Abstract
As natural peroxisome proliferator-activated receptor-α (PPARα) ligands, high levels of fatty acids and glucose could lead to hyperactivation of PPARα, like that seen in diabetes. Important diabetes research goals are to uncover new metabolic or signaling pathways involved in hyperglycemic cellular injury and to develop therapeutics for preventing or reversing this injury. Consequently, 1040 putative antidiabetic agents were screened for their ability to 1) affect PPARα lipid binding, 2) directly bind PPARα, and 3) alter PPARα transactivation in the presence of high glucose. A high-throughput fluorescent binding assay was developed to examine each compound’s ability to restore fatty acyl-CoA binding to PPARα in the presence of high glucose concentrations. Approximately 1% of the compounds restored acyl-CoA binding by 60% or more. These compounds directly interacted with PPAR α with high affinity (nM Kds), validating the primary screen. Furthermore, these compounds altered PPARα transactivation, and 1 strongly reversed the hyperactivation of PPARα found in the presence of clofibrate and high glucose levels.
Keywords: nuclear transcription factors, peroxisome proliferator-activated receptors, fluorescent binding assays, glucose
INTRODUCTION
AN IMPORTANT BUT ELUSIVE GOAL of diabetes research is to uncover new pathways involved in hyperglycemic cellular injury and to develop therapeutics to ameliorate this injury. Diabetes pathophysiology involves elevated glucose and long-chain fatty acid (LCFA) levels, both of which function as nuclear signals, regulating transcription of metabolic genes. Although glucose and/or its metabolites regulate several nuclear regulatory proteins that enhance transcriptional genes encoding glycolytic and lipogenic enzymes,1 the mechanisms linking glucose and its metabolites to these regulators are largely unknown. In contrast, LCFA and LCFA metabolites (e.g., long-chain fatty acyl-CoAs [LCFA-CoA]) are established endogenous ligands of several nuclear receptors, including the peroxisome proliferator-activated receptor-α (PPARα),2,3 which initiates transcription of multiple genes involved in β-oxidation and glucose metabolism.4–6
Abnormal PPAR activation by elevated LCFA levels contributes to lipotoxicity associated with obesity, insulin resistance, type 2 diabetes, and hyperlipidemia.4,5 Cardiac-specific overexpression of PPARα hyperactivates genes for fatty acid uptake and oxidation while downregulating genes for glucose utilization.7,8 Conversely, PPARα-null mice demonstrate reduced fatty acid oxidation and protection from high-fat diet–induced insulin resistance.9,10 This suggests that PPARα plays a pivotal role in diabetic cardiomyopathy through the overinduction of β-oxidation at the expense of glucose metabolism.
The finding that glucose is a high-affinity endogenous ligand of PPARα11 provides a mechanistic role of PPARα in energy homeostasis and a possible target for the treatment of diabetic complications. Herein we demonstrate that hyperglycemic glucose concentrations alter PPARα secondary structure, displace bound fluorescent LCFA-CoA, and potentiate fibrate-induced β-oxidation. A novel fluorescence-based screening assay was designed to identify compounds that restore PPARα binding of LCFA-CoA in the presence of high glucose. Such compounds bound PPARα with high affinity and altered PPARα transactivation of acyl-CoA oxidase but did not hyperactivate PPARα in the presence of high glucose.
MATERIALS AND METHODS
Protein expression and purification
The vector pET-PPARαΔAB2 was expressed in BL21-(DE3)pLysS cells and purified as described.3,11 For comparing results obtained herein with earlier findings,11 the murine PPARα was used, which shares >90% homology with the human PPARα-LBD. This truncated version was used because of solubility issues with the full-length protein that complicated its purification. This PPARα construct is expected to show ligand-binding properties identical to the full-length receptor based on similar experiments with PPARγ.4,12,13
Circular dichroism
Circular dichroic (CD) spectra of 0.8 μM PPARαΔAB were obtained with a J-710 spectropolarimeter (JASCO Inc., Easton, MD).11 Ten scans were averaged for secondary structure analysis by 3 different methods (SELCON3, CDSSTR, and CONTIN/LL) using CDPro software.3
PPARα binding assays to BODIPY-C16 fatty acid or fatty acyl-CoA
PPARαΔAB protein (100 nM) was titrated with increasing BODIPY-C16-CoA (Invitrogen, Carlsbad, CA), and emission spectra (300–400 nm) were obtained at 24 °C after excitation at 280 nm using a PC1 photon-counting spectrofluorometer (ISS Inc., Champaign, IL). Data were corrected for background (buffer, fluorescent ligand, solvent) and maximal intensities used to calculate the binding affinity.3 To determine the effect of glucose on lipid binding, 50 nM BODIPY-C16 or BODIPY-C16-CoA was bound to 100 nM PPARαΔAB and titrated with glucose, and emission spectra (490–540 nm) were obtained upon excitation at 460 nm. Data were corrected for background effects, and maximal intensities were used to calculate the percentage change in BODIPY-C16 and BODIPY-C16-CoA binding versus no glucose controls.
Primary drug-screening assay
A fluorescence-based assay was developed to allow high-throughput screening of 1040 putative antidiabetic agents (MicroSource Discovery Systems Inc., NIH/JDRF custom collection, Gaylordsville, CT) for their ability to restore LCFA-CoA binding to PPARα in the presence of 20 mM glucose. PPARαΔAB protein (200 nM) in phosphate-buffered saline (PBS) was incubated with 10 nM BODIPY-C16-CoA at 20 °C for 10 min, 20 mM glucose was added, and it was incubated for 10 more minutes at 20 °C. Samples were prepared in 10-ml master mixes, and 100 μl per well was transferred to white 96-well plates (Corning, Corning, NY). Compounds (1.25 μM) were added to the appropriate well, mixed, and incubated at 20 °C for 10 min to reach equilibrium. Fluorescence (100-ms integration time; 5 scans/sample) was quantitated at 24 °C with a Fluoroskan Ascent fluorometer (Thermo Scientific, Waltham, MA) using 460-nm excitation and 510-nm emission filters. Samples were run in triplicate on 3 different plates, and measurements were assigned arbitrary units (a.u.). Background fluorescence of the 96-well plate, PBS, and each compound was measured and subtracted from the BODIPY fluorescence obtained for samples and plate controls. Controls for each plate consisted of PBS, protein, and BODIPY-C16-CoA in the absence and presence of 20 mM glucose. Data were shown as the mean percentage recovery of BODIPY-C16-CoA binding.
Direct binding of compounds by PPARα
Direct binding to PPARαΔAB was determined by quenching of intrinsic PPARαΔAB aromatic amino acid fluorescence.3,11 Briefly, 0.1 μM PPARαΔAB in 2 ml of 20 mM glucose in PBS was titrated with each compound, excited at 280 nm, and emission (300–380 nm) obtained with the above PC1 photon-counting spectrofluorometer. Fluorescence was corrected for background (buffer, compound fluorescence, solvent), and maximal intensities were used to calculate the binding affinity (Kd)3.
Transactivation assays
COS-7 cells (ATCC, Manassas, VA) seeded onto 24-well culture plates were transfected with 0.4 μg of each full-length mammalian expression vector (pSG5-PPARα and pSG5-mRXRα) or empty plasmid (pSG5; Stratagene, La Jolla, CA), 0.4 μg of reporter PPRE3-TK-LUC14, and 0.04 μg of transfection control (pRL-CMV; Promega, Madison, WI).11 Following transfection, media were replaced with serum-free, glucose-free DMEM (Invitrogen, Carlsbad, CA) for 2 h, ligands were added, and cells were grown for 24 additional hours. Each compound (10 μM) was examined at 0 mM or 20 mM glucose, with or without 10 μM clofibrate. Firefly luciferase activity, normalized to Renilla luciferase (for transfection efficiency), was determined with the dual-luciferase reporter assay (Promega, Madison, WI), and luminescence was measured with a Synergy-2 microplate reader (BioTek Instruments Inc., Winooski, VT).
RESULTS AND DISCUSSION
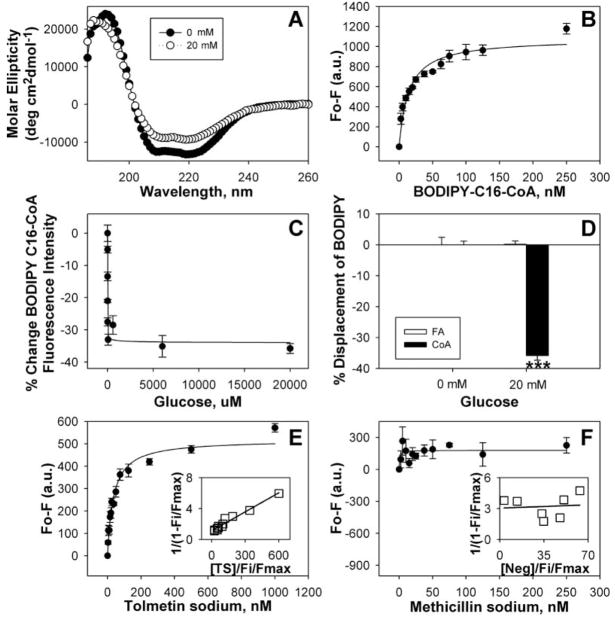
Low glucose concentrations alter PPARα secondary structure and decrease BODIPY-C16-CoA binding.11 To determine if this effect was exacerbated by higher glucose concentrations as seen in diabetes, PPARα protein was incubated with 20 mM glucose and examined by CD (Fig. 1A). The α-helical structures were decreased concomitant with increased β-sheets and unordered structures (Table 1), significantly more than observed with lower glucose levels.11 Strong, saturable binding of BODIPY-C16-CoA by PPARα (Fig. 1B; Kd = 13.9 ± 1.9 nM) allowed for the determination of glucose effects on acyl-CoA binding. Although high glucose significantly decreased BODIPY-C16-CoA binding to PPARα, this decrease was only slightly lower than with lower glucose concentrations (Fig. 1C). Reduced LCFA-CoA binding to PPARα was specific because PPARα bound fatty acid (BODIPY-C16) was not affected by high glucose (Fig. 1D). Compared with low glucose, diabetic concentrations of glucose exacerbated alterations in PPARα secondary structure but not fatty acyl-CoA binding in vitro.
FIG. 1.
Circular dichroic (CD) spectra and ligand binding assays with peroxisome proliferator-activated receptor-α (PPARα). (A) CD spectra of PPARαΔAB with 0 mM (filled circles) and 20 mM (open circles) glucose. Each spectrum represents an average of 10 scans for a given representative spectrum from 3 replicates. (B) Binding curve of the change in fluorescence intensity (Fo-F) of PPARαΔAB titrated with BODIPY-C16-CoA. (C) The percentage change in PPARα binding to BODIPY-C16-CoA as a function of glucose concentration. (D) The percentage change in PPARαΔ AB binding to BODIPY-C16 (open bars) and BODIPY-C16-CoA (filled bars) in the presence of 20 mM glucose, measured as the change in fluorescence intensity after background subtraction. Asterisks represent significant differences as compared with the no glucose control. *p < 0.05, **p < 0.01, ***p < 0.001. (E, F) Binding curves of the change in PPARαΔAB intrinsic fluorescence in the presence of 20 mM glucose upon titration with tolmetin sodium (E) and methicillin sodium (negative control) (F). Values are the mean ± SEM (n = 3–4). Insets are double reciprocal plots of the mean binding curve data presented in each panel.
Table 1.
Percentage Composition of Peroxisome Proliferator-Activated Receptor-α (PPARα) Secondary Structures in the Absence and Presence of 20 mM Glucose
| α-Helices Regular, Hr (%) | α-Helices Distorted, Hd (%) | β-Sheets Regular, Sr (%) | β-Sheets Distorted, Sd (%) | Turns (%) | Unordered (%) | |
|---|---|---|---|---|---|---|
| PPARα | 23.8 ± 0.6 | 15.7 ± 0.2 | 8.2 ± 0.2 | 6.9 ± 0.2 | 19.1 ± 0.4 | 26.6 ± 0.2 |
| 20 mM glucose | 14.3 ± 0.3*** | 10.8 ± 0.3*** | 14.4 ± 1.2*** | 8.8 ± 0.5*** | 20.2 ± 0.7 | 30.7 ± 0.8*** |
Values represent the mean ± SEM (n = 4). Significant differences were determined by Student’s t-test.
p < 0.001.
A high-throughput assay was developed for screening putative antidiabetic agents and used to examine a commercially available library of 1040 compounds. Because glucose inhibits PPARα binding to a fluorescent LCFA-CoA analogue, this assay examines compounds for their ability to restore acyl-CoA binding to PPARα at high glucose. Restoration of LCFA-CoA binding could be important in treating diabetes because high glucose inhibits LCFA-CoA binding while not affecting LCFA binding. Although PPARα normally binds to both lipids to control energy homeostasis, the presence of hyperglycemic glucose may shift the equilibrium such that fatty acid oxidation is favored over glucose metabolism. By allowing both lipids to bind in the presence of glucose, equilibrium may be reestablished such that both fatty acids and glucose are available for energy production.
To prevent micelle formation and fluorescent artifacts with this assay, minimal amounts of protein and BODIPY-C16-CoA (submicellar) were required to obtain a strong, reproducible signal that was significantly altered by 20 mM glucose. This combination resulted in a ~30% decrease in fluorescence intensity in the presence of 20 mM glucose. Although this was slightly less of a difference than in the cuvette-based assay (Fig. 1B), which used higher BODIPY-C16-CoA concentrations, these values gave an acceptable Z′ factor of 0.6. Preliminary screening of a small subset of compounds to optimize compound concentration and incubation time suggested a target compound concentration of 1.25 μM, because concentrations <1 μM were difficult to distinguish from background and concentrations >3 μM resulted in nonspecific effects by some compounds. For each incubation step, multiple time points were examined to determine the time required for binding equilibrium. In each case, no significant differences in fluorescence intensity were noted among time points beyond 7 min.
Compounds fell into 1 of 4 categories: 1) 75 restored acyl-CoA binding, 2) 235 further decreased acyl-CoA binding, 3) 673 resulted in no change to acyl-CoA binding (<3 standard deviations from control values), and 4) 57 were undeterminable because of compound interference with the fluorescence assay. The top 1% of compounds capable of restoring acyl-CoA binding ranged from gedunin with 62.3% recovery to tolmetin sodium with 97.1% recovery (Table 2), and these compounds were further examined. This tripartite assay differed from previous PPARα assays in that it 1) used fatty acyl-CoA binding rather than fatty acid binding, 2) used high glucose, and 3) separated compounds capable of binding PPARα into groups that increased or decreased acyl-CoA binding. Although competition binding assays, ligand-induced complex formation assays, coactivator-dependent receptor ligand assays, and differential protease sensitivity assays have shown fibrates to be ligands of PPARα,4 clofibric acid, gemfibrozil, and bezafibrate had no effect on acyl-CoA binding (group 3), whereas fenofibrate further reduced acyl-CoA binding (group 2). Although statins are known PPARα activators,15 lovastatin and rosuvastatin had no effect on acyl-CoA binding (group 3), whereas pravastatin and atorvastatin further reduced acyl-CoA binding (group 2). Through the selection of compounds capable of restoring acyl-CoA binding, a different set of PPARα ligands than from previous assays was examined.
Table 2.
Most Efficient Compounds at Reversing the Effects of High Glucose on Acyl-CoA Binding to Peroxisome Proliferator-Activated Receptor-α (PPARα)
| Compound | Recovery (%) | Kd (nM) |
|---|---|---|
| Tolmetin sodium | 97.1 | 35.1 ± 3.7 |
| Hyoscyamine | 86.4 | 14.8 ± 2.8 |
| g-aminobutyric acid | 85.5 | 29.5 ± 4.1 |
| Salicylamide | 83.1 | 25.8 ± 6.0 |
| Budesonide | 82.0 | 26.3 ± 3.2 |
| Saccharin | 81.3 | 22.5 ± 2.0 |
| Tolazoline hydrochloride | 73.9 | 31.4 ± 3.6 |
| Pyrazinamide | 68.6 | 28.4 ± 2.3 |
| Targinine hydrochloride | 67.5 | 33.5 ± 3.5 |
| Enalapril maleate | 64.7 | 26.3 ± 2.8 |
| Acetylglucosamine | 63.8 | 33.3 ± 3.0 |
| Gedunin | 62.3 | 21.9 ± 1.8 |
Recovery is defined as the ability to restore acyl-CoA binding, and Kd is defined as the binding affinity of PPARα for each compound. Values represent the mean ± SEM (n = 3–4).
To confirm the primary screen, the affinity of PPARα for the above 12 compounds was determined in the presence of high glucose. This assay was used, rather than titration with the BODIPY-C16-CoA, to examine each compound’s ability to directly bind PPARα (regardless of lipid status) and to ensure that fluorescent changes in the primary screen were not an artifact of the compound interacting with the BODIPY-C16-CoA. Titration of PPARα with each of the 12 compounds resulted in strong, saturable binding at a single binding site, as indicated by the sharp saturation curve obtained from the change in intrinsic fluorescence intensity with increasing compound concentration (Fig. 1E; tolmetin sodium). However, titration of PPARα with a compound that did not affect acyl-CoA binding (methicillin sodium) did not alter PPARα intrinsic fluorescence (Fig. 1F), demonstrating no binding. Multiple replicates yielded high PPAR α affinity (nM Kds) for the 12 compounds, whereas no Kd value could be obtained for the negative compound (Table 2), confirming the primary screen as a valid assay for compounds bound by PPARα. The similar binding affinities of these 12 compounds is most likely explained by the facts that 1) only compounds most capable of restoring acyl-CoA binding were examined and 2) PPARα binds both BODIPY-C16-CoA and glucose very strongly, so for a compound to affect these interactions, it would also need to bind strongly.
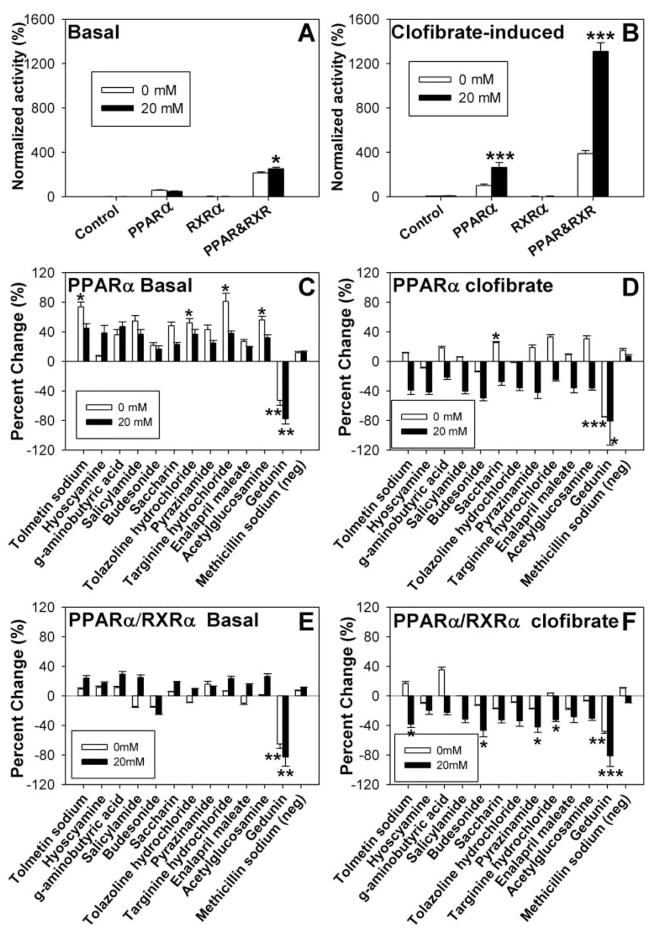
Because PPARα forms heterodimers with the retinoid X receptor-α (RXRα) to induce transactivation,14 transactivation experiments were performed with the 12 compounds (with or without 20 mM glucose) in cells expressing PPARα, RXRα, PPARα, and RXRα, or pSG5 empty vector (negative control). Transactivation was measured as the expression of firefly luciferase upon protein interaction with the acyl-CoA oxidase promoter normalized for Renilla luciferase.11 In the absence of any other ligand, PPARα and RXRα heterodimers showed increased activity in the presence of 20 mM glucose, although no significant effect on PPARα alone was noted (Fig. 2A). However, in the presence of 10 μM clofibrate, a lipid-lowering xenobiotic known to activate PPARα,14 the addition of glucose also increased transactivation (both PPARα alone and PPARα-RXRα heterodimers), 3- to 4-fold more than clofibrate alone (Fig. 2B).
FIG. 2.
Glucose and compound effects on peroxisome proliferator-activated receptor-α (PPARα) transactivation. COS-7 cells transfected with PPARα, retinoid X receptor-α (RXRα), PPARα and RXRα, or pSG5 empty vector were analyzed for basal (A) and clofibrate-induced (B) transactivation of an acyl-CoA oxidase reporter construct in the absence (open bars) and presence of 20 mM glucose (filled bars). Transactivation values are presented as the percentage firefly luciferase activity normalized to Renilla luciferase (internal control), where clofibrate-induced PPARα activity in the absence of ligand is arbitrarily set to 100%. COS-7 cells transfected with PPARα (C, D) or PPARα and RXRα ( E, F ) were examined for compound effect on basal (C, E) and clofibrate-induced (D, F) transactivation of the acyl-CoA oxidase reporter construct in the absence (open bars) and presence (filled bars) of 20 mM glucose. Transactivation values are presented as the mean percentage change from the no-compound control ± SEM (n = 3). Asterisks (*) represent significant differences as compared with the no-glucose control for panels A and B or the no-compound control for panels C through F. *p < 0.05, **p < 0.01, ***p < 0.001.
To compare the effects of each compound on PPARα transactivation, all values are presented as the percentage change for the addition of the compound for a given condition. Although most compounds increased PPARα activity, both in the presence and absence of glucose, only a few of these changes were significant (Fig. 2C). Tolmetin sodium, tolazine hydrochloride, targinine hydrochloride, and acetylglucosamine increased PPARα transactivation by 50% or more in the absence of glucose (Fig. 2C, open bars), similar to clofibrate effects. However, in the presence of glucose, the addition of the compounds had little effect, an effect opposite that of clofibrate (Fig. 2C, filled bars). With or without glucose, the addition of 10 μM gedunin significantly reduced PPARα activity (Fig. 2C). Although slight changes were noted for compound effects on PPARα-RXRα heterodimers, only gedunin elicited significant changes, resulting in decreased activity with or without glucose (Fig. 2E). No significant differences were noted for RXRα only or pSG5 empty vector by addition of any of the compounds (data not shown).
Because clofibrate is a strong PPARα activator that affects PPARα similarly to LCFA, both in the absence and presence of glucose,11 it was used to imitate the PPARα hyperactivation seen under diabetic conditions (e.g., high glucose and high LCFA). Each compound was examined for effects on clofibrate-induced PPARα transactivation in the absence of glucose (i.e., competition, synergistic increases, or no effect), as well as its ability to reverse the hyperactivation seen with high glucose. Only minor changes were noted in the absence of glucose, except for significant increases in response to saccharin and decreases to gedunin (Fig. 2D, open bars). Each of the compounds slightly decreased the PPARα hyperactivation, although only gedunin resulted in significant changes (Fig. 2D, filled bars). For the most part, this trend was echoed for PPARα-RXRα heterodimer activity (Fig. 2F). In the presence of clofibrate and glucose, tolmetin sodium, budesonide, pyrazinamide, targinine hydrochloride, and gedunin significantly decreased PPARα-RXRα activity, whereas only gedunin significantly affected activity in the absence of glucose (Fig. 2F). Again, no significant differences were noted for RXRα only or pSG5 empty vector by addition of any of the compounds (data not shown).
Because the pathophysiology of diabetes includes both elevated LCFA and glucose levels, ideally a therapeutic would increase both glucose and fatty acid metabolism. Unfortunately, current therapeutics involve PPARα function to increase fatty acid oxidation at the expense of glucose metabolism, leading to increased susceptibility to injury during ischemia.6 Although further testing is required to determine the effect of these compounds on glucose uptake and utilization, by increasing fatty acid oxidation without hyperactivating fatty acid oxidation in the presence of high glucose, these compounds may help maintain energy homeostasis.
This work presents a rapid fluorescence assay for examining potential therapeutics for the treatment of diabetic complications by examining compounds for their ability to restore acyl-CoA binding to PPARα. The compounds most capable of restoring acyl-CoA binding were found to 1) bind to PPARα with high affinity, 2) alter PPARα secondary structure, and 3) affect PPARα transactivation. Each of the examined compounds bound to PPARα with affinities only slightly lower than those previously determined for PPARα binding to endogenous ligands,2,3 and compounds that did not alter LCFA-CoA binding did not bind to or interact with PPARα. Furthermore, compounds found as potential therapeutics by the primary screen also altered PPARα transactivation, although not all changes were significant at the examined concentration. Taken together, these data validate the primary screen as a high-throughput method for examining potential therapeutics for PPARα interactions.
Acknowledgments
We gratefully thank Noa Noy (Case Western University, Cleveland, OH) for PPARα bacterial expression vector, Sander Kersten (Wageningen University, the Netherlands) for PPARα mammalian expression vector, Pierre Chambon (Université Louis Pasteur, France) for RXRα mammalian expression vector, and Ronald Evans (Salk Institute, San Diego, CA) for PPRE3-TK-LUC reporter construct. This work was supported in part by an administrative supplement to USPHS NIH grant R01DK41402 (to F.S. and A.B.K.) and NIH grant K99DK77573 (to H.A.H.).
References
- 1.Girard J, Ferre P, Foufelle F. Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Ann Rev Nutr. 1997;17:325–352. doi: 10.1146/annurev.nutr.17.1.325. [DOI] [PubMed] [Google Scholar]
- 2.Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor-α. Biochemistry. 1999;38:185–190. doi: 10.1021/bi9816094. [DOI] [PubMed] [Google Scholar]
- 3.Hostetler HA, Petrescu AD, Kier AB, Schroeder F. Peroxisome proliferator activated receptor-α interacts with high affinity and is conformationally responsive to endogenous ligands. J Biol Chem. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- 4.Desvergne B, Wahli W. Peroxisome proliferator activated receptors: nuclear control of metabolism. Endocrine Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 5.Frederiksen KS, Wulf EM, Wassermann K, Sauerberg P, Fleckner J. Identification of hepatic transcriptional changes in insulin-resistant rats treated with peroxisome proliferator activated-receptor-alpha agonists. J Mol Endocrinol. 2003;30:317–329. doi: 10.1677/jme.0.0300317. [DOI] [PubMed] [Google Scholar]
- 6.Panagia M, Gibbons GF, Radda GK, Clarke K. PPAR-α activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. Am J Physiol Heart Circ Physiol. 2005;288:H2677–H2683. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- 7.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S-H, Cho Y-R, Finck BN, Kim H-J, Higahimori T, Hong E-G, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, et al. Altered constitutive expression of fatty acid metabolizing enzymes in mice lacking PPARα. J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 10.Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, et al. PPARα null mice are protected from high fat diet-induced insulin resistance. Diabetes. 2001;50:2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- 11.Hostetler HA, Huang H, Kier AB, Schroeder F. Glucose directly links to lipid metabolism through high-affinity interaction with peroxisome proliferator-activated receptor-α. J Biol Chem. 2008;283(4):2246–2254. doi: 10.1074/jbc.M705138200. [DOI] [PubMed] [Google Scholar]
- 12.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta(12,14)-prostaglandin J(2) is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J(2) metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 14.Kliewer SA, Umesono K, Noon DJ, Heyman RA, Evans RM. Convergence of 9-cis-retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasinska M, Owaczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483–499. [PubMed] [Google Scholar]