Abstract
The principal nucleus of the bed nucleus of the stria terminalis (BNSTp) is larger in males than in females of several species. We previously demonstrated that in mice lacking the pro-death gene, bax, total BNSTp cell number is increased and sex differences in cell number are eliminated. This suggests that Bax-dependent cell death underlies sexual differentiation of the BNSTp. However, it is not known what cells in the BNSTp are affected by bax deletion. Here we used immunohistochemistry and stereological techniques to quantify phenotypically-identified cells in the BNSTp of adult male and female bax -/- and bax +/+ mice. Sections were thionin-stained, or double-labeled for NeuN and GFAP to identify mature neurons and astrocytes, respectively; an additional series was labeled for androgen receptor (AR). As previously demonstrated, sex differences in BNSTp area and overall cell number were seen in wildtype mice, but absent in bax -/- animals. In addition, sex differences (favoring males) were present in the number of NeuN+ and AR+ cells in wildtype mice. Bax gene deletion significantly increased the number of NeuN+ and AR+ cells and reduced or eliminated the sex differences in these cell types. The number of astrocytes in the BNSTp was not sexually dimorphic, nor significantly affected by bax gene status, although there was a trend for more GFAP+ cells in bax -/- mice. Overall brain weight was also greater in bax -/- animals compared to controls. We conclude that the sex differences in neuron and AR+ cell number are due at least in part to Bax-mediated cell death. Increased NeuN+ and AR+ cell number in bax -/- mice suggests that supernumerary cells in bax knockouts differentiate similarly to those in wildtype mice, and retain the capacity to respond to androgens.
Keywords: apoptosis, cell death, differentiation, GFAP, sex difference, testosterone
A major facet of sexual differentiation of the vertebrate nervous system is the sculpting of cell number in a sex typical manner. Many of the best-studied neural sexual dimorphisms manifest as either a sex difference in the total number of cells, or in the number of neurons of a particular phenotype within a neural region. Sex differences in cell number could result from differences in cell birth, differentiation, migration, or death, although hormone regulated cell death is currently the best established mechanism underlying sex differences in adult cell number (reviewed in Forger, 2006).
Support for the role of cell death in neural sex differences comes from recent work using mice genetically altered to over- or under-express particular cell death proteins. Specifically, members of the Bcl-2 family of proteins are key regulators of cell death in a variety of tissue types (Merry and Korsmeyer, 1997) and Bax, a pro-death member of the family, is critically important for apoptosis of many developing neurons (White et al., 1998). In mice lacking the bax gene, sex differences in cell number are eliminated in several brain regions, including the anteroventral periventricular nucleus (AVPV) and the principal nucleus of the bed nucleus of the stria terminalis (BNSTp) (Forger et al., 2004).
The BNSTp is a key component of an integrated neural circuit that regulates sexual and social behaviors in rodents. Pheromonal and olfactory information are relayed from the accessory olfactory bulb to the BNSTp, which in turn projects to several sexually dimorphic hypothalamic nuclei (Simerly, 2002). The BNSTp has a high density of both androgen and estrogen receptors (Simerly et al., 1990). It is larger overall in male than in female rats, mice, guinea pigs, and humans (Hines et al., 1985; del Abril et al., 1987; Guillamon et al., 1988; Allen and Gorski, 1990; Forger et al., 2004), and the number of androgen receptor (AR)-expressing cells, or the ‘cloud’ of AR-expressing cells, is also larger in males (Wersinger et al., 1997; Shah et al., 2004).
Although deletion of the bax gene in mice eliminates the sex differences in BNSTp volume and total cell number (Forger et al., 2004), this finding is based on cells identified in a thionin stain. The BNSTp is a heterogeneous nucleus consisting of multiple cell types, including, but not limited to cells expressing AR, estrogen receptors α and β, progestin receptors, galanin, vasopressin (VP), cholecystokinin, and substance P (Malsbury and McKay, 1987; Micevych et al., 1988; Wersinger et al., 1997; Han and De Vries, 1999; Greco et al., 2001; Mitra et al., 2003; Shah et al., 2004). These categories are not mutually exclusive; for example since most neurons in the mouse BNSTp express AR (Shah et al., 2004), many markers co-localize with AR. The only subset of BNSTp neurons to be examined thus far in bax -/- mice is those that express the neuropeptide VP. VP cells are much more numerous in the BNSTp of males than of females, but deletion of the bax gene does not affect this sex difference (De Vries et al., 2008). In the present study, we used immunohistochemistry to quantify three phenotypically identified cell types in the BNSTp of bax +/+ and bax -/- animals. Counts of total cell number (based on a thionin stain) were compared with counts of cells expressing NeuN, a marker of mature neurons, glial fibrillary acidic protein (GFAP), a marker of astrocytes, and the AR.
Experimental Procedures
Animals and tissue collection
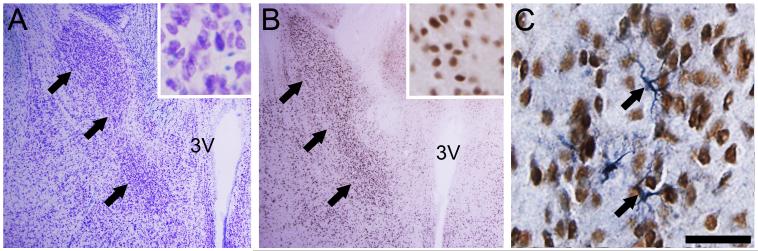
Bax +/+ and bax -/- mice were generated by pairing bax +/- males and females from our breeding colony. Genotyping was performed by PCR amplification of tail DNA using established primer sequences (White et al., 1998). The mice used in the present experiment served in a previous study of motor and sexual behaviors (Cohort II in Jyotika et al., 2007). All animals were gonadectomized at 2-3 months of age and received a subcutaneous Silastic capsule (1.02 mm ID × 2.16 mm OD) packed with 5 mm of crystalline testosterone. Two to three months later, and one week after the last behavioral test, animals were subcutaneously injected with 500 μg of dihydrotestosterone (Zhou et al., 1994). This bolus of androgen was provided to maximize the translocation of AR to the nucleus and ensure that all AR+ cells would be detected. Thirty minutes later, animals were weighed, deeply anesthetized, and transcardially perfused with 4% paraformaldehyde. Brains were extracted, post-fixed for 4 hours and transferred to 30% sucrose for cryoprotection. Brains were then blocked (cerebellum and olfactory bulbs removed), weighed by an investigator blind to experimental group, and frozen-sectioned at 30 μm in the coronal plane into four series. One series was stained with thionin (Figure 1A) and the others were stored at -20°C until processing.
Figure 1.
Photomicrographs of cross-sections through the BNSTp in tissue stained for thionin (A), androgen receptor immunohistochemistry (B), or NeuN/GFAP double-label immunohistochemistry (C). (A) and (B) are adjacent sections from a bax -/- male mouse; the lateral border of the BNSTp is marked by black arrows and insets are taken from the center of the cell group. In (C), NeuN+ cells are identified by brown reaction product and GFAP+ cells are identified by blue/grey reaction product; arrows point to two GFAP+ cells. Scale bar = 500 μm for A and B (50 μm for inserts) and 30 μm for C.
Immunohistochemistry
One series of sections was processed for double-label immunohistochemistry (IHC) for NeuN and GFAP, markers for mature neurons and mature astrocytes, respectively (Figure 1C). Tissue was rinsed in 0.05M tris buffered saline (TBS), and incubated for 45 minutes in TX100 [TBS + 10% normal horse serum (NHS) + 0.35% Triton X] with avidin blocking solution and 1% H2O2 at room temperature. Next, tissue was incubated in NeuN primary antibody [1:1000 mouse anti-NeuN monoclonal antibody (Chemicon International, Temecula, CA)] in TX100 + biotin blocking solution for 40 hours at 4°C. Secondary antibody incubation [1:200 horse anti-mouse (Vector Laboratories, Burlingame, CA) in TBS with NHS and Triton X for 90 min at room temperature] was followed by a 90 min ABC incubation (Vector Laboratories, Burlingame, CA). NeuN staining was visualized using a diaminobenzidine (DAB) reaction and tissue was rinsed 3x5 min with TBS between all steps. GFAP IHC (1:1000 rabbit anti-GFAP polyclonal antibody; Chemicon International) immediately followed the DAB reaction. All steps were the same except that normal goat serum (NGS) and goat anti-rabbit were used and visualization was performed using Vector SG (Vector Laboratories). Tissue was then mounted onto gel coated slides, dehydrated and coverslipped using Permount (Fisher Scientific).
A separate series was processed for AR IHC (Figure 1B). Sections were rinsed with TBS, and incubated in 1.47% sodium citrate at 80°C for 30 min. Following a 30 min incubation in blocking solution (TBS + 4% NGS + 0.35% Triton X-100) with 1% H2O2, tissue was transferred to the primary antibody solution (blocking solution plus 2 μl/ml of the polyclonal AR antiserum, PG21, a generous gift from Dr. Gail Prins) for 40 hours at 4°C. A 90 min secondary antibody incubation [1:400 goat anti-rabbit (Vector Laboratories) in TBS with 4% NGS] was followed by a 90 min ABC wash. Visualization was achieved using a nickel-enhanced DAB reaction. TBS washes were performed between all steps and after the DAB reaction.
Morphometry and statistical analyses
All analyses were performed on slides coded to conceal the sex and genotype of the animals. Stereological analyses of overall volume, thionin cell number, NeuN+ cell number, GFAP+ cell number and AR+ cell number were performed using StereoInvestigator software (MicroBrightfield, Williston, VT). To calculate overall volume of the BNSTp in thionin and AR stained tissue, outlines were bilaterally traced in each section, and the summed areas multiplied by section thickness. Unbiased estimates of the number of each cell type were obtained using the optical disector method. A counting frame of 20 × 20 μm was systematically moved through the traced area, with counts performed every 100 μm (i.e., a 100 × 100 μm sampling grid, as in Forger et al., 2004). Cells were counted bilaterally throughout the rostral-caudal extent of the BNSTp for each stain; estimates of the total number for each cell type incorporate the number of cells counted per section, section thickness, and sampling ratio. Thionin stained cells were counted if they had the size and staining characteristics consistent with neuronal morphology. Boundaries for NeuN and GFAP cell counts were based on tracings of thionin-stained tissue.
Data were analyzed by two-way ANOVAs (sex-by-genotype) except where indicated below. Planned comparisons using Fisher’s LSD were performed following significant main effects or interactions in the ANOVA; tests are two-tailed unless otherwise noted.
Results
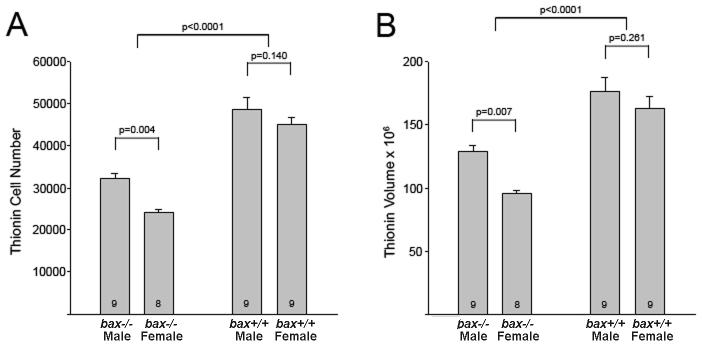
As expected, we observed a main effect of sex, favoring males, on BNSTp volume (F1,31 = 8.1, p = 0.008) and total cell number (F1,31 = 10.9, p = 0.002) in thionin stained tissue. Bax deletion increased both volume (F1,31 = 49.3, p < 0.0001) and cell number (F1,31 = 108.8, p < 0.0001) in males and females. Planned comparisons indicate that cell number and volume were sexually dimorphic in wildtype mice (p < 0.007 in both cases) but not in bax knockouts (Figures 2A and B). Thus, in confirmation of previous findings (Forger et al., 2004), bax deletion increases total cell number and eliminates the sex difference in cell number in the BNSTp.
Figure 2.
Mean (+SEM) BNSTp cell number (A) and volume (μm3) (B) in wildtype (bax +/+) and bax -/- mice based on thionin stained tissue.
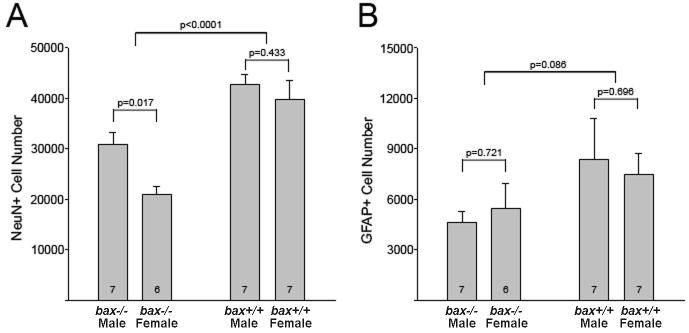
IHC demonstrates that the sex difference in total cell number is due to a greater number of neurons in males. Wildtype males had more NeuN+ cells than wildtype females (p = 0.017). Bax deletion increased NeuN+ cell number in both sexes (F1,23 = 32.5; p < 0.001) and eliminated the sex difference (p = 0.433) (Figure 3A). We observed no sex difference in the number of GFAP+ cells (F1,23 = 0.001, p = 0.99). There also was no significant effect of bax deletion on glial cell number, despite a trend for an increased number of GFAP+ cells in bax-/- mice (F1,23 = 3.2, p = 0.086) (Figure 3B).
Figure 3.
Mean (+SEM) NeuN+ (A) and GFAP+ (B) cell number in the BNSTp of wildtype (bax +/+) and bax -/- mice.
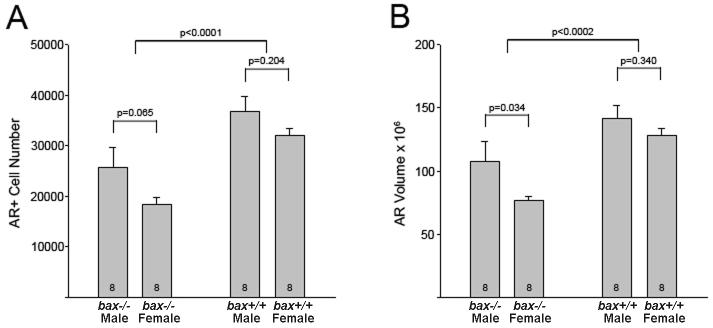
Consistent with Shah et al. (2004), we report a greater number of AR+ cells in the BNSTp of males than of females (F1,28 = 5.2, p = 0.030) and further demonstrate that bax deletion increased AR+ cell number (F1,28 = 21.7, p < 0.001) (Figure 4A). In planned comparisons the sex difference in AR+ cell number did not reach significance for either wildtype or bax -/- mice (p > 0.05 in both cases), although we note that the two-tailed value was very close to significance for the wildtype mice (p = 0.065) and the previous report by Shah and colleagues (2004) might justify a 1-tailed test here. Similarly, the volume of the cluster of AR cells was greater in wildtype males than in females (p = 0.034) and bax deletion eliminated this sex difference (p = 0.34; Figure 4B).
Figure 4.
Mean (+SEM) AR+ cell number (A) and volume (μm3) of AR+ ‘cloud’ (B) in the BNSTp of wildtype (bax +/+) and bax -/- mice.
It has been suggested that bax gene deletion does not affect gross brain morphology or brain size (White et al., 1998). However, direct quantification of brain size or weight has not been reported. We find that bax -/- mice in fact do have significantly heavier brains than wildtype mice (F1,34 = 87.44, p < 0.0001; Table 1). There was no effect of genotype on body weight although, as expected, males were heavier than females (F1,34 = 37.006, p < 0.0001; Table 1). The effect of bax deletion on brain weight remains if brain weights are corrected by body size (F1,34 = 41.84, p < 0.0001).
Table 1.
Body and brain weight (+/- SEM) of bax -/- and +/+ mice
| Body weight, g | Brain weight*, mg | |
|---|---|---|
| bax +/+ males (n=10) | 29.72 (0.61) a | 356 (6) |
| bax +/+ females (n=10) | 24.89 (0.54) | 344 (5) |
| bax -/- males (n=9) | 28.60 (0.54) a | 416 (13)b |
| bax -/- females (n=9) | 26.67 (0.51) | 433 (7)b |
Brain weight after removal of olfactory bulbs and cerebellum.
Significantly different from females of the same genotype (p<0.05).
Significantly different from same sex bax +/+ animals (p<0.05).
Discussion
The present data demonstrate that the sex difference in BNSTp cell number is due to an increased number of neurons in males relative to females. Overall, bax-/- mice have more NeuN+ cells in the BNSTp than do wildtypes, resulting in the elimination of the sex difference. A similar pattern is seen for AR+ cell number. Shah and colleagues (2004) have demonstrated that the majority of neurons in the mouse BNSTp express AR (80-90%) and, conversely, fewer than 5% of AR+ cells fail to express NeuN. Similarly, our AR+ cell counts are 85-90% of our NeuN+ counts across all groups. The most parsimonious interpretation is that bax deletion rescues neurons, the majority of which are AR+, and that sex differences in both neuron number and AR+ cell number are due, at least in part, to Bax-dependent cell death.
We did not find a sex difference in the number of astrocytes in the BNSTp, as identified by GFAP labeling. We also did not find an effect of bax deletion, although the number of GFAP+ cells tended to be higher in knockout animals. In addition to Bax, many cell types express a second multidomain pro-death member of the bcl-2 family, Bak. Neurons, however, lack full-length Bak (Uo et al., 2005). As a result, while Bax and Bak are functionally redundant in many non-neuronal tissue types including glia (Lindsten et al., 2000; Wei et al., 2001; Degenhardt et al. 2002; Itoh et al., 2003), Bax is singularly important for initiating apoptosis in many populations of developing neurons (White et al., 1998; Uo et al., 2005). This may explain why bax deletion selectively increases neuron number. However, apoptotis in glial cells is often dependent on the presence or absence of neuron-derived signals (Winseck et al., 2002), and an increase in the number of neurons results in a corresponding increase in astrocytes in some systems (Burne et al., 1996). Thus, one might expect an increase in GFAP+ cell number in bax -/- mice as an indirect result of the greater numbers of neurons.
Previously, we examined VP cell number in the BNSTp and dopaminergic cell number in AVPV of wildtype and bax -/- mice. Males have more VP neurons than do females, even after hormone levels have been made equivalent in adulthood (De Vries and al-Shamma, 1990). Bax gene deletion does increase VP cell number in the BNSTp, but the magnitude of the sex difference is unchanged in knockout animals (De Vries et al., 2008). The same pattern is seen for mice overexpressing the pro-survival protein, Bcl-2 (De Vries et al., 2008). Thus, cell death may determine the total number of cells with the potential to become VP neurons in the BNSTp, but is not the mechanism underlying the sex difference. In the AVPV, females have many more dopaminergic cells than do males (Simerly et al., 1997). However, bax deletion neither alters the total number of dopaminergic cells, nor affects the sex difference in dopaminergic cell number in AVPV (Forger et al., 2004). Recently, a novel cell death pathway has been identified for cultured dopaminergic neurons of the mouse midbrain that is independent of Bax and other Bcl-2 family proteins (Yu et al., 2008); it is possible that a similar mechanism controls sexually dimorphic cell death of dopaminergic neurons in AVPV. Thus, while Bax-dependent cell death is clearly important for establishing sex differences in neuron number, and is required for the sex differences in NeuN+ and AR+ cells in the BNSTp, other mechanisms contribute to the sexual differentiation of cell number throughout the brain.
Previous investigators report that some cells rescued by bax deletion may be atrophic and not differentiate or migrate normally (e.g., Sun et al., 2003; 2004; Jacob et al., 2005; Buss et al., 2006; Jung et al., 2008). In contrast, other neural populations show little or no change in cell size in bax-/- mice, perhaps because the ‘extra’ neurons are able to maintain functional connections (e.g., Fan et al., 2001; Sun et al., 2003). The present study demonstrates that most, if not all, of the extra cells in the BNSTp of bax -/- mice become mature neurons, at least to the point of expressing NeuN. It must be acknowledged that expression of NeuN does not necessarily infer complete differentiation and/or functionality. For example, adult-generated dentate gyrus neurons express the NeuN and GFAP proteins in a similar ratio in bax -/- and wildtype mice, although expression of another neuronal marker, calbindin, is markedly decreased in cells rescued by bax deletion (Sun et al., 2004). However, as we demonstrate that supernumerary cells in the BNSTp can also express the AR protein (present results) and the VP peptide (De Vries et al., 2008), these neurons may be responsive to circulating steroids and involved in neuroendocrine function. Interestingly, both sexual and social behaviors that are sexually dimorphic in wildtype animals are altered in bax -/- mice. Specifically, lordosis quotients and social affiliation are masculinized in bax-/- mice of both sexes, and a sex difference in olfactory preference is eliminated in the knockouts (Jyotika et al., 2007; MM Holmes, L Niel, DA Monks, & NG Forger, unpublished observations). Determining how the elimination of cell death influences the circuitry underlying these and other socio-sexual behaviors is the next challenge.
Acknowledgements
This work was funded by NIH RO1 MH068482 and K02 MH072825 to NGF and a Canadian Institutes of Health Research postdoctoral fellowship to MMH.
Abbreviations
- AR
androgen receptor
- AVPV
anteroventral periventricular nucleus
- BNSTp
principal nucleus of the bed nucleus of the stria terminalis
- DAB
diaminobenzidine
- GFAP
glial fibrillary acidic protein
- IHC
immunohistochemistry
- NeuN
antigen expressed in neuronal nuclei
- NGS
normal goat serum
- NHS
normal horse serum
- TBS
tris buffered saline VP vasopressin
- VP
vasopressin
Footnotes
Section Editor (Behavioral Neuroscience): Dr. Joan I. Morrell, Rutgers, The State University of New Jersey, Center for Molecular and Behavioral Neuroscience, 197 University Avenue, Newark, New Jersey, 07102, USA
Literature Cited
- Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16:2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Gould TW, Ma J, Vinsant S, Prevette D, Winseck A, Toops KA, Hammarback JA, Smith TL, Oppenheim RW. Neuromuscular development in the absence of programmed cell death: phenotypic alteration of motoneurons and muscle. J Neurosci. 2006;26:13413–13427. doi: 10.1523/JNEUROSCI.3528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, al-Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21:686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervations of the brain: cell death versus phenotypic differentiation. Endocrinol. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Fan H, Favero M, Vogel MW. Elimination of Bax expression in mice increases cerebellar purkinje cell numbers but not the number of granule cells. J Comp Neurol. 2001;436:82–91. [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neurosci. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinol. 2001;142:5172–81. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Neurogenesis of galanin cells in the bed nucleus of the stria terminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neurobiol. 1999;38:491–8. [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Itoh A, Pleasure D. Bcl-2-related protein family gene expression during oligodendroglial differentiation. J Neurochem. 2003;85:1500–1512. doi: 10.1046/j.1471-4159.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AR, Kim TW, Rhyu IJ, Kim H, Lee YD, Vinsant S, Oppenheim RW, Sun W. Misplacement of Purkinje cells during postnatal development in Bax knock-out mice: a novel role for programmed cell death in the nervous system? J Neurosci. 2008;28:2941–2948. doi: 10.1523/JNEUROSCI.3897-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsbury CW, McKay K. A sex difference in the pattern of substance P-like immunoreactivity in the bed nucleus of the stria terminalis. Brain Res. 1987;420:365–370. doi: 10.1016/0006-8993(87)91258-3. [DOI] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Matt DW, Go VL. Concentrations of cholecystokinin, substance P, and bombesin in discrete regions of male and female rat brain: sex differences and estrogen effects. Exp Neurol. 1988;100:416–25. doi: 10.1016/0014-4886(88)90119-7. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Gould TW, Vinsant S, Prevette D, Oppenheim RW. Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion. J Neurosci. 2003;23:7298–7310. doi: 10.1523/JNEUROSCI.23-19-07298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J Biol Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winseck AK, Caldero J, Ciutat D, Prevette D, Scott SA, Wang G, Esquerda JE, Oppenheim RW. In vivo analysis of Schwann cell programmed cell death in the embryonic chick: regulation by axons and glial growth factor. J Neurosci. 2002;22:4509–4521. doi: 10.1523/JNEUROSCI.22-11-04509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LY, Saarma M, Arumae U. Death receptors and caspases but not mitochondria are activated in the GDNF- or BDNF-deprived dopaminergic neurons. J Neurosci. 2008;28:7467–7475. doi: 10.1523/JNEUROSCI.1877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinol. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]