spared VM pathways (Baussart et al., 2006). The relative percent of spared bulbospinal inputs to phrenic motoneurons in our minimal VM sparing group was not determined. However, Lipski et al. (1994) observed that of a total of 16 VRC neurons identified with intracellular recordings, three (19%) had axonal projections in close proximity to the VM fissure (see Fig. 2 in Lipski et al., 1994). Our supplemental anterograde labeling experiments were consistent with Lipski’s data. Specifically, BDA-positive processes were observed in the VM cervical spinal cord following bilateral injections of this anterograde tracer in the area of the VRC. Thus, bulbospinal projections in the VM cord presumably underlie our observations of different breathing patterns between rats with complete C2HS vs. those with minimal VM sparing at C2.
Activation of these medially located projections to phrenic motoneurons appears to depend critically on the post-lesion time interval suggesting they contribute to recovered rather than spared respiratory function. For example, Vinit et al. (2008) reported that ipsilateral phrenic output is absent seven days after an incomplete hemilesion (similar to our “moderate VM sparing lesion”. However, at three months post-injury, robust ipsilateral phrenic bursting was observed, and this bursting persisted after hemisection of the contralateral spinal cord at C1. The authors concluded that inputs to phrenic motoneurons in the medial portion of the spinal cord are strengthened over time post-injury. To what extent this reflects a form of neuroplasticity, remyelination, or some other endogenous repair mechanism is unknown.
Our data also indicate that lateral C2 hemilesions with greater tissue sparing than described above (i.e. “moderate sparing”, Fig. 1) cause enduring changes in ventilation. Prior work has shown that similar white matter loss attenuates phrenic motor output in anesthetized rats (Baussart et al., 2006; Vinit et al., 2006, 2007), but it has not previously been determined if ventilation was impacted. Ventilation will not necessarily be altered after such lesions as rats can compensate for unilateral lesions with increased reliance on contralateral motor pathways (Doperalski and Fuller, 2006; Golder et al., 2001, 2003; Muir et al., 1998). Nevertheless, our data suggest that rats with moderate sparing are unable to fully compensate during a respiratory challenge, at least when studied at 1 mo post-injury. An examination of breathing at later time points may have indicated additional recovery. For example, several reports note that phrenic motor output is progressively enhanced over weeks to months after lateral cervical SCI (Nantwi et al., 1999; Golder and Mitchell, 2005; Vinit et al., 2007), and Choi et al. (2005) demonstrated full recovery of hypercapnic ventilatory responses at six weeks post- C2 hemicontusion injury in rats.
4.2. Quantifying phrenic motor output after SCI
Phrenic neurograms represent the ensemble output of ipsilateral phrenic motoneurons and are thus an important index of recovered phrenic function after SCI (especially in the context of the CPP). However, there is currently no consensus opinion regarding the optimal method for presenting phrenic neurogram data obtained from animals with cervical SCI. A simple approach is to report the unprocessed (“raw”) phrenic burst amplitude (i.e. absolute voltage; Fuller et al. 2005; Golder et al., 2005). However, technical issues which may vary between experiments have the potential to influence the raw burst and thus confound interpretations (Eldridge, 1975). Burst amplitude is therefore usually expressed relative to another parameter (i.e. “normalized” bursting). For example, the phrenic burst can be normalized relative to a maximum (i.e. bursting during a chemical respiratory challenge; Fuller et al. 2005) or a minimum (i.e. baseline bursting; Fuller et al., 2008; Golder et al., 2008). However, if the ability to recruit phrenic motoneurons is impaired (e.g. after SCI), then normalizing phrenic activity to a maximum (or minimum) may have the unintended effect of eliminating physiologically meaningful differences between experimental groups. Another approach is to express ipsilateral phrenic bursting relative to bursting recorded in the contralateral phrenic nerve (Nantwi et al., 1999; Vinit et al., 2008) or to bursting recorded in uninjured control animals (Nantwi et al., 1999). However, these procedures are subject to the same concerns raised above regarding raw phrenic burst amplitude. Normalization procedures also are hindered in cases where ipsilateral phrenic bursting is absent during conditions of low respiratory drive (i.e. when baseline ∫Phr amplitude = 0). In these cases, subsequent normalization to baseline (e.g. “% baseline”) is impossible.
Our bias has thus been that it is more effective to present both the raw (i.e. voltage) and normalized burst amplitudes (see Fuller et al., 2005; Golder and Mitchell, 2005; Doperalski et al., 2008). Here we observed that the raw signal was significantly correlated with the amount of tissue (white matter) sparing at the lesion site (Table 4). These data are consistent with prior reports that indicate non-normalized phrenic burst amplitude (Golder and Mitchell 2005) or the ratio of non-normalized ipsilateral and contralateral phrenic burst amplitude (“%contralateral”; Nantwi et al. 1999, Vinit et al. 2007) track phrenic recovery better than normalized data. For example, Golder and Mitchell (2005) report that ipsilateral ∫Phr bursting (V) grows stronger over time post-C2HS, but this effect was abolished when data were expressed as percentage of maximum bursting. Taken together, the available data support the inclusion of raw phrenic burst amplitudes as one component of a comprehensive analysis of phrenic recovery after SCI.
Acknowledgments
The authors thank Heather Carr, Sandy Walker and Whitney Bour for technical assistance. These studies were supported by the Christopher Reeve Paralysis Foundation (DDF), the Oscar and Anne Lackner Chair in Medicine (PJR), and grants from the National Institute of Neurological Disorders and Stroke RO3 NS050684 (DDF), NIH 1 R01 NS054025 (PJR), and the Craig H. Neilsen Foundation (MAL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baussart B, Stamegna JC, Polentes J, Tadie M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–74. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25:4550–9. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats Resp Physiol Neurobio. 2008;162:160–7. doi: 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Duffin J, Li YM. Transmission of respiratory rhythm: midline-crossing connections at the level of the phrenic motor nucleus? Respir Physiol Neurobiol. 2006;153:139–47. doi: 10.1016/j.resp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975;39:567–74. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2005;100:800–6. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–6. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–9. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–32. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–42. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–25. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–9. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Kastner A, Gauthier P. Are rodents an appropriate pre-clinical model for treating spinal cord injury? Examples from the respiratory system. Exp Neurol. 2008;213:249–56. doi: 10.1016/j.expneurol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory Plasticity and Spinal Cord Injury. Trends in Neuroscience. 2008a;31:538–47. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical pre-phrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–31. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–84. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse-The crossed phrenic phenomenon. Exp Neurol. 2006;200:486–95. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- Muir GD, Katz SL, Gosline JM, Steeves JD. Asymmetric bipedal locomotion--an adaptive response to incomplete spinal injury in the chick. Exp Brain Res. 1998;22:275–82. doi: 10.1007/s002210050515. [DOI] [PubMed] [Google Scholar]
- Nantwi K, El-bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchell GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–16. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–47. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci. 2003;23:4182–9. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–46. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25:3551–60. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Darlot F, Stamegna JC, Sanchez P, Gauthier P, Kastner A. Long-term reorganization of respiratory pathways after partial cervical spinal cord injury. Eur J Neurosci. 2008;27:897–908. doi: 10.1111/j.1460-9568.2008.06072.x. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol. 2007a;209:399–406. doi: 10.1016/j.expneurol.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007b;30:319–30. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]