Abstract
The primary aims of this trial are: 1) to compare surgical outcomes following sacrospinous ligament fixation to uterosacral vaginal vault suspension in women undergoing vaginal surgery for apical or uterine pelvic organ prolapse and stress urinary incontinence and 2) to examine the effects of a structured perioperative program consisting of behavioral techniques and pelvic floor muscle training compared to usual care. This trial is performed through the Pelvic Floor Disorders Network (PFDN), which is funded by National Institute of Child Health and Human Development. Subjects will be enrolled from hospitals associated with seven PFDN clinical centers across the United States. A centralized biostatistical coordinating center will oversee data collection and analysis. Two approaches will be investigated simultaneously using a 2×2 randomized factorial design: a surgical intervention (sacrospinous ligament fixation versus uterosacral vaginal vault suspension) and a perioperative behavioral intervention (behavioral and pelvic floor muscle training versus usual care). Surgeons have standardized essential components of each surgical procedure and have met specific standards of expertise. Providers of the behavioral intervention have undergone standardized training. Anatomic, functional, and health-related quality of life outcomes will be assessed using validated measures by researchers blinded to all randomization assignments. Cost-effectiveness analysis will be performed using prospectively collected data on health care costs and resource utilization. The primary surgical endpoint is a composite outcome defined by anatomic recurrence, recurrence of bothersome vaginal prolapse symptoms and/or retreatment and will be assessed 2 years after the index surgery. Endpoints for the behavioral intervention include both short-term (6-month) improvement in urinary symptoms and long-term (2-year) improvement in anatomic outcomes and prolapse symptoms. This article describes the rationale and design of this randomized trial, focusing on several key design features of potential interest to researchers in the field of female pelvic floor disorders and others conducting randomized surgical trials.
Keywords: pelvic organ prolapse, vaginal prolapse, uterine prolapse, stress urinary incontinence, randomized surgical trial, factorial design, pelvic floor muscle training, behavioral therapy, sacrospinous ligament fixation, uterosacral ligament suspension
Introduction
Pelvic floor disorders, including pelvic organ prolapse (a condition in which the uterus, vagina, bladder and/or rectum bulge into or outside of the vagina) and urinary incontinence (involuntary urinary leakage), are common in women. One in nine American women will undergo at least one surgery for prolapse and/or urinary incontinence by the age of 80.[1] Within five years of their first surgery, approximately 13% undergo a repeat operation, and over their lifetime, as many as 29% will undergo another surgery for prolapse or a related condition.[1, 2] Given these high rates of initial and repeat surgery, there is clearly a need for high quality trials to improve surgical management strategies.
While prolapse surgery can be performed through an abdominal or vaginal route, current data suggest the preferred route for most prolapse surgery in the United States is vaginal, with as many as 80%-90% of surgeries being performed through this approach.[1, 3, 4] Prolapse often involves a combination of support defects, but loss of apical support is usually present in women with more advanced degrees of prolapse that extends beyond the hymen.[5-7]
There is growing recognition that adequate support of the vaginal apex is an essential component of a durable surgical repair for women with advanced prolapse.[5, 8-10] Numerous vaginal surgical procedures have been described for treatment of apical prolapse, with two of the most popular being the sacrospinous ligament fixation (SSLF) and the uterosacral vaginal vault suspension (ULS). The use of these procedures varies geographically and by training.
The SSLF procedure attaches the vaginal apex to the sacrospinous ligament either unilaterally or bilaterally, typically using an extraperitoneal approach. Available data suggests that while apical recurrence after SSLF is infrequent (<10%), recurrence of anterior vaginal prolapse affects approximately 30% of patients. [11-23] The ULS procedure attaches the vaginal apex to the uterosacral ligaments using an intraperitoneal approach. Data from uncontrolled case series have been used to suggest that the ULS may have greater anatomic success than SSLF, particularly with regard to the anterior segment.[8, 24-28] Unfortunately, no comparative data exist to provide information about which technique is safer, more durable, and/or provides greater symptomatic relief. While both surgical techniques are clinically useful, it is essential to establish whether one is better, to optimize current clinical care, as well as inform the design of future trials, possibly comparing traditional vaginal apical repairs with mesh-augmented repairs or comparing routes of surgery (abdominal vs. vaginal).
Behavioral therapy, including pelvic floor muscle training (PMT) with or without biofeedback is an effective therapy for stress urinary incontinence (SUI), urge urinary incontinence, and fecal incontinence with almost no adverse consequences.[33-36] There is growing interest in evaluating behavioral and physical therapies as an adjunct to prolapse surgery in order to minimize pelvic floor disorders symptoms postoperatively and perhaps even improve anatomic outcomes of the prolapse surgery.[36-39] One published study reported results of perioperative PMT in women undergoing prolapse surgery, finding fewer urinary symptoms and better quality of life after surgery among women receiving perioperative PMT compared to a control group receiving usual care.[38]
The principal aims of the Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trial are 1) to compare surgical outcomes after SSLF versus ULS and 2) to assess the role of perioperative behavioral and pelvic floor muscle training versus usual care in women undergoing vaginal surgery for apical or uterine prolapse and SUI using a 2×2 randomized factorial design. The purpose of this paper is to describe the rationale, design and challenges of planning the trial, focusing on several key design features of interest to researchers in the field of female pelvic floor disorders and others conducting randomized surgical trials.
Methods
Design Overview
The OPTIMAL trial is a collaborative multi-centered surgical trial performed by the Pelvic Floor Disorders Network (PFDN), a cooperative network of investigators from seven clinical sites and a Data Coordinating Center (DCC) supported by the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) (Appendix). This protocol was developed as a collaborative effort of members from all seven PFDN clinical sites and the DCC. All participating sites in the PFDN, received institutional review board approval for this randomized surgical trial.
The OPTIMAL trial compares SSLF to ULS with or without perioperative behavioral and pelvic floor muscle training (BPMT) in women undergoing vaginal surgery for Stage 2-4 pelvic organ prolapse and SUI using a 2×2 factorial study design. The overall design is shown in Figure 1. A standardized common protocol for enrollment, treatment and data collection will be employed by all sites and coordinated by the DCC. Women with Stage 2-4 prolapse and SUI symptoms that plan vaginal surgery for treatment of prolapse of the vaginal apex (with or without a uterus) will be approached for enrollment. Each enrolled subject will undergo two distinct randomizations: (1) the method of vaginal apical suspension to either SSLF or ULS (Surgery Intervention) and (2) BPMT vs. usual care during the perioperative period. The use of a factorial design will allow us to evaluate these two independent interventions in a single study population.
Figure 1.
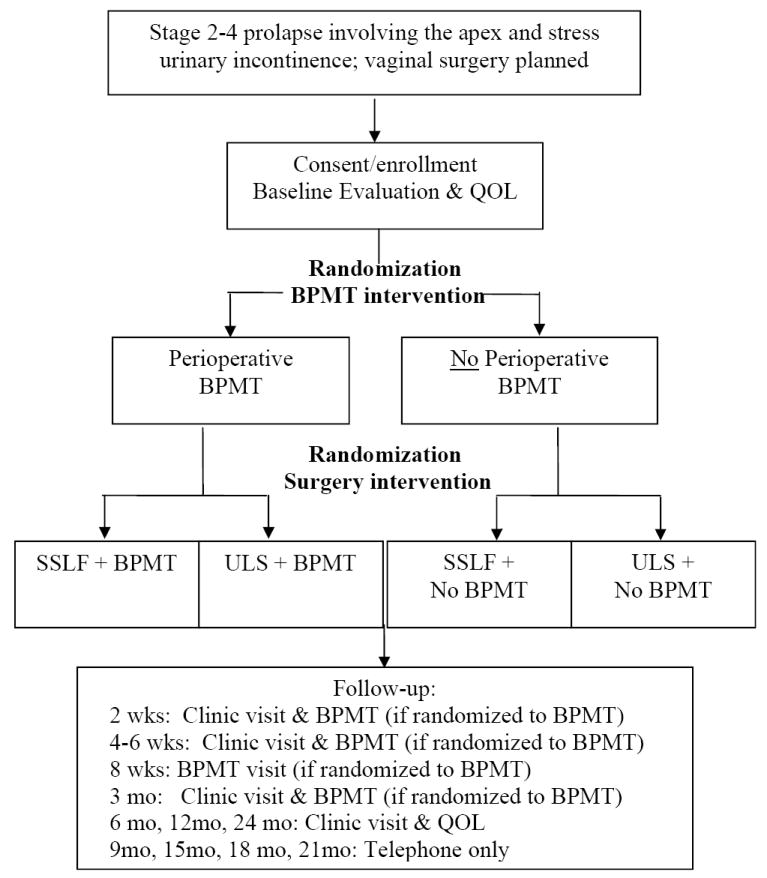
Study Flow Chart. BPMT = behavioral and pelvic floor muscle training; QOL = quality of life assessment by the data coordinating center’s Quality of Life Interviewing Center; SSLF, sacrospinous ligament fixation; ULS, uterosacral ligament suspension
Prior to surgery, the surgeon will describe their intended concomitant surgery for prolapse. Following randomization, subjects will receive the allocated vaginal vault suspension and any additional vaginal prolapse surgery, as clinically indicated prior to randomization. All subjects will receive a Tension-Free Vaginal Tape (TVT®) (Ethicon Women’s Health and Urology, Somerville, NJ) for surgical management of their SUI. The primary objective of the Surgery Intervention is to compare the anatomic success of vaginal prolapse surgery using SSLF to surgery using ULS 2 years postoperatively. Secondary objectives include comparing the change in pelvic floor disorders symptoms, sexual function and health-related quality of life (HRQOL) and the incidence of perioperative complications.
Subjects who are randomized to receive Perioperative Intervention will participate in a formal individualized BPMT program that begins two to four weeks prior to surgery and continues for three months after surgery. The primary short-term objective of the Perioperative Intervention is to evaluate the efficacy of perioperative training for reducing urinary symptoms six months after vaginal surgery for prolapse. The primary long-term objective of the Perioperative Intervention is to assess the efficacy of perioperative training on prolapse symptoms and anatomic outcomes two years after surgery. Secondary objectives include evaluating the effect of perioperative BPMT on postoperative pain and return to normal activities, as well as the short-term (six month) effect on sexual function and HRQOL. We also plan to evaluate the long-term (two year) effect of perioperative BPMT on other pelvic floor disorders symptoms, sexual function, and HRQOL. Additionally, there is scant information on the cost-effectiveness of perioperative BPMT; in this trial, we will also compare the cost-effectiveness of perioperative intervention with usual care in women undergoing prolapse surgery.
Study Population
The study population will consist of adult women with Stage 2-4 prolapse and coexisting SUI symptoms who have prolapse at the vaginal apex (with or without a uterus) and have opted for vaginal surgery for prolapse repair. We will document the symptomatic impact of prolapse by requiring that subjects have an affirmative response to one of two questions from the Pelvic Floor Distress Inventory (PFDI)[40]. Anatomically, participants must have at least Stage 2 prolapse that includes prolapse of the vaginal apex at least half-way into the vaginal canal using the Pelvic Organ Prolapse Quantification (POPQ) system.[41] Subjects must also have SUI symptoms (demonstrated by an affirmative response to one or more of the three items on the PFDI stress subscale) and have documentation of a positive office stress test or urodynamic stress incontinence[42] in the previous 12 months. Because prior prolapse surgery alters vaginal topography and is an independent risk factor for prolapse recurrence, subjects who have previously undergone either of the two surgical interventions (SSLF or ULS) will be excluded. Similarly, because of an altered risk/benefit ratio for patients with SUI symptoms despite a previous synthetic sling for SUI, these potential participants are excluded. Subjects are also excluded if they have a clinical contraindication to SSLF, ULS, TVT or a pelvic floor muscle strengthening program in the opinion of their surgeon. The protocol requires that there be sufficient time prior to the planned surgical procedure (at least two weeks, but not more than four weeks) to schedule the preoperative BPMT visit for those who are randomized to this intervention. A complete list of the inclusion and exclusion criteria is summarized in Table 1.
Table 1.
Protocol Inclusion and Exclusion Criteria
Inclusion Criteria
|
Exclusion Criteria
|
Baseline Assessment
After enrollment, standardized baseline data collection will include: demographics, current medications, a medical history and a physical examination that includes POPQ evaluation[41] and pelvic floor muscle strength assessment using the Brinks standardized grading system.[43] A series of validated instruments will be administered via standardized telephone interviews to assess pelvic symptoms, sexual function and condition-specific and generic HRQOL. Standardized interviews will be performed by trained female interviewers, blinded to treatment assignments, at the DCC’s centralized Quality of Life Interviewing Center at the DCC and will include the following instruments: 1) PFDI [40], 2) Pelvic Floor Impact Questionnaire (PFIQ) [40], 3) Incontinence Severity Index [44], 4) Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire short form (PISQ-12)[45], 5) EuroQOL (EQ-5D)[46], and 6) a body image scale [47, 48]. Pre-intervention measures of pain and functional activity will be assessed by the Surgical Pain Scale [49] and Activity Assessment Scale [50]. Additionally, the Medical Outcome Study Short Form 36 (SF-36) [51] will be collected by study personnel at the baseline visit.
Randomization
Eligible consenting subjects will each undergo two distinct randomizations with equal probability of assignment to one of the two groups for each intervention (Surgery Intervention: SSLF or ULS; Perioperative Intervention: perioperative BPMT or usual care).
Randomization for the Perioperative Intervention will take place preoperatively to allow for scheduling of the preoperative training visit and will be stratified by clinical site. Separate randomization schedules will be generated by the DCC for each clinical site using a random permutated block design. The DCC will provide treatment allocations to the Clinical Sites in sequentially numbered, sealed, opaque envelopes, such that each enrolled subject will be assigned two sealed envelopes (one for each intervention).
Randomization for the Surgical Intervention will take place in the operating room on the day of surgery and will be stratified by surgeon and by performance of concomitant hysterectomy. Although all study surgeons have experience and skill with both surgical approaches, differences in training and experience may impact the study outcomes. Similarly, we will stratify by concomitant hysterectomy because some study surgeons believe concomitant hysterectomy may favor localization of the uterosacral ligaments, improving outcomes from ULS in this setting.
Blinding
Because the study surgeon will be providing clinical care to subjects, blinding the surgeon to treatment allocation or subject symptoms is not practical or feasible. However, it is our intent that when feasible and ethical, all outcomes assessors and the subjects will be blinded to the surgical treatment allocation. To help maintain blinding, the procedure summary in the medical record will not state the specific apical procedure, instead listing the non-study procedures and “apical suspension per OPTIMAL protocol.” While the details of the actual procedure will be readily available within the text of the operative report, study personnel are alerted to maintain blinding by the procedure summary. Study surgeons will instruct housestaff to limit their post-operative notes to similar text that does not unmask the hospital team, which can inadvertently unblind the subject.
For the Perioperative Intervention, neither the subject nor the study coordinator will be blinded to the allocation. It is not feasible to blind the study coordinator because of the scheduling requirements in those subjects undergoing perioperative training. The telephone interviewer will remain blinded for the duration of the study. For the Surgery Intervention, the subject, study coordinator and telephone interviewer will be blinded to the assignment for the duration of the study. Additionally, all postoperative POPQ evaluations and assessments of pelvic floor muscle strength will be performed by a trained examiner (Evaluator) who is blinded to both allocations and who is not the operating surgeon. All BPMT providers (Interventionists) will be blinded to the Surgery Intervention treatment allocation. A summary of masking for each of the two study interventions is shown in Table 2.
Table 2.
Summary of Blinding
| Blinding | Surgical Intervention | BPMT Intervention |
|---|---|---|
| Subject | Yes | No |
| Study coordinator | Yes | No |
| Telephone interviewer* | Yes | Yes |
| Study surgeon | No | Yes |
| Evaluator** | Yes | Yes |
| Interventionist# | Yes | No |
BPMT, behavioral and pelvic floor muscle training
Telephone interviewer: individual from the Quality of Life Interviewing Center, Data Coordinating Center, University of Michigan
Evaluator: the individual(s) at the clinical sites performing outcome assessments
Interventionist: the individual(s) at the clinical sites providing the PMT Intervention
Surgical Intervention
In order to maximize the generalizability of our study while maintaining standardization within surgical intervention, the protocol requires strict standardization of essential components of the surgical technique for the two treatment arms (SSLF and ULS) and the stress incontinence surgery (TVT), while allowing individual surgeons some flexibility in the use and technique of concomitant prolapse surgery. These components were developed with the input of non-network expert surgeons who have published their case series for the study procedures.
Participating study surgeons are required to have performed a minimum of 20 of each vault suspension procedure in their career including at least 5 of each in the 12 months prior to beginning subject enrollment. A surgical videotape illustrating essential components of each vault suspension technique was developed, reviewed as a group, and distributed to each participating surgeon along with a detailed written description of the technique and guidelines for concurrent procedures.
The SSLF procedure used for this protocol is a modification of the Michigan 4-wall technique originally described by Morley and DeLancey.[12, 16] Subjects randomized to this arm will receive a unilateral SSLF using two permanent and two delayed absorbable (four sutures total), 0 or 2-0 monofilament stitches placed in the left or right sacrospinous ligament (bilateral placement is not allowed). The ULS procedure used for this protocol is a modification of the technique originally described by Shull.[52] Subjects randomized to this arm will receive a bilateral uterosacral vault suspension with one permanent and one delayed absorbable 0 or 2-0 monofilament suture placed in each uterosacral ligament (two sutures per side; four sutures total). Midline plication of the uterosacral ligaments and/or culdoplasty is not allowed.
In the event that circumstances prohibit safe or effective completion of the assigned vault suspension procedure, surgeons will perform the alternative surgical intervention (e.g., if randomized to SSLF but SSLF can not be safely performed then a ULS is attempted). In the unlikely event that both ULS and SSLF cannot be performed safely or effectively, the choice of vaginal suspension procedure is left to the surgeon’s discretion and recorded. Cystoscopy with intravenous indigo carmine will be routinely performed after either procedure to assess bladder integrity and ureteral patency.
All concomitant procedures must be declared and recorded prior to randomization. Anterior and posterior colporrhaphies will be performed at the discretion of the surgeon such that the anterior and posterior vaginal walls are located at least 1 cm above the hymen (i.e., POPQ points Aa, Ba, Ap and Bp are less than or equal to -1 cm) at the end of the procedure. Colporrhaphies, when performed, will be performed with 2-0 or 0 monofilament delayed absorbable sutures. Use of mesh or biologic graft materials in the anterior or posterior compartment or to suspend the vaginal apex is not permitted. Women with a uterus in situ will undergo a hysterectomy, as this is the current standard of care for both vault suspension procedures. All subjects will receive a TVT placed using the standard retropubic approach for treatment of the SUI symptoms. The TVT will be positioned after completion of the apical and anterior prolapse corrective surgery. An assessment of pelvic support will be performed at the completion of the procedure by noting standard POPQ points under anesthesia without Valsalva. All concomitant procedures, whether planned or not, and intraoperative complications will be recorded.
Perioperative Intervention
Subjects randomized to the Perioperative Intervention (BPMT) will receive a formal, individualized program that includes progressive pelvic floor muscle training and exercise and education in behavioral strategies to prevent/reduce lower urinary tract and colorectal symptoms. The intervention was modeled after the program described by Jarvis et al.[38] and includes behavioral intervention components used in the PFDN’s Ambulatory Treatment for Leakage Associated with Stress Incontinence (ATLAS) study.[53]
To ensure standardization in the implementation of OPTIMAL behavioral interventions across clinical sites, all behavioral interventionists attended standardized training, including didactic lectures (on urogenital prolapse, the behavioral intervention protocol, anatomy and physiology of pelvic floor muscle function, exercise physiology and exercise progression, behavioral principles and strategies, and optimizing exercise/behavioral adherence), problem-oriented patient case discussions, and certification in the conduct of patient visits (including examination and evaluation of pelvic floor muscle function, teaching PMT and behavioral strategies to prevent or reduce lower urinary tract and colorectal symptoms, and promoting long-term adherence) by direct observation of structured role play interactions. A minimum of two trained interventionists are available at each clinical site. Interventionists include physical therapists, registered nurses and certified registered nurse practitioners.
Each subject will attend five separate sessions with the interventionists and receive one interventionist telephone call following hospital discharge. The first visit will occur 2-4 weeks prior to surgery and include evaluation of the subject’s pelvic floor muscle function via vaginal palpation, instruction on correct pelvic floor muscle exercise, and recommendations for a preoperative pelvic floor muscle exercises based on the subject’s baseline muscle function. Verbal and written instructions will be individualized with a maximum of 45 contractions per day, an initial muscle contraction duration ranging from 1-3 seconds, and a schedule to increase the contraction duration 1-2 seconds per week to a maximum of 7-seconds, as well as instructions for resuming exercises postoperatively. In addition, the interventionist will educate and provide the subject with written instructions on strategies to prevent or reduce symptoms of stress incontinence (stress strategy), urge incontinence (urge strategy), obstructive symptoms and dysfunctional voiding, (including proper toilet posture and pelvic floor muscle relaxation to facilitate bladder emptying), and colorectal symptoms and dysfunctional defecation (including management of stool consistency, proper toilet posture, pelvic floor relaxation to facilitate stool passage, and responding to natural defecation urges).
The subject will attend four postoperative visits with the interventionist (2 weeks, 4-6 weeks, 8 weeks and 3 months after surgery). At each visit, subjects will be screened for symptoms of stress incontinence, urge incontinence, urinary urgency and frequency, voiding dysfunction, and defecatory dysfunction. If a subject is not experiencing any of these symptoms, the emphasis of the visit will be PMT instruction and education on maintaining healthy bowel and bladder habits. If a subject reports one or more of these symptoms, then behavioral techniques that address these symptoms will be emphasized during the visit in addition to PMT instruction.
During the postoperative visits, the interventionist will reexamine the subject’s pelvic floor muscle function to determine whether she is performing the exercises correctly and to remediate any skill deficits. This will be done via vaginal palpation, except for at the 2-week postoperative visit, during which the perineum will be visually inspected to insure that the subject is not straining during exercise. At the 2 week, 4-6week and 8 week postoperative visits, the interventionist adjusts the subject’s exercise regimen by gradually increasing the number (maximum ranging from 45 to 60 per day) and duration of each contraction (maximum = 10 seconds). At the final postoperative session, the interventionist will provide subjects with a maintenance exercise program consisting of 15 contractions per day at the maximum contraction duration achieved during the intervention period.
At all postoperative visits, subjects will complete an exercise and behavioral adherence questionnaire to determine the number of exercises subjects performed daily and whether they encountered any barriers that interfered with exercise and implementation of behavioral strategies, which the interventionist will use to guide treatment progression and to assist subjects in problem-solving strategies for improving exercise and strategy adherence and success.
To promote exercise adherence during the two-year follow-up period, the interventionist will mail a flyer to subjects on a quarterly basis that details the exercise regimen and encourages exercise and behavioral strategy adherence. Beyond this, subjects will receive no other contact to encourage exercise and behavior strategy adherence. However, adherence to the behavioral intervention will be assessed at the time of each clinic visit (at 6, 12 and 24 months).
Those subjects randomized to “usual care” will receive no behavioral or pelvic floor muscle training, other than routine perioperative teaching and a standardized set of postoperative instructions given to all subjects.
Data Collection
A timeline of visits, events and data collection is listed in Table 3. Scheduled in-person follow-up will occur at 2 and 4-6 weeks and 3, 6, 12, and 24 months. At the 6, 12, and 24 month visits, a POPQ examination and a pelvic floor muscle strength assessment will be performed by an examiner blinded to the intervention assignments. In addition, an update of current medications, an assessment of new or continuing pelvic floor disorders, adverse events that occurred since the previous evaluation, and an assessment of health care costs and resources used will be obtained by the clinical site study coordinator at the 3-, 6-, 12-, and 24-month in-person visits and by phone 9, 15, 18 and 21 months after surgery. Telephone interviews by the Quality of Life Interviewing Center will also occur at 6, 12 and 24 months and will include administration of each of the instruments used in the baseline assessment, as well as the Patient’s Global Impression of Improvement (PGI-I).[54] Two years after the index surgery subjects will be asked whether they believe they have been unblinded to the surgical intervention.
Table 3.
Timeline of visits events and data collection
| Baseline | Surgery & Hosp | 2 wk | 4-6 wk | 8 wk* | 3 mo | 6 mo | 9 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Window | -2mo | ±1 wk | ±0 wk | ±1 wk | ±1 wk | ±2 wk | ±1 mo | ±1 mo | ±1 mo | ±1 mo | ±1 mo | ±2 mo | |
| Informed Consent | X | ||||||||||||
| Demographics | X | ||||||||||||
| Medical History | X | ||||||||||||
| Medications | X | X | X | X | X | X | X | X | X | X | X | ||
| Physical examination w/POPQ | X | X$ | X | X | X | ||||||||
| Pelvic muscle strength | X | X | X | X | |||||||||
| Randomization | BPMT | SURG | |||||||||||
| BPMT visit* | X | X | X | X | |||||||||
| Telephone interview/QOL# | X | X | X | X | |||||||||
| SF-36 | X | X | X | X | |||||||||
| Adverse events | PRN | PRN | PRN | PRN | PRN | PRN | PRN | PRN | PRN | PRN | PRN | PRN | |
| Pain/Activities assessment | X | X | X | X | X | X | X | ||||||
| Cost/Resources assessment | X | X | X | X | X | X | X | X | X | X |
BPMT = Behavioral and pelvic floor muscle training; SURG = Surgical intervention; PRN= as needed; Hosp.= hospital stay; f/u= follow-up.
BPMT visits will only be attended by subjects randomized to this intervention; subjects randomized to usual care will not have an 8 week follow-up visit.
Data collected during telephone interview: PFDI, PFIQ, PISQ-12, EQ-5D, Body image scale, and Hunskaar measure, Adaptation measure, PGI-I (follow-up only).
A vaginal examination for mesh/suture erosion without POPQ will be performed at the 4-6 week visit
Outcome Measures
The primary outcome measure for the Surgical Intervention will be surgical “success” or “failure” assessed two years after surgery. The primary outcome measure has three components: (1) an anatomic assessment of prolapse, using the POPQ examination, (2) the presence or absence of bulge symptoms specific to prolapse, using two questions from the PFDI and (3) an assessment of additional treatment (surgical or non-surgical) for prolapse after the index surgery. Specific details of this composite primary endpoint are provided in Table 4.
Table 4.
Primary outcome measures for the study interventions
| Intervention
|
Outcome Assessment
|
Outcome
|
|---|---|---|
| Surgery | 2 years | Surgical success/failure
|
| Perioperative BMT | 6 month | Urinary Distress Inventory (UDI) scale of the PFDI |
| 2 years | Pelvic Organ Prolapse Distress Inventory (POPDI of the PFDI) Anatomic outcomes described for the Surgery intervention (above) |
BPMT = Behavioral and pelvic floor muscle training; POPQ, pelvic organ prolapse quantification[41];TVL, total vaginal length; PFDI, pelvic floor distress inventory[40]
Subjects who do not meet any of these criteria will be considered a surgical success.
Subjects will be considered as having bothersome vaginal bulge symptoms if they report an affirmative response to either of the following questions from the PFDI, “Do you usually have a sensation of bulging or protrusion from the vaginal area?” or “ Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?” and report any degree of bother from these symptoms (i.e., any response other than “not at all” to the question “How much does this bother you?”
For the Perioperative Intervention, both short-and long-term primary outcomes will be assessed. The primary short-term outcome measure will be urinary symptoms measured by the Urinary Distress Inventory (UDI) subscale of the PFDI at six months after surgery. The six-month time frame was chosen because it is early enough after the formal therapy program to be within the time frame of a maximal impact on symptoms, yet far enough from the intervention that the duration of effect on symptoms would be clinically meaningful. The primary long-term outcomes will be prolapse symptoms measured by the Pelvic Organ Prolapse Distress Inventory (POPDI) subscale of the PFDI and anatomic outcomes at two years after surgery.
Secondary outcomes that will be assessed independently for both the Surgical and Perioperative Interventions include anatomic outcomes of each vaginal segment (anterior, posterior and apical); time to prolapse recurrence; urinary, bowel and sexual symptoms; generic and condition-specific HRQOL; retreatment for prolapse or urinary incontinence; functional activity; and postoperative pain. Additionally, pelvic floor muscle strength is a secondary outcome specific to the BPMT intervention. Surgical complications will be collected and categorized using a modification of the Dindo Classification [55].
Economic Evaluation and Analysis
One of the aims of the OPTIMAL trial is to investigate the cost-effectiveness of providing perioperative training at the time of vaginal surgery for prolapse. The analysis will be performed from the U.S. societal perspective. We will account for the direct medical costs such as hospitalization and outpatient care, direct non-medical costs such as transportation to health care and use of incontinence products, and indirect costs (i.e., productivity loss) for each subject. Detailed data on each subject’s utilization of health care resources associated with the Perioperative Intervention and subsequent health care over the two-year follow-up period will be collected using each clinical site’s administrative records supplemented by subject self-report. Medicare fee schedules will be used as unit cost measures for medical services.[56] Because the Perioperative Intervention is specifically developed for the OPTIMAL trial, we will collect detailed data on the use of personnel time, space/facility cost, and materials/supplies (e.g., workbooks) when delivering the BPMT sessions. National median wage rates for the specific type of personnel will be applied to estimate personnel costs.
Subject-specific quality-adjusted life years (QALYs) will be calculated based on the EQ-5D[57] assessments collected at baseline and 6 months, 12 months and 24 months post-surgery. We will assume linear changes in each subject’s utility values over time between every two assessments in order to calculate the area under the curve over the two-year period. An annual discount rate of 3% will be applied to both costs and QALYs. The incremental cost-effectiveness ratio (ICER) will be calculated as the differential costs between the perioperative BPMT group and the usual care group, divided by the differential QALYs between the two groups. General linear regression analysis will be conducted to estimate the differential mean costs and differential mean QALYs between the BPMT group and the usual care group, while adjusting for surgical treatment (SSLF or ULS), hysterectomy (concomitant or prior), surgeon, and other baseline measures identified as unbalanced between the two groups. The measures of costs and QALYs may be transformed before analysis if the variables demonstrate skewed distributions. Our base case cost-effectiveness analysis will include only subjects with complete cost and utility data. Sensitivity analysis will include subjects with missing data (e.g., lost to follow-up in one or more of the follow-up assessments) using the multiple imputation method.[58] Non-parametric bootstrapping resampling technique will be used to derive the 95% confidence intervals for the ICER.[59]
Sample Size
The OPTIMAL trial uses a 2×2 factorial design such that subjects will undergo two separate randomizations, one for the Surgery Intervention (SSLF vs. ULS) and one for the Perioperative Intervention (BPMT vs. usual care). The intent is to assess the superiority of each of the two interventions. Although we will perform a test for interaction between the two interventions and between the two interventions and clinical site, we do not expect the effect of BPMT to differ by the type of surgery performed, nor do we expect the efficacy of the two surgical techniques to be differentially affected by the performance of perioperative BPMT. Thus, sample size calculations were based on the comparisons between the Surgical Intervention groups, and separately, on the comparisons between the Perioperative Intervention groups.
Using definitions of anatomic surgical success similar to the one we propose, the anatomic success of SSLF in the randomized trials performed by Benson et al and Maher et al were 69% and 67%, respectively.[14, 23]. Retrospective case series, with follow-up of at least three years using relatively similar definitions of anatomic success (Baden-Walker Grade 0 or 1), demonstrate that the cure rates for SSLF were between 74% [11] and 82% [20]. Based on this information, we assumed anatomic success of 70% for SSLF at 2 years. The investigators believe that a difference in success less than 15% will not change clinical practice, but also that the sample size should be large enough to detect an observed difference in success of 10%; i.e., an inability to differentiate between the procedures will require an observed difference that is less than 10%. With 170 subjects per group (a total of 340 subjects randomized), there will be 80% power to differentiate between success rates of 70% and 83% using a two-tailed test with a 5% level of significance. In addition, the observed difference must be less than 10% in order for the two procedures not to differ significantly. Projecting a 15% drop-out or loss to follow-up rate over two years, we anticipate recruiting and randomizing 400 subjects in this protocol.
The primary short-term (6-month) outcome measure for the Perioperative Intervention is the UDI subscale of the PFDI. If efficacious, the Perioperative Intervention should reduce the UDI score. With 340 subjects (170 per group), there will be 80% power to identify a difference of 0.3 standard deviations (SD) in the mean UDI score using a two-tailed test with 5% level of significance. According to unpublished data from a prospective surgical cohort, in women 6 months after receiving vaginal prolapse surgery and TVT, the mean (±SD) UDI score on the PFDI was 55 ± 32. Assuming similar results in OPTIMAL, this sample size will provide 80% power to identify a difference of approximately 10 points in mean UDI score between the two intervention groups (i.e., SD 32 × 0.3 = 9.6 points). Analysis of baseline data in the ATLAS study[53] demonstrated an 11-point difference between in mean UDI scores for subjects who leak urine up to once per week (31±26) and subjects who leak urine up to once per day (42±27) (i.e., SD 26 or 27 with 11-point difference = 0.4 SD). These data suggest that a difference of 11 points (0.4 SD) or more would be of clinical interest. With our sample size, there is more than 80% power to identify a change of this magnitude.
Analytic Plan
The primary outcomes of both interventions will be analyzed according to the original randomized treatment assignment (intent to treat principle). The primary endpoint for the Surgical Intervention is dichotomous: success or failure at two years after surgery. The primary outcome measure uses the POPQ results at two years unless retreatment for prolapse occurred at an earlier time. When there is retreatment, or when the two-year POPQ result is missing and the last observed POPQ measurement is consistent with the definition of failure, the POPQ result at two years will be imputed as a failure. Sensitivity analyses will be performed to study the effect on the primary endpoint of different methods of handling drop-outs and missing data. A logistic regression model will be fitted to the outcome measure at two years. Factors for treatment assignment (both surgery and perioperative intervention), hysterectomy (concomitant or prior), and surgeon will be included in the model. If baseline measures are unbalanced between treatment groups, the logistic model will be fitted with and without these measures.
The primary outcome measure for the Perioperative Intervention is the comparison of mean UDI scores between the two groups at six months post-surgery. This measure is preferable to change in the UDI score, since the TVT and prolapse surgery are expected to have large effects on the UDI score; therefore, comparing the level of symptoms after the index surgery is more relevant than comparing changes in the symptom score. Because the UDI score is expected to be skewed, it may be transformed prior to analysis. A general linear model will be fitted to the UDI score (or transformed UDI score). The model will include factors for treatment assignment (both surgery and the perioperative intervention) and clinical site. Missing values will be imputed by the method of Brown [60]; a sensitivity analysis to assumptions will be performed. Subjects who are receiving treatment for lower urinary tract symptoms at the time of the six-month outcome assessment will be considered treatment failures for the BPMT intervention and their UDI values will be imputed using the method of Brown.[60]
Secondary outcome measures will be analyzed similar to the UDI score when they are continuous or similar to the surgical failure measure when they are discrete. However, imputation of missing values will not be done for secondary measures. Time-to-event outcomes will be analyzed using appropriate methods, such as log-rank tests, Kaplan-Meier survival curve estimation, and proportional hazards models. Since the primary endpoint is at two years, all subjects are expected to be enrolled and to have completed the intervention prior to the earliest time of any interim analysis. Therefore, we are not planning a formal interim analysis. A data safety and monitoring board will monitor for excess morbidity in either arm of both interventions.
Discussion
Despite the substantial rates of surgery for pelvic organ prolapse and the rapidity of newly proposed procedures, there are few high quality studies available to help guide surgeons who wish to use an evidence-based approach to management of this condition. Most prolapse surgery studies consist of uncontrolled, single-surgeon case series and are limited due to small numbers, inadequate power to detect clinically important differences, lack of standardization of technique, inadequate follow-up, and failure to blind the evaluator. [8, 26] The OPTIMAL trial is adequately powered to compare the safety and efficacy of two popular surgical procedures for treating apical and uterine prolapse, using standardized surgical techniques and validated outcome measures in multiple domains. The subjects are assessed by evaluators blinded to treatment assignment and are followed for two years after surgery in a multi-site setting.
A factorial design is preferred in order to evaluate two independent interventions in a single trial without the cost and effort of an increased in sample size. Because we have assumed that there is no interaction between the Surgical and Perioperative Interventions, the current sample size is powered to detect the main effects of each intervention. Although it is not adequately powered to detect an interaction between the interventions, we believe that the obvious advantage of the factorial design far outweighs the risk of this potential problem. A recent survey of the International Urogynecological Association (IUGA) found that the preferred vaginal vault suspension among members was SSLF (78%).[61] Uncontrolled retrospective case series and clinical trials in which SSLF was used in one arm suggest that while apical recurrence after SSLF is uncommon (2.4% to 19%), recurrence of anterior vaginal prolapse is more problematic (6% to 28.5%)[11-23]. Although ULS has been adopted as the preferred vaginal vault suspension among many academic urogynecologists in the U.S. in hopes of reducing anterior vaginal prolapse recurrence, current evidence supporting the use of ULS is limited to seven uncontrolled retrospective case-series. [24, 25, 52, 62-65] There are currently no comparative studies, randomized or not, that compare SSLF to ULS. Moreover, most studies of both procedures focus on anatomic outcomes and complications, and generally lack information on functional, HRQOL and sexual outcomes.
The results of the OPTIMAL trial are essential for establishing which of these two popular vaginal apical prolapse repairs is superior. This knowledge could potentially improve surgical outcomes, and may also inform the design of trials that compare “traditional” surgery with trans-vaginal mesh-augmented repairs. The theoretical advantage of these kits is improved long-term anatomic results, although such data are currently unavailable. Unfortunately, early studies suggest that these kits may be associated with more frequent complications than traditional repairs including mesh erosion, pelvic pain and dyspareunia. [66] A major impetus for implementing the OPTIMAL trial is to determine the best traditional vaginal approach so that adequate comparisons between traditional prolapse surgery and prolapse kits can be made in future clinical trials.
Women undergoing surgery for prolapse often have concurrent urinary, bowel and other pelvic symptoms and may develop de novo symptoms following surgery. The OPTIMAL trial limits participation to patients planning vaginal surgery for apical or uterine prolapse who also have SUI symptoms, as there is currently no consensus on the management of women undergoing vaginal prolapse surgery who do not have SUI symptoms. Limiting eligibility to stress incontinent women in OPTIMAL, we were able to develop a standardized management protocol using a widely accepted procedure whose efficacy and durability is well-documented for surgical treatment of SUI (i.e., TVT). Because the prevalence of stress incontinence symptoms in patients with ≥ stage 2 prolapse is high, there will be an adequate number of subjects eligible for recruitment and our findings will be applicable to a large number of patients.
One notable unique challenge of a surgical trial is that the technical aspects of a particular treatment can vary greatly between surgeons, as can the participating surgeons’ operative skill and experience. To minimize variability of surgical technique within OPTIMAL, we established strict standardization of suture type, number, and placement for the two apical suspension techniques. The Michigan 4-wall technique of SSLF was chosen because uncontrolled studies suggest a lower anterior prolapse recurrence than other approaches.[8, 12] Since no scientific evidence existed to guide the standardization or identify essential components of either procedure, decisions were made by group consensus. To minimize variability related to surgeon experience, only surgeons who use both apical vault suspension techniques in their practice and have performed at least 20 of each procedure during their career and at least 5 in the last 12 months are able to participate in OPTIMAL.
It is rare that surgery to correct apical or uterine prolapse is performed in isolation; concurrent surgery to correct anterior and/or posterior prolapse is common. However, the degree and location of vaginal support loss can vary considerably from patient to patient and surgeons often use intraoperative judgment to decide whether or not to perform concurrent procedures. To avoid introducing bias in performing or selecting concurrent procedures after surgical randomization, surgeons are required to indicate planned procedures at the time of preoperative planning. Surgeons can alter their plan intraoperatively if necessary to provide patients with the best possible care; however, this will be recorded as a protocol deviation and closely monitored. To standardize the performance of concurrent surgery, guidelines establishing a minimum degree of anatomic support of the anterior and posterior vaginal wall at the end of the surgery were developed and included in the protocol.
Another challenge in surgical trials is maintenance of blinding the treatment assignment, particularly in this study with two interventions. We are expending considerable effort to maintain blinding of the subject to the surgical treatment assignment for the duration of the study. It is not possible to mask the subject to Perioperative Intervention assignment; however, all outcomes assessors will be blinded for both interventions. Blinding at the clinical sites requires careful attention to the medical records (whether paper, electronic, or some combination) especially the surgical consent form, operative notes, and medical bills. Our template for the surgical consent form will state: “Vaginal apical suspension (sacrospinous ligament suspension or uterosacral ligament suspension per OPTIMAL Protocol), Tension-Free Vaginal Tape (TVT), and other procedures as required for that patient (hysterectomy, bilateral salpingo-oophorectomy, etc.)” In addition, the operative note is organized to reflect protocol participation in the “Procedure” portion, and details of the specific procedures performed are only available within the text of the operative note. This adds a modicum of protection to alert blinded investigators to avoid seeking details within the operative note. On-going education of hospital staff including nurses, fellows, residents and medical students about the importance of blinding will be essential.
Perioperative physical therapy is associated with improved outcomes in a number of areas including orthopedic, spine and cardiac surgery. The pelvic floor muscles play a critical role in pelvic organ support and contribute to continence of both urine and stool by involvement with urethral and anal sphincter function.[67, 68] Moreover, diminished pelvic floor muscle strength is associated with more frequent prolapse recurrence and increasing reoperation for pelvic floor disorders within five months after prolapse surgery.[69] Thus, perioperative PMT has significant potential as adjuvant treatment in women undergoing surgery for prolapse and/or SUI. Jarvis et al randomized 60 women undergoing surgery for prolapse and/or SUI to either perioperative BPMT (preoperative pelvic floor muscle exercise teaching reinforced postoperatively, and instruction in healthy voiding and defecation techniques to reduce the need for straining) or usual care.[38] Three months after surgery, subjects in the intervention group had significantly greater reduction in urinary symptoms, including reduction in daytime urinary frequency, and greater improvement in quality of life compared to the control group. Subjects were not followed beyond three months postoperatively, however, and only urinary symptoms were assessed. Whether perioperative BPMT is effective in reducing the recurrence of prolapse or its symptoms in the long-term is unknown.
The OPTIMAL study will assess both the short- and long-term effects of perioperative BPMT on a wide range of pelvic floor disorders symptoms as well as sexual, HRQOL and anatomic outcomes of women undergoing vaginal surgery for advanced prolapse and SUI. In addition, the study will generate much needed information on the cost-effectiveness of perioperative BPMT in vaginal surgery for prolapse. Collectively, these findings will provide substantial insight into the potential merits of this adjunctive treatment.
The OPTIMAL BPMT program is a simple program that can be easily applied in the perioperative period with minimal subject burden and is flexible enough to address the varying pelvic symptoms likely to be encountered in women after surgery for advanced prolapse and SUI. Behavioral programs typically consist of four to six visits over an eight to twelve week period.[33-35] To achieve maximal benefit and allow any postoperative pelvic floor symptoms to stabilize after surgery, while not overburdening the patient, we expanded the three-visit six-week program outlined by Jarvis to a five-visit, twelve-week program.
The relatively poor compliance with PMT over the long-term is well known.[36] Subjects randomized to undergo perioperative training may not be compliant with their home program over the 2-year course of the study. This potential decline in compliance with time is one of the reasons we have chosen to assess the primary outcome of the Perioperative Intervention at 6 months. However, over the long-term, we are interested in a pragmatic comparison of the long-term impact of perioperative training including the effect of compliance. Thus, we plan to assess adherence throughout the follow-up phase. Subjects randomized to BPMT will receive a mailed reminder quarterly to encourage continued compliance with the intervention.
An estimated 225,000 women undergo prolapse operations every year in the United States, with medical costs over $1 billion (in 1997 dollars).[70] Since many of these are repeat operations[1, 2] strategies improving outcomes of prolapse surgeries will not only lessen the illness burden of patients, but may also reduce the cost of medical care in the long run.[38] Pelvic floor muscle exercises are successful in improving symptoms associated with a wide variety of pelvic floor disorders [33-36] and may reduce the incidence of recurrent prolapse and incontinence and hence the need for reoperation.[69] However, data are lacking on the cost effectiveness of providing behavioral or pelvic floor muscle training at the time of vaginal surgery for prolapse. The OPTIMAL trial will provide useful information to fill this gap.
Each intervention and assessment in the OPTIMAL trial has been carefully considered and chosen to provide maximum opportunity for reliable assessment of the relative impact of two accepted vaginal surgical methods for treatment of women with pelvic organ prolapse with or without SUI. Furthermore, we have designed the trial to allow a reasonably independent assessment of an established nonsurgical intervention in the form of BPMT. Although ambitious goals, we believe they are feasible primarily because of our efforts at standardization of each intervention, use of power analysis to estimate necessary sample size, utilization of valid and reliable outcome measures, and emphasis on long-term subject retention and assessment. The discussion we present in this article is relevant to other surgical trials with similar objectives.
Acknowledgments
Grant Support: Supported by grants from the National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health (U01 HD41249, U10 HD41250, U10 HD41261, U10 HD41267, U10 HD54136, U10 HD54214, U10 HD54215, and U10 HD54241).
Appendix - Pelvic Floor Disorders Network Contributors
Cleveland Clinic
Mathew D. Barber, MD, MHS, Principal Investigator
Marie Fidela R. Paraiso, MD, Co-Investigator
Mark D. Walters, MD, Co-Investigator
J. Eric Jelovsek, MD, Co-Investigator
Firouz Daneshgari, Co-Investigator
Linda McElrath, RN, Research Nurse Coordinator
Donel Murphy, RN, MSN, Research Nurse
Cheryl Williams, Research Assistant
Duke University
Anthony G. Visco, MD, Principal Investigator
Jennifer Wu, MD, Co-Investigator
Alison Weidner, MD, Co-Investigator
Cindy Amundsen, MD, Co-Investigator
Mary J. Loomis, RN, BSN, Research Coordinator
Loyola University, Chicago
Linda Brubaker, MD, MS, Principal Investigator
Kimberly Kenton, MD, MS, Investigator
MaryPat FitzGerald, MD, MS, Investigator
Elizabeth Mueller, MD, MSME, Investigator
Kathy Marchese, RN, Study Coordinator
Mary Tulke, RN, Study Coordinator
University of Alabama at Birmingham
Holly E. Richter, PhD, MD, Principal Investigator
R. Edward Varner, MD, Co-Investigator
Robert L. Holley, MD, Co-Investigator
Thomas L. Wheeler, MD, Co-Investigator
Patricia S. Goode, MD, Co-Investigator
L. Keith Lloyd, MD, Co-Investigator
Alayne D. Markland, DO, Co-Investigator
Velria Willis, RN, BSN, Research Coordinator
Nancy Saxon, BSN, Research Nurse Clinician
LaChele Ward, LPN, Research Specialist
Lisa S. Pair, CRNP
University of Michigan
Morton B. Brown, PhD, Co-Investigator
Cathie Spino, PhD, Principal Investigator
John T. Wei, MD, MS, Co-Principal Investigator
Beverly Marchant, RN, BS, Project Manager
Donna DiFranco, BS, Clinical Monitor
John O.L. DeLancey, MD, Co-Investigator
Dee Fenner, MD, Co-Investigator
Nancy K. Janz, PhD, Co-Investigator
Wen Ye, PhD, Statistician
Zhen Chen, MS, Statistician
Yang Wang Casher, MS, Database Programmer
University of Texas, Southwestern
Joseph Schaffer MD – Principal Investigator
Clifford Wai, MD - Co-Investigator
Marlene Corton, MD - Co-Investigator
Gary Lemack, MD - Co-Investigator
Kelly Moore - Research Coordinator
David Rahn, MD
Amanda White, MD
Shanna Atnip, NP
Margaret Hull, NP
Pam Martinez, NP
Deborah Lawson, NP
University of Utah
Ingrid Nygaard, MD, Principal Investigator
Peggy Norton, MD, Co-Investigator
Linda Freeman, RN, Research Coordinator
NIH Project Scientist
Anne M. Weber, MD, MS
Susan Meikle, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Gregory T, Smith VJ, Edwards R. Epidemiologic evaluation of reoperation for surgically treated pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2003;189:1261–7. doi: 10.1067/s0002-9378(03)00829-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;186:712–6. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 4.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol. 2003;188:108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108:324–32. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowder JL, Park AJ, Ellison R, et al. The role of apical vaginal support in the appearance of anterior and posterior vaginal prolapse. Obstet Gynecol. 2008;111:152–7. doi: 10.1097/01.AOG.0000297309.25091.a0. [DOI] [PubMed] [Google Scholar]
- 7.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195:1837–40. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker L, Bump RC, Fynes M, et al. Surgery for Pelvic Organ Prolapse. In: Abrams P, Cordozo L, Koury S, Wein A, editors. International Consultation on Incontinence. 3. Paris: Health Publication Ltd; 2005. [Google Scholar]
- 9.Shull BL. Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol. 1999;181:6–11. doi: 10.1016/s0002-9378(99)70427-8. [DOI] [PubMed] [Google Scholar]
- 10.Toozs-Hobson P, Boos K, Cardozo L. Management of vaginal vault prolapse. Br J Obstet Gynaecol. 1998;105:13–7. doi: 10.1111/j.1471-0528.1998.tb09343.x. [DOI] [PubMed] [Google Scholar]
- 11.Colombo M, Milani R. Sacrospinous ligament fixation and modified McCall culdoplasty during vaginal hysterectomy for advanced uterovaginal prolapse. Am J Obstet Gynecol. 1998;179:13–20. doi: 10.1016/s0002-9378(98)70245-5. [DOI] [PubMed] [Google Scholar]
- 12.DeLancey JO, Morley GW, Howard D. Sacrospinous suspension: Michigan 4-wall offers better support. OBG Management. 2001 March;:18–29. [Google Scholar]
- 13.Lantzsch T, Goepel C, Wolters M, Koelbl H, Methfessel HD. Sacrospinous ligament fixation for vaginal vault prolapse. Arch Gynecol Obstet. 2001;265:21–5. doi: 10.1007/s004040000116. [DOI] [PubMed] [Google Scholar]
- 14.Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20–6. doi: 10.1016/j.ajog.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Meschia M, Bruschi F, Amicarelli F, Pifarotti P, Marchini M, Crosignani PG. The sacrospinous vaginal vault suspension: Critical analysis of outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:155–9. doi: 10.1007/s001920050037. [DOI] [PubMed] [Google Scholar]
- 16.Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872–81. doi: 10.1016/0002-9378(88)90088-9. [DOI] [PubMed] [Google Scholar]
- 17.Paraiso MF, Ballard LA, Walters MD, Lee JC, Mitchinson AR. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423–30. doi: 10.1016/s0002-9378(96)70085-6. discussion 1430-1. [DOI] [PubMed] [Google Scholar]
- 18.Pasley WW. Sacrospinous suspension: a local practitioner’s experience. Am J Obstet Gynecol. 1995;173:440–5. doi: 10.1016/0002-9378(95)90264-3. discussion 445-8. [DOI] [PubMed] [Google Scholar]
- 19.Penalver M, Mekki Y, Lafferty H, Escobar M, Angioli R. Should sacrospinous ligament fixation for the management of pelvic support defects be part of a residency program procedure? The University of Miami experience. Am J Obstet Gynecol. 1998;178:326–9. doi: 10.1016/s0002-9378(98)80020-3. [DOI] [PubMed] [Google Scholar]
- 20.Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764–8. doi: 10.1016/0002-9378(92)91567-t. discussion 1768-71. [DOI] [PubMed] [Google Scholar]
- 21.Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390–3. doi: 10.1007/s001920050066. [DOI] [PubMed] [Google Scholar]
- 22.Imparato E, Aspesi G, Rovetta E, Presti M. Surgical management and prevention of vaginal vault prolapse. Surg Gynecol Obstet. 1992;175:233–7. [PubMed] [Google Scholar]
- 23.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418–21. doi: 10.1016/s0002-9378(96)70084-4. discussion 1421-2. [DOI] [PubMed] [Google Scholar]
- 24.Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402–10. doi: 10.1067/mob.2000.111298. discussion 1410-1. [DOI] [PubMed] [Google Scholar]
- 25.Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339–42. doi: 10.1067/mob.2001.119077. discussion 1342-3. [DOI] [PubMed] [Google Scholar]
- 26.Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2004:CD004014. doi: 10.1002/14651858.CD004014.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466–75. doi: 10.1016/S0029-7844(96)00337-7. [DOI] [PubMed] [Google Scholar]
- 28.Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106:615–634. doi: 10.1097/01.AOG.0000175832.13266.bb. [DOI] [PubMed] [Google Scholar]
- 29.Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332–7. doi: 10.1067/mob.2001.119078. discussion 1337-8. [DOI] [PubMed] [Google Scholar]
- 30.Barber MD. Symptoms and outcome measures of pelvic organ prolapse. Clin Obstet Gynecol. 2005;48:648–61. doi: 10.1097/01.grf.0000170424.11993.73. [DOI] [PubMed] [Google Scholar]
- 31.Roovers JP, van der Vaart CH, van der Bom JG, van Leeuwen JH, Scholten PC, Heintz AP. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. Bjog. 2004;111:50–6. doi: 10.1111/j.1471-0528.2004.00001.x. [DOI] [PubMed] [Google Scholar]
- 32.Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299–304. doi: 10.1067/mob.2001.119081. discussion 1304-6. [DOI] [PubMed] [Google Scholar]
- 33.Berghmans LC, Hendriks HJ, Bo K, Hay-Smith EJ, de Bie RA, van Waalwijk van Doorn ES. Conservative treatment of stress urinary incontinence in women: a systematic review of randomized clinical trials. Br J Urol. 1998;82:181–91. doi: 10.1046/j.1464-410x.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Berghmans LC, Hendriks HJ, De Bie RA, van Waalwijk van Doorn ES, Bo K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU Int. 2000;85:254–63. doi: 10.1046/j.1464-410x.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- 35.Norton C, Kamm MA. Anal sphincter biofeedback and pelvic floor exercises for faecal incontinence in adults--a systematic review. Aliment Pharmacol Ther. 2001;15:1147–54. doi: 10.1046/j.1365-2036.2001.01039.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson PD, Berghmans B, Hagen S, et al. Adult Conservative Management. In: Abrams P, Cordozo L, Koury S, Wein A, editors. International Consultation on Incontinence. 3. Paris: Health Publication Ltd; 2005. [Google Scholar]
- 37.Hagen S, Stark D, Maher C, Adams E. Conservative management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2004:CD003882. doi: 10.1002/14651858.CD003882.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Jarvis SK, Hallam TK, Lujic S, Abbott JA, Vancaillie TG. Peri-operative physiotherapy improves outcomes for women undergoing incontinence and or prolapse surgery: results of a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2005;45:300–3. doi: 10.1111/j.1479-828X.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 39.Thakar R, Stanton S. Management of genital prolapse. Bmj. 2002;324:1258–62. doi: 10.1136/bmj.324.7348.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–95. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 41.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 42.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 43.Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R. A digital test for pelvic muscle strength in women with urinary incontinence. Nurs Res. 1994;43:352–6. [PubMed] [Google Scholar]
- 44.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497–9. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:164–8. doi: 10.1007/s00192-003-1063-2. discussion 168. [DOI] [PubMed] [Google Scholar]
- 46.EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 47.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37:189–97. doi: 10.1016/s0959-8049(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 48.Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obstet Gynecol. 2006;194:1455–61. doi: 10.1016/j.ajog.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy M, Jr, Chang CH, Pickard AS, et al. Visual analog scales for assessing surgical pain. J Am Coll Surg. 2005;201:245–52. doi: 10.1016/j.jamcollsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy M, Jr, Jonasson O, Chang CH, et al. Assessment of patient functional status after surgery. J Am Coll Surg. 2005;201:171–8. doi: 10.1016/j.jamcollsurg.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 52.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365–73. doi: 10.1067/mob.2000.110910. discussion 1373-4. [DOI] [PubMed] [Google Scholar]
- 53.Richter HE, Burgio KL, Goode PS, et al. Non-surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Clin Trials. 2007;4:92–101. doi: 10.1177/1740774506075237. [DOI] [PubMed] [Google Scholar]
- 54.Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189:98–101. doi: 10.1067/mob.2003.379. [DOI] [PubMed] [Google Scholar]
- 55.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Medicaid & Medicare Servies. Fee schedule-general information. Available from: www.cms.hhs.gov/FeeScheduleGenInfo/
- 57.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43:1078–86. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 58.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 59.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994;3:309–19. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 60.Brown MB. A test for the difference between two treatments in a continuous measure of outcome when there are dropouts. Control Clin Trials. 1992;13:213–25. doi: 10.1016/0197-2456(92)90004-j. [DOI] [PubMed] [Google Scholar]
- 61.Davila GW, Ghoniem GM, Kapoor DS, Contreras-Ortiz O. Pelvic floor dysfunction management practice patterns: a survey of members of the International Urogynecological Association. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:319–25. doi: 10.1007/s001920200069. [DOI] [PubMed] [Google Scholar]
- 62.Comiter CV, Vasavada SP, Raz S. Transvaginal culdosuspension: technique and results. Urology. 1999;54:819–22. doi: 10.1016/s0090-4295(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 63.Amundsen CL, Flynn BJ, Webster GD. Anatomical correction of vaginal vault prolapse by uterosacral ligament fixation in women who also require a pubovaginal sling. J Urol. 2003;169:1770–4. doi: 10.1097/01.ju.0000061472.94183.26. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins VR., 2nd Uterosacral ligament fixation for vaginal vault suspension in uterine and vaginal vault prolapse. Am J Obstet Gynecol. 1997;177:1337–43. doi: 10.1016/s0002-9378(97)70073-5. discussion 1343-4. [DOI] [PubMed] [Google Scholar]
- 65.Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108:255–63. doi: 10.1097/01.AOG.0000224610.83158.23. [DOI] [PubMed] [Google Scholar]
- 66.Ho MH, Bhatia NN. Introduction of newer surgical prostheses and procedures in pelvic reconstruction: a challenge for pelvic surgeons. Curr Opin Obstet Gynecol. 2007;19:461–3. doi: 10.1097/GCO.0b013e3282f09edd. [DOI] [PubMed] [Google Scholar]
- 67.DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897–909. doi: 10.1097/00003081-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 68.DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–20. doi: 10.1016/s0002-9378(94)70346-9. discussion 1720-3. [DOI] [PubMed] [Google Scholar]
- 69.Vakili B, Zheng YT, Loesch H, Echols KT, Franco N, Chesson RR. Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse. Am J Obstet Gynecol. 2005;192:1592–8. doi: 10.1016/j.ajog.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]


