Summary
Objective
Altered loading is an important etiological factor for temporomandibular joint (TMJ) disorders. Studies examining altered loading of the TMJ have been done in rats but the response of the TMJ to altered loading in mice is largely unknown. Therefore, due to the potential usefulness of genetically engineered mice, the goal of this study was to develop a mouse TMJ altered functional loading model.
Methods
One hundred and thirty four, 21-day-old CD-1 female mice were divided into two groups: (1) normal loading (hard pellet diet) for 2–6 weeks and (2) altered functional loading (incisor trimming every other day and soft dough diet) for 2–6 weeks. The mandibular condylar cartilage was evaluated by histology, the subchondral bone was evaluated by microcomputed tomography (micro-CT) analysis and gene expression was evaluated by real time polymerase chain reaction (PCR) analysis.
Results
Altered functional loading for 2–6 weeks caused significant reduction in the thickness of the condylar cartilage whereas, only at 4 weeks was there a significant decrease in the bone volume fraction and trabecular thickness of the subchondral bone. Gene expression analysis showed that altered functional loading for 4 weeks caused a significant reduction in the expression of SRY-box containing gene 9 (Sox9), Collagen type X (Col X), Indian hedgehog (lhh), Collagen type II (Col II) and Vascular endothelial growth factor (Vegf) and altered loading for 6 weeks caused a significant decrease in the expression of Sox9, Col II, Vegf and Receptor activator of NF-κB ligand (Rankl) compared to the normal loading group.
Conclusion
Altered functional TMJ loading in mice for 2–6 weeks leads to a loss of the condylar cartilage and a transient loss in the density of the mandibular condylar subchondral bone.
Keywords: Temporomandibular joint, Mouse model, Mechanical loading, Fibrocartilage, Subchondral bone
Introduction
Approximately 10% of the population over the age of 18 has pain in the temporomandibular joint (TMJ) region1 and about 15% of the people who have TMJ pain have degenerative diseases of the TMJ (TMJ-DD)2. The TMJ is formed by the mandibular condyle and the mandibular fossa of the temporal bone. Separating these two bones from direct contact is the articular disc. Unlike other joints, which are composed of hyaline cartilage, the articular portion of the mandibular condyle and disc is comprised of fibrocartilage. The mandibular condylar cartilage can be organized into four zones. The most superficial layer is called the articular zone and cells in this zone are characterized by their expression of Proteoglycan 4 (Prg4)3. The second zone is the polymorphic zone, which contains the precursor cells for the flattened and hypertrophic zones4. The third zone is the flattened zone. The cartilage cells in this layer are characterized by the expression of Collagen type II (Col II)5. The fourth and deepest zone is the hypertrophic zone. In this zone, the chondrocytes are characterized by the expression of Collagen type X (Col X)5.
The exact etiology for TMJ-DD is unknown; however, most dentists and physicians have been inclined to believe that the single most important etiological factor is mechanical loading that surpasses the adaptive capacity of the joint6,7. In order to examine the effects of mechanical loading-induced adaptation of the TMJ in rodents, investigators have manipulated the masticatory sequence that can be classified into two stages, incision and chewing8. Trimming the incisors out of occlusion (believed to decrease the occlusal force during the incision stage and decrease the amount of protrusion that occurs during the incision stage9,10), replacing the standard hard pellet diet with a mushy soft diet (believed to decrease the amount of molar force required during the chewing stage9,10) or a combination of both are all methods used to manipulate the masticatory sequence.
In rats, there are numerous studies examining the effects of incisor trimming and soft diet administration (altered functional loading) on the TMJ. For example, Pirttiniemi et al. and Hinton et al. have shown that altered functional loading caused a decrease in proliferation and sulphate incorporation in the mandibular condylar cartilage10,11. Pirttiniemi et al. have also shown in rats that altered functional loading caused an increase in MMP-3 expression11, an increase in the height of the condylar process12 and a decrease in maturation and differentiation of the condylar cartilage cells13,14. In contrast, there is only one study that examined altered functional loading on the TMJ in mice15. Sasguri et al. found that altered functional loading for 2 weeks caused a decrease in the mRNA expression of Bone sialoprotein (Bsp), Osteopontin (Opn), Osteocalcin (Oc) and Collagen type I (Col I) from the mandibular condyle. In addition, histomorphometric measurements showed that there was a thinner layer of cartilage and fewer bone trabeculae in the mandibular condyle from the altered functional loading group compared to the normal loading group after 2 weeks, although these changes were not quantitated. The goal of this study was to expand on the one mouse study by quantitating the changes in micro architecture of the mandibular condylar head and to examine the effects of altered functional loading on the expression of other genes found in the various zones of the mandibular condylar cartilage. The development of the altered functional loading mouse model will enable further investigation elucidating the role of specific genes in mediating the mechanical loading response of the TMJ.
Materials and methods
MICE
All experiments were performed under an institutionally approved protocol for the use of animals in research (University of Connecticut Health Center #2005-195). One hundred and eighty two, 21-day-old CD-1 female mice (Charles River Wilmington, MA) were used for this study. Eruption of molars and occlusion are complete at this age16. The mice were divided into five groups – (1) normal pellet diet (n = 67) for 2, 4 and 6 weeks, (2) soft dough diet with the same nutritional composition as the normal pellet diet (Transgenic Dough Diet, BioServ, Frenchtown, NJ) (n = 9) for 4 weeks, (3) mandibular incisors trimmed out of occlusion (approximately 1 mm of tooth structure was removed with an orthodontic light wire clipper every other day) and normal pellet diet (n = 9) for 4 weeks, (4) soft dough diet and mandibular incisor trimming every other day (n = 67) for 2, 4, and 6 weeks and (5) soft dough diet and mandibular incisor trimming every other day for 4 weeks followed by cessation of incisor trimming and normal pellet diet for 2 weeks (return to normal loading, n = 30). The mice were weighed twice a week and were sacrificed after 2, 4, or 6 weeks of treatment.
RNA EXTRACTION AND POLYMERASE CHAIN REACTION (PCR) AMPLIFICATION
The mandibular condyle was carefully isolated with all the soft tissues removed using a dissecting microscope [Fig. 3(a, b)]. Total RNA was obtained from the condylar head, which contains both condylar cartilage and subchondral bone and extracted with Trizol Reagent (Invitrogen life technologies, Carlsbad, CA) following the manufacturer’s protocol. Total RNA obtained from the left and right TMJ of one mouse was pooled. Total RNA was converted to cDNA by ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster city, CA) following the manufacturer’s protocol. Real time PCR was performed for expression of different genes in separate wells (singleplex assay) of 96-well plate in reaction volume of 20 μl. Gapdh was used as endogenous control. Three replicates of each sample were amplified using Assays-on-Demand Gene Expression for the particular gene of interest, using pre-designed unlabeled gene-specific PCR primers and TaqMan MGB FAM dye-labeled probe. The PCR reaction mixture (including 2× TaqMan Universal PCR Master Mix, 20× Assays-on-Demand Gene Expression Assay Mix, 50 ng of cDNA) was run in Applied Biosystems ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. For the genes for which the efficiencies of target and endogenous control amplification were approximately equal, relative expression in a test sample compared to a reference calibrator sample (ΔΔCt Method) was used for data analysis. For the genes that were not amplified with the same efficiency as the endogenous control, the Relative Standard Curve method in which target quantity was determined from the standard curve and divided by the target quantity of the calibrator was used. Gene expression was performed for Prg4, Runx2 (Runx2), Col I, Parathyroid hormone related protein (Pthrp), SRY-box containing gene 9 (Sox9), Col II, Indian hedgehog (lhh), Col X, Vascular endothelial growth factor (Vegf), Opn, Oc, Osteoprotegerin (Opg), and Receptor activator of NF-κB ligand (Rankl).
Fig. 3.
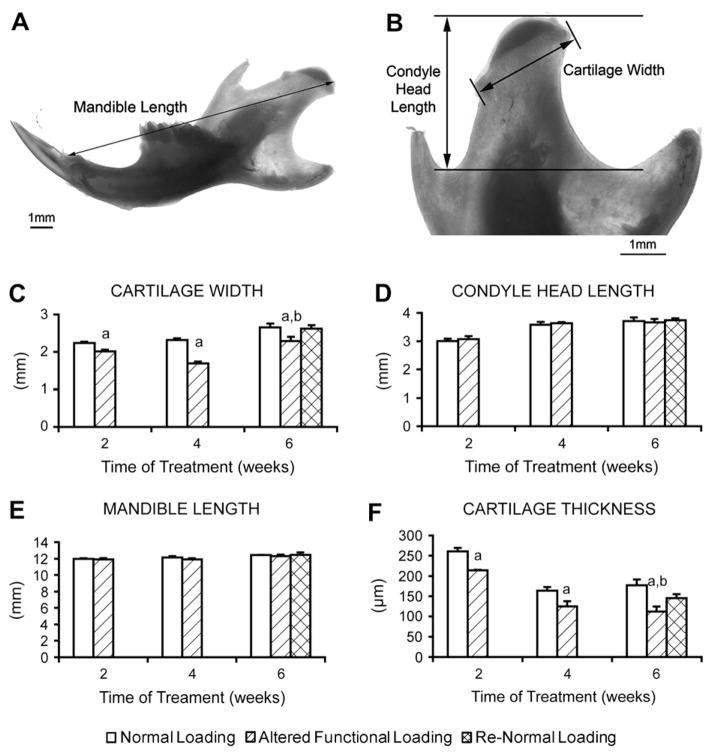
Measurements of mandibles from 21 -day-old female CD-1 mice subjected to normal loading (2,4 or 6 weeks), altered functional loading (2, 4 or 6 weeks) and return to normal loading (Re-Normal) (6 weeks). (A and B) Gross mandibular and condylar measurements of the whole mandible and the distal condylar end of the mandible. (C—F) Statistical results of the measurements of cartilage width (C), condyle head length (D), mandible length (E) and cartilage thickness (F). Points are the mean and S.E.M. for n = 9 for the normal loading group, n = 9 for the altered functional loading group and n = 9 for the return to normal loading group. aSignificant difference between altered functional loading and normal loading (P<0.05). bSignificant difference between altered functional loading and return to normal loading (P<0.05).
HISTOLOGY AND IMMUNOHISTOCHEMISTRY
The TMJ was dissected and fixed in 10% formalin for 3 days at room temperature. The samples were washed with tap water for 5 min, decalcified in 14% EDTA for 1 week and then processed for standard paraffin embedding. Serial sagittal sections of the TMJ were performed with every fifth section stained with hematoxylin and eosin (H&E) or Safranin O17. Safranin O is a cationic dye that binds to the negatively charged glycosaminoglycans and is used to determine proteoglycan content.
Mandibular condylar cartilage thickness
H&E sagittal sections corresponding to the mid-coronal portion of the mandibular condylar head of each animal were selected and captured using a digital camera. For each mouse, the condyle cartilage thickness was measured in a blinded, nonbiased manner using the OsteoMeasure computerized image analysis system (OsteoMetrics, Inc. Atlanta, GA) interfaced with an Optiphot Nikon microscope (Nikon Inc., Melville, NY). Briefly, a 200 × 200 μm2 field was placed at the central portion of the mandibular condylar cartilage and the outline of the condylar cartilage was demarcated by the same investigator who did not know from which mice the sections originated. The OsteoMeasure program then calculated the average of the cartilage thickness for the field. Measurements were performed from three sections from each mouse and from three mice from each of the groups.
Mandibular condyle measurements
Right mandibles were dissected under a dissection microscope to remove the attached soft tissue. Images of the hemisected mandible were captured with a digital camera. The following measurements were made with Adobe Photoshop CS2 software version 9.02 [Fig. 3(a, b)]: (1) mandibular length from the most distal point on the condyle articular surface to the most anterior point on the incisor alveolus; (2) condyle length from the most distal point on the condyle articular surface to the line connecting the two notches of the mandibular ramus; and (3) condylar cartilage width from the most anterior to the most posterior point on the condylar articular surface. Three mice from each group were analyzed.
Immunohistochemistry
Tissue sections were deparaffinized with xylene and rehydrated with decreasing concentrations of ethanol. Following rehydration, the sections were treated with 3% peroxide to block endogenous peroxidase activity and digested for 60 min with pepsin for unmasking (Lab Vision, Cat #AP-9007-006, Fremont, CA). Immunohistochemistry staining was performed using the LSAB + System-HRP Kit (DakoCytomation, code #K0690, Carpinteria, CA) following the procedure recommended by the manufacturer. The antibodies for COL II and SOX9 were obtained from Chemicon (Billerica, Ma, cat #8887, 1:200 in PBS buffer) and Abeam (Cambridge, Ma, cat #59265, 1:75 in TBS buffer), respectively. In order to evaluate for non-specific binding, substitution of the primary antibody with rabbit IgG (Upstate, cat #12-370, Charlottesville, VA) was performed.
Semiquantitative analysis of COL II and SOX9 expression was performed by calculating the area of COL II visible/total area of the mandibular condylar cartilage and by counting the number of SOX9 positive cells/total number of cells within a rectangular box of fixed area corresponding to center portion of the mandibular condylar cartilage. Measurements were made by using Adobe Photoshop CS2 software version 9.02 from at least three different sections from three mice from each group. The determination of the SOX9 positive cells and area of COL II visible was subjectively made by the same investigator while viewing both the immunostaining of interest and the corresponding negative control.
MICRO-CT
The subchondral bone of the mandibular condylar cartilage from 21 -day-old (n = 4), 35-day-old (n = 11), 49-day-old (n= 10) and 63-day-old (n= 19) female CD-1 mice was analyzed. The three-dimensional morphology of the subchondral bone of the mandibular condyle using micro-CT was evaluated by the micro-CT facility at University of Connecticut Health Center headed by Dr. Doug Adams. Bone was segmented from cartilage and soft tissue at a threshold of 690 mg/cm3. At this threshold, the attenuation for cartilage is similar to the bathing fluid making it undetectable. The analysis included bone surface, bone volume, total volume, trabecular spacing, and trabecular thickness.
STATISTICAL ANALYSIS
Statistical significance of differences among means was determined by analysis of variance (ANOVA) with post hoc comparison of more than two means by the Bonferroni method using GraphPad Prism (San Diego, CA).
Results
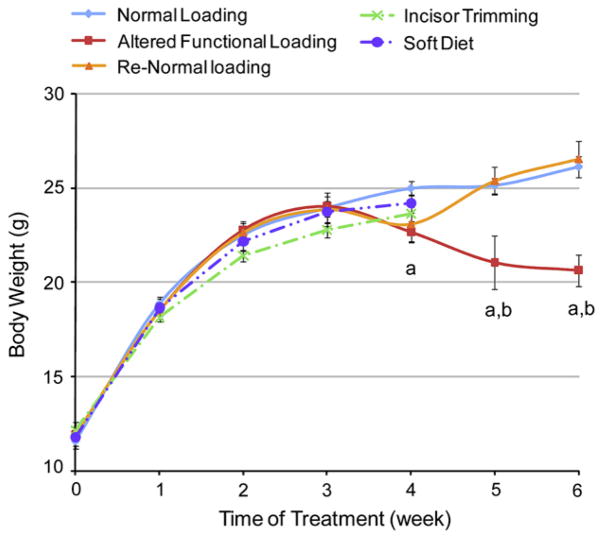
Incisor trimming and soft diet administration caused a progressive significant decrease in the weight of the mice starting at experimental week 4 and continuing to experimental week 6, at which time the weight was reduced 21% in the incisor trimming and soft diet compared to the normal loading group. Return to normal loading of the TMJ by the cessation of incisor trimming and the administration of a hard diet at experimental week 4 caused a quick recovery in the weight of the mice (Fig. 1).
Fig. 1.
Body weight of female CD-1 mice exposed to different masticatory conditions. Points are the mean and S.E.M. for n = 58 for normal loading group, n = 9 incisor trimming, n = 9 soft diet, n = 59 for altered functional loading (incisor trimming in combination with soft diet administration) and n = 30 for the return to normal loading (Re-Normal). aSignificant difference between altered functional loading and normal loading (P< 0.05). bSignificant difference between altered functional loading and return to normal loading (P< 0.05).
GENE EXPRESSION
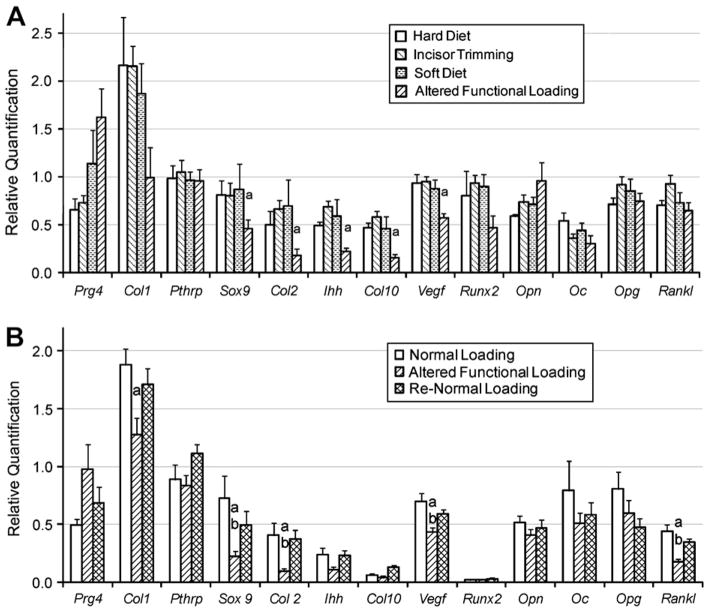
The initial experiment we performed was to examine the effects of soft diet alone, incisor trimming alone and the combination of both for 4 weeks on gene expression from the mandibular condylar head. Real time PCR gene expression analysis revealed incisor trimming and soft dough diet in combination caused a significant reduction in the expression of Sox9, Col X, lhh, Col II and Vegf whereas, incisor trimming alone or soft dough diet alone did not cause any significant change in gene expression after 4 weeks compared to the normal loading group [Fig. 2(a)]. Since incisor trimming alone and soft dough diet alone did not produce any significant changes in gene expression after 4 weeks, we decided to perform the rest of our experiments using the combination of both (altered functional loading).
Fig. 2.
21-day-old female CD-1 mice were exposed to different masticatory conditions. Real time PCR analysis for Prg4, Pthrp, Sox9, Col II, lhh, Col X, Vegf, Opn, Oc, Opg, Runx2, Col I and Rankl gene expression from the mandibular condylar head from (A) 4 weeks of normal loading, soft diet, incisor trimming or soft diet and incisor trimming (altered functional loading), and (B) 6 weeks of normal loading, altered functional loading or return to normal loading conditions (Re-Normal). Points are the mean and SEM for n = 5 for the 4-week and n = 6 for the 6-week loading groups. aSignificant difference between altered functional loading and normal loading (P < 0.05). bSignificant difference between altered functional loading and return to normal loading (P< 0.05).
Altered functional loading for 2 weeks of the TMJ did not cause any significant changes in the expression of the genes we examined in the condylar head compared to their expression in the condylar heads of the normal loading group (data not shown). Altered functional loading for 6 weeks, caused a significant decrease in the expression of Sox9, Col II, Col I, Vegf and Rankl in the altered loading group compared to the normal and return to normal loading groups [Fig. 2(b)]. In addition, there was no significant difference in any of the genes examined between the return to normal group and the normal loading group at 6 weeks [Fig. 2(b)].
MANDIBLE AND CONDYLAR CARTILAGE MEASUREMENTS
Altered functional loading of the TMJ did not cause a significant change in the length of the mandible or the length of the condyle compared to the other groups at any time point [Fig. 3(d, e)]. However, altered functional loading for 2–6 weeks did cause a significant decrease in the width of the condylar cartilage [Fig. 3(c)] and in the thickness of the mandibular condylar cartilage [Fig. 3(f)] compared to the normal loading group. Return to normal loading of the TMJ for 2 weeks after 4 weeks of altered functional loading was able to restore the condylar cartilage width and partially restore the cartilage thickness measurements back to the level of the 6-week normal loading group [Fig. 3(c, f)].
SAFRANIN O STAINING
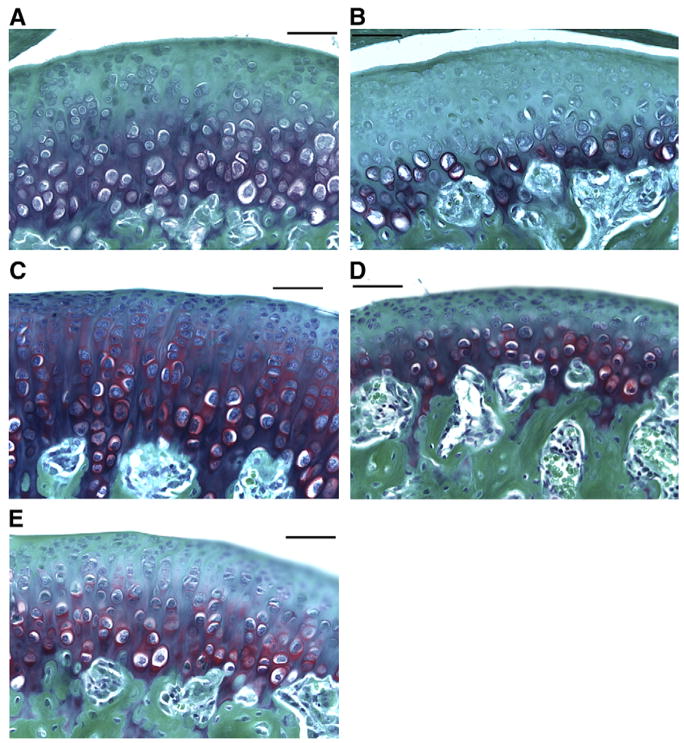
To further characterize the structural changes in the mandibular condylar head in the altered functional loading and return to normal loading groups compared to normal loading group, TMJ sections were stained with Safranin O Safranin O is a cationic dye that binds to the negatively charged glycosaminoglycans. At 2 weeks there was no difference in Safranin O staining between the altered functional and normal loading groups (data not shown). However, at 4 weeks there was a marked decrease of Safranin O staining in the altered functional loading group compared to the normal loading group [Fig. 4(a, b)]. At 6 weeks there was a decrease in Safranin O staining in the mandibular condylar cartilage from the altered functional loading group compared to the normal loading [Fig. 4(c, d)]. There was partial recovery of Safranin O staining after returning to normal loading of the joint for 2 weeks following 4 weeks of altered functional loading [Fig. 4(e)].
Fig. 4.
Representatives of the Safranin O staining (red) of the condylar cartilage. The slides were counter stained with Fast Green. (A) 4-Week normal loading; (B) 4-week altered functional loading; (C) 6-week normal loading; (D) 6-week altered functional loading; and (E) return to normal loading (4-week altered loading followed by 2-week normal loading).
SUBCHONDRAL BONE
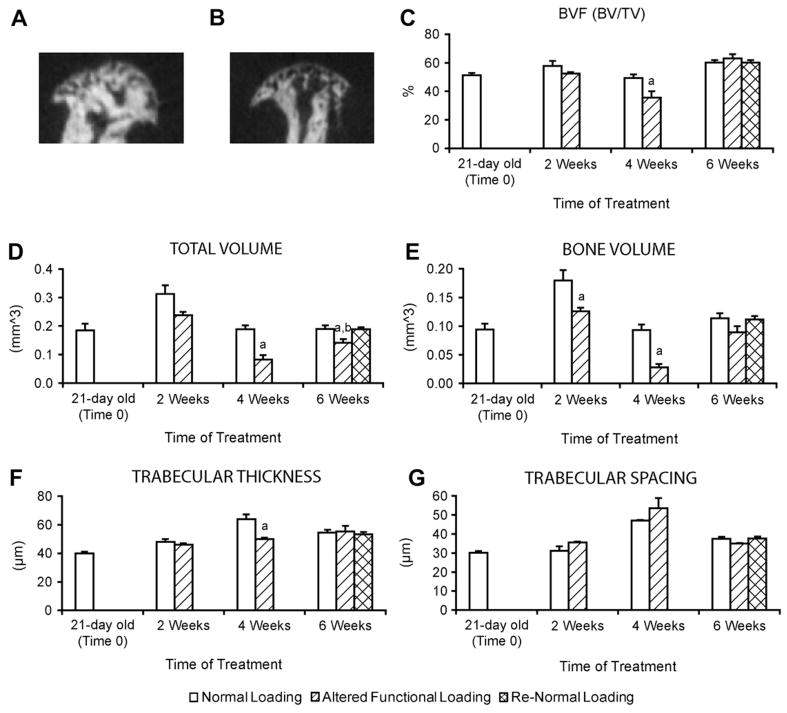
To examine if altered functional TMJ loading caused changes in the micro architecture of the mandibular condylar subchondral bone, micro-CT analysis was performed. Micro-CT analysis revealed that altered functional TMJ loading for 2 weeks caused a significant reduction in the bone volume compared to the normal loading group [Fig. 5(e)]. After 4 weeks, altered functional loading caused a significant decrease in the trabecular thickness [Fig. 5(f)], total volume [Fig. 5(d)], bone volume [Fig. 5(e)], and bone volume fraction [Fig. 5(c)] compared to the normal loading group. After 6 weeks there were no significant differences in any of the micro-CT parameters between the normal loading, altered functional loading, and return to normal loading groups except for a significant decrease in the total volume in the altered functional loading group.
Fig. 5.
Micro-CT analysis of the subchondral bone. Representative images of mid-sagittal cross sections from the mandibular condylar head of 21-day-old CD-1 mice subjected to 4 weeks Normal Loading (A) or Altered functional loading (B). (C–G) Micro-CT analysis of bone volume fraction (C), total volume (D), bone volume (E), trabecular thickness (F) and trabecular spacing (G) from the mandibular condylar subchondral bone of 21 -day-old CD-1 mice subjected to 0 weeks (n = 4), 2 weeks (n = 11), 4 weeks (n = 10), or 6 weeks (n = 19) of normal loading, altered functional loading or return to normal loading TMJ conditions. aSignificant difference between altered loading and normal loading (P < 0.05). bSignificant difference between altered loading and return to normal loading (P< 0.05).
PROTEIN EXPRESSION
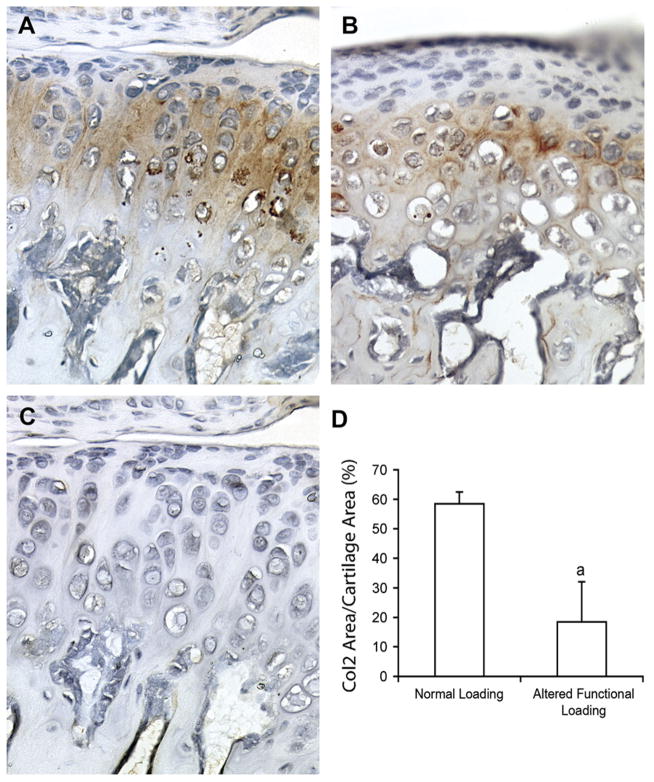
In order to examine if gene expression corresponded to protein expression, immunohistochemistry for COL II and SOX9 was performed. Semiquantitative analysis of COL II and SOX9 expression revealed an approximately 50% significant reduction (P < 0.05) in the percent area of positive COL II area/total area of the condylar cartilage (Fig. 6) and a significant 69% decrease (P < 0.05) in the number of SOX9 positive cells/total number if cells (data not shown) from mice exposed to altered functional loading conditions for 4 weeks compared to mice exposed to normal loading conditions. This 50% decrease in protein expression of COL II and 69% decrease of SOX 9 by altered functional loading corresponded to the decrease in the mRNA expression of Col II of 64% and Sox9 of 43% by altered functional loading at 4 weeks.
Fig. 6.
Immunohistochemistry of Col II (A – normal loading, B – altered functional loading) and negative control (C) performed on sagittal sections of the TMJ area from female CD-1 49-day-old mice. (D) Semiquantitative analysis of COL II positive area normalized by total mandibular condylar cartilage area from 49-day-old CD-1 mice exposed to 4 weeks of normal loading or altered loading. Points are the mean and SEM for n= 11 for the normal loading group and n= 11 for the altered loading group. aSignificant difference between altered loading and normal loading (P<0.05).
Discussion
We found that incisor trimming and soft diet administration in mice caused similar effects in the mandibular cartilage as reported in rats10,11,13,14. Altered functional loading for 4 and 6 weeks caused a decrease in the expression of chondrocyte differentiation markers and a decrease in proteoglycan staining in the mandibular condylar cartilage. However, unlike previous reports in rats12 we found that altered functional loading in mice did not cause a change in the length of the condyle. We also found similarities with the previous report of altered functional loading in a mouse model15. In that study, Sasguri et al. reported by histological observations that there was a thinner layer of cartilage in the mandibular condyle from the altered functional loading group compared to the normal loading group after 2 weeks. We were able to quantitate these results and found a significant decrease in the thickness and width of the condylar cartilage after 2, 4 and 6 weeks of altered functional loading compared to the normal loading group. However, unlike the previous report in mice15, we did not find a significant decrease in the expression of Opn and Oc at anytime point and we found a decrease in Col I expression only at 6 weeks in the altered functional loading group compared to the normal loading group. Interestingly, in that report male mice were examined while in this report we only examined female mice, which may suggest gender differences in the mandibular condyle in response to altered functional loading.
To verify that differences in the TMJ were due to altered functional loading (incisor trimming and soft diet administration), we included at 6 weeks a return to normal loading group, which consisted of altered functional loading for 4 weeks followed by normal loading for 2 weeks. In the study, we found no significant difference in any of the parameters we examined in the return to normal loading group compared to the normal loading group at 6 weeks. Besides serving as a control, the return to normal loading could also be used in future studies examining the effects of aging, gender or various genes (by the use of transgenic mouse technology) on the adaptive mechanical loading capacity of the TMJ.
In growth plate cartilages from long bones of mice, a feedback loop between IHH and PTHrP exists. In these growth plate cartilages, periarticular chondrocytes differentiate into flat, actively proliferating columnar chondrocytes. The columnar chondrocytes then stop proliferating and differentiate into hypertrophic chondrocytes (Col X +). PTHrP has been shown to keep the chondrocytes proliferating and to delay their further differentiation, while IHH is synthesized by chondrocytes that have just stopped proliferating and is required for synthesis of PTHrP. Therefore, the rate of growth and differentiation of the chondrocytes of the growth plate are controlled by the expression of PTHrP and IHH18,19. The IHH–PTHrP feedback loop has also been proposed to be present in the mouse mandibular condylar cartilage because there is decreased expression of PTHrP in the mandibular condylar cartilage from lhh deficient mice compared to normal wildtype controls5. Examination of gene expression in the condylar head in our model showed that incisor trimming and soft diet administration for 4 weeks caused significant decreases in the expression of the chondrocyte differentiation genes markers Sox9, lhh, Col II, and Col X with no decrease in the expression of PTHrP. This is somewhat surprising because one would suspect that a decrease of IHH would cause a decrease in PTHrP because of the IHH–PTHrP feedback loop. One possible explanation is that altered functional loading directly stimulates the expression of PTHrP in the mandibular condylar cartilage, preventing further differentiation and compensating for the decrease in IHH expression. In support of this, altered TMJ loading by the placement of a functional appliance in the mandible of rats has been shown to cause a upregulation of PTHrP expression in the mandibular condylar cartilage20.
Continued altered functional loading for 6 weeks caused similar effect in the mandibular condylar cartilage as 4 weeks of altered functional loading. Examination of gene expression in the 6-week altered loading group revealed that chondrocyte differentiation markers Col II and Sox 9 are significantly decreased, there is a trend for a decrease in lhh and Col X expression and there is no decrease in Pthrp expression compared to the normal loading group. The lack of significance in expression of Col X and lhh in the altered loading group at 6 weeks may be due to the fact that the hypertrophic zone of the mandibular cartilage shrinks as the mice age21.
In the subchondral bone altered functional loading for 4 weeks caused a significant decrease in the bone volume, trabecular thickness, total volume, and bone volume fraction in the subchondral bone compared to the normal loading group. Surprisingly, in the subchondral bone, continued altered functional loading for 6 weeks did not cause a further decrease in micro-CT parameters used to examine the subchondral bone but instead a return of all of them back to the normal loading control values except for total volume. The increase in the bone volume fraction between 4 weeks and 6 weeks of altered functional TMJ loading might be due to a decrease in osteoclastogenesis. The formation of mature bone-resorbing osteoclasts from hematopoietic precursors requires cell–cell interaction with cells from the osteoblastic lineage. The ratio of the expression of OPG/RANKL is a major regulator in the control of osteoclastogenesis, the higher the ratio the less the number of newly formed osteoclasts and vice versa22. For 2–4 weeks of altered functional loading, there was no significant difference in the expression of Opg and Rankl between the altered functional loading groups and the normal loading group. On the other hand, at 6 weeks there was significant decrease in Rankl expression with little change in Opg expression from the altered functional loading compared to the normal and return to normal loading groups. Changes in Rankl expression in the mandibular condylar head from the altered functional loading group at 6 weeks may be due to alterations in the mechanical microenvironment of the subchondral bone. One could imagine that, as the overlaying mandibular condylar cartilage becomes thinner, the underlying subchondral bone is experiencing different forces during masticatory behaviors. In fact, it has been shown that mechanical loading-induced fluid flow on bone marrow stromal cells causes a significant decrease in the RANKL/OPG ratio and a subsequent suppression of the formation of osteoclasts23.
In summary, we have developed an altered functional loading mouse model and found that incisor trimming and soft diet administration for 4–6 weeks caused consistent and transient changes in the mandibular condylar head, including a significant decrease in the size of the mandibular condylar cartilage and a significant decrease in the expression of Col II, Vegf and Sox9 compared to the normal masticatory loading group. Transient changes caused by altered functional loading include a decrease of bone volume fraction in the mandibular subchondral bone at 4 weeks and a return to baseline after 6 weeks of altered functional loading compared to the normal loading group. Greater understanding of how altered functional loading effects the temporal adaptive remodeling changes in the bone and cartilage of the mandibular condylar head is needed to find novel therapies for patients who suffer from degenerative diseases of the TMJ.
Acknowledgments
This work was supported by NIDCR K-22 DE017193 grant (SW), an American Association of Orthodontists Foundation Faculty Development Award (SW) and by the DIR, NIDCR, of the 1RP, NIH, DHHS (TK, MFY).
Footnotes
Conflict of interest statement
All authors have no conflict of interest.
References
- 1.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 2.Emshoff R, Brandlmaier I, Gerhard S, Strobl H, Bertram S, Rudisch A. Magnetic resonance imaging predictors of temporomandibular joint pain. J Am Dent Assoc. 2003;134:705–14. doi: 10.14219/jada.archive.2003.0256. [DOI] [PubMed] [Google Scholar]
- 3.Ohno S, Schmid T, Tanne Y, Kamiya T, Honda K, Ohno-Nakahara M, et al. Expression of superficial zone protein in mandibular condyle cartilage. Osteoarthritis Cartilage. 2006;14:807–13. doi: 10.1016/j.joca.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- 5.Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–34. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 6.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 7.Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 8.Thomas NR, Peyton SC. An electromyographic study of mastication in the freely-moving rat. Arch Oral Biol. 1983;28:939–45. doi: 10.1016/0003-9969(83)90090-0. [DOI] [PubMed] [Google Scholar]
- 9.Hinton RJ, Carlson DS. Response of the mandibular joint to loss of incisal function in the rat. Acta Anat (Basel) 1986;125:145–51. doi: 10.1159/000146153. [DOI] [PubMed] [Google Scholar]
- 10.Hinton RJ. Effect of altered masticatory function on [3H]-thymidine and [35S]-sulfate incorporation in the condylar cartilage of the rat. Acta Anat (Basel) 1988;131:136–9. doi: 10.1159/000146501. [DOI] [PubMed] [Google Scholar]
- 11.Pirttiniemi P, Kantomaa T, Sorsa T. Effect of decreased loading on the metabolic activity of the mandibular condylar cartilage in the rat. Eur J Orthod. 2004;26:1–5. doi: 10.1093/ejo/26.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Tuominen M, Kantomaa T, Pirttiniemi P. Effect of altered loading on condylar growth in the rat. Acta Odontol Scand. 1994;52:129–34. doi: 10.3109/00016359409027586. [DOI] [PubMed] [Google Scholar]
- 13.Kantomaa T, Tuominen M, Pirttiniemi P. Effect of mechanical forces on chondrocyte maturation and differentiation in the mandibular condyle of the rat. J Dent Res. 1994;73:1150–6. doi: 10.1177/00220345940730060401. [DOI] [PubMed] [Google Scholar]
- 14.Pirttiniemi P, Kantomaa T, Salo L, Tuominen M. Effect of reduced articular function on deposition of type I and type II collagens in the mandibular condylar cartilage of the rat. Arch Oral Biol. 1996;41:127–31. doi: 10.1016/0003-9969(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 15.Sasaguri K, Jiang H, Chen J. The effect of altered functional forces on the expression of bone-matrix proteins in developing mouse mandibular condyle. Arch Oral Biol. 1998;43:83–92. doi: 10.1016/s0003-9969(97)00075-7. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Suzuki S, Tengan T, Yamashita Y. A histochemical study of apoptosis in the reduced ameloblasts of erupting mouse molars. Arch Oral Biol. 1995;40:677–80. doi: 10.1016/0003-9969(95)00021-g. [DOI] [PubMed] [Google Scholar]
- 17.Kiraly K, Lammi M, Arokoski J, Lapvetelainen T, Tammi M, Helminen H, et al. Safranin O reduces loss of glycosaminoglycans from bovine articular cartilage during histological specimen preparation. Histochem J. 1996;28:99–107. doi: 10.1007/BF02331414. [DOI] [PubMed] [Google Scholar]
- 18.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–42. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabie AB, Tang GH, Xiong H, Hagg U. PTHrP regulates chondrocyte maturation in condylar cartilage. J Dent Res. 2003;82:627–31. doi: 10.1177/154405910308200811. [DOI] [PubMed] [Google Scholar]
- 21.Hossain KS, Amizuka N, Ikeda N, Nozawa-Inoue K, Suzuki A, Li M, et al. Histochemical evidences on the chronological alterations of the hypertrophic zone of mandibular condylar cartilage. Microsc Res Tech. 2005;67:325–35. doi: 10.1002/jemt.20211. [DOI] [PubMed] [Google Scholar]
- 22.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–7. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]