Abstract
Background
US national guidelines recommend assessing short-acting β-agonist (SABA) medication use as a marker of asthma severity and control. However, the relationship between recent SABA use and asthma exacerbations is not currently known.
Objective
To evaluate the proximal relationship between the type and frequency of SABA use and asthma-related outcomes.
Methods
We evaluated SABA use among patients with asthma ages 5 to 56 years who were members of a large health maintenance organization in southeast Michigan. Frequency of use was estimated from pharmacy data assessing the timing and amount of SABA fills. Cox proportional hazards models were used to examine the prospective relationship between average daily SABA use for 3 months and outcomes associated with poor asthma control (ie, oral corticosteroids use, asthma-related emergency department visits, and asthma-related hospitalizations). We separately accounted for SABA metered-dose inhaler (MDI) and SABA nebulizer use.
Results
Of the 2,056 patients who met study criteria, 1,569 (76.3%) had used a SABA medication in their baseline year. After adjusting for potential confounders, SABA nebulizer use was associated with asthma-related emergency department visits (adjusted hazard ratio [aHR], 6.32; 95% confidence interval [CI], 2.38 to 16.80) and asthma-related hospitalizations (aHR, 21.62; 95% CI, 3.17 to 147.57). In contrast, frequency of SABA MDI use was not associated with these outcomes.
Conclusions
Frequency of SABA use during a 3-month period was associated with poor asthma outcomes. The relationship with poor asthma outcomes was strongest for SABA nebulizer use, suggesting that the type of SABA used is also of prognostic importance.
INTRODUCTION
Asthma is a common chronic disease in the United States, and its prevalence is increasing.1 Despite better understanding of asthma pathophysiology and new treatment modalities, cases have more than doubled since 1980 and death rates have increased during that time as well.2 Furthermore, during the last 20 years, asthma hospitalizations have doubled in adults and increased 5-fold in children.3 Therefore, identifying individuals at risk for asthma exacerbations is of utmost importance in the clinical management of this disease.
To help clinicians achieve this goal, various clinical parameters have been proposed. Symptom scores, spirometry data, and short-acting β-agonist (SABA) use (ie, rescue medication use) have been used to predict poor asthma control and to help guide therapy.4 Short-acting β-agonist use as a marker for disease activity seems to be intuitive because patients with uncontrolled asthma probably will use this type of medication more frequently to relieve symptoms when compared with patients with well-controlled asthma.5 Accordingly, current asthma guidelines use SABA rescue medication as a central component of ascertaining both asthma severity and control.6 However, existing studies looking at the relationship between SABA use and asthma exacerbations have either been retrospective in design or do not examine rescue medication use proximal to time of outcomes.7,8
There are different preparations of SABA medication, such as metered-dose inhaler (MDI) or nebulized solution.9 Many patients report superior subjective improvement with nebulized SABA medication when compared with MDI administration, although objective data at equipotent dosages do not support such a benefit.10,11 Accordingly, patients may select different SABA preparations, based on the severity of their symptoms. Therefore, the association between asthma exacerbations and SABA use may differ by preparation. Current asthma guidelines do not distinguish types of SABA used when assessing disease severity and control.6
In this report, we prospectively examine the relationship between SABA use and asthma outcomes. In addition, our predictive model accounts for the separate use of nebulizer and MDI medication.
METHODS
Study Setting and Patient Population
This study was approved by the institutional review board at Henry Ford Health System and was in compliance with its Health Insurance Portability and Accountability Act policy. Patients were part of the AFFIRM trial (ClinicalTrials.gov No. NCT00459368), a cluster-randomized study to improve adherence to inhaled corticosteroid medication, and as such had been followed up prospectively for asthma medication use and outcomes related to poor control. Patients were also members of a large health maintenance organization (HMO) in southeast Michigan and they received their care from a large, multispecialty medical group. To be eligible for inclusion, patients had to meet the following criteria: an electronic prescription for an inhaled corticosteroid (ICS) between January 1, 2005, and December 31, 2006 (the first electronic prescription for an ICS during this interval defined the index date); ages 5 to 56 years at the time of the index date; membership in the HMO with both medical and pharmacy benefit coverage at the time of and subsequent to the index prescription; at least 1 physician diagnosis of asthma in the year before the index date (ie, the baseline year); no diagnosis of chronic obstructive pulmonary disease or congestive heart failure in the baseline year; and continuous enrollment in the HMO beginning at least 1 year before the index date. We have previously demonstrated that a physician diagnosis of asthma in our patient population has a high concordance with patient-reported asthma.12 By restricting our sample to patients with an outpatient, electronic prescription for an ICS, we implicitly were limiting our population to patients deemed to have persistent asthma by their outpatient physicians. The period of observation for this study preceded and did not overlap randomization into study arms for the parent-randomized control trial. The last date of follow-up was December 31, 2006.
Information on patient characteristics, including age, sex, and race/ethnicity, was available from electronic data sources maintained by the health care system. Race-ethnic categories were entered at the time of registration into the health system; usually this information was self-identified but on occasion could have been assigned by heath care personnel.
Calculating SABA Exposure
Pharmacy claims were used to estimate daily inhaled SABA use. Inhaled SABA preparations and their associated national drug codes were separated into those representing MDIs and those representing nebulizer preparations. Since each SABA pharmacy dispensing included the national drug codes for the medication dispensed, we used the latter to identify the number of doses available (eg, 100 puffs per canister) at each dispensing. We calculated separate average use for SABA MDI preparations and SABA nebulizer preparations for each study individual. “Current” use (for MDI and nebulizer preparations separately) was defined for each day of follow-up as the sum doses dispensed during the preceding 3 months divided by 90 days. In other words, for each day after the index date, study patients had a separate measure of SABA MDI and SABA nebulizer use. “Historic” use (for MDI and nebulizer preparations separately) was defined as the sum doses dispensed during the year preceding the index date divided by 365 days.
Outcome Evaluation
Outcomes and baseline events (ie, oral corticosteroids fills, asthma-related emergency department visits, and asthma-related hospitalizations) were ascertained from electronic data maintained by the health care system (ie, from claims and administrative databases). For the outcomes of asthma-related emergency department visits and asthma-related hospitalizations, asthma was the primary diagnosis for these events.
Statistical Analysis
We compared the average number of oral steroid fills, asthma-related emergency department visits, and asthma-related hospitalization between study individuals who in the baseline year had no SABA fills, SABA MDI fills alone, SABA nebulizer fills alone, or both SABA MDI and nebulizer fills. The Jonckheere-Terpstra test, a nonparametric test for trend, was used to evaluate an increase in the number of these asthma-related events by type of SABA used.13 We also evaluated overall significance for differences in these events using the Kruskal Wallis test.13 Nonparametric tests were used because these events (ie, oral steroid fills, asthma-related emergency department visits, and asthma-related hospitalization) were not normally distributed. Confidence intervals (CIs) around category means were calculated as 1.96 times the SEM.
We used Cox proportional hazards models to examine the relationship between current daily SABA MDI use and current average daily SABA nebulizer use (ie, both averaged during the preceding 3-month period) and time to outcomes associated with poor asthma control. Specifically, we examined the following asthma-related outcomes: use (ie, fill) of oral corticosteroids, asthma-related emergency department visits, and asthma-related hospitalizations. Proportional hazards models were used because they model the time to the given event and they allow for exposure variables (eg, SABA use) to change over time.14 From these models, the reported hazards ratio for a given exposure is analogous to a risk ratio. Because we were interested in the relationship of current SABA use and outcomes, multivariable Cox models were used to adjust for potential confounders, including the following baseline variables from the year before the index date: oral steroid use, asthma-related emergency department visits, and asthma-related hospitalizations, historic SABA MDI use, and historic SABA nebulizer use. These latter 2 variables were log-transformed to normalize their distribution and minimized the effects of outliers. These models also adjusted for age, race-ethnicity, and sex. Confidence intervals were calculated as the exponentiation of each parameter estimate 1.96 times the square root of the model-based variance estimate. Data were analyzed using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, North Carolina).15 P <.05 was considered statistically significant.
RESULTS
We identified 2,056 patients who met our study criteria of having been diagnosed as having asthma and having an electronic prescription for an ICS. For these individuals, we had 1,221.3 person-years of follow-up or a mean (SD) of 0.59 (0.39) years of follow-up per person. The baseline characteristics of those individuals are given in Table 1. As can be seen, most patients (76.3%) had used a SABA in their baseline year (ie, the year before their index ICS prescription); 1,138 (55.4%) used SABA MDI, 85 (4.1%) used SABA nebulizer medication, and 346 (16.8%) used both SABA MDI and nebulizer medication. During this time, 780 (37.9%) had at least 1 fill of an oral corticosteroid, 387 (18.8%) an asthma-related emergency department visit, and 102 (5.0%) had an asthma-related hospitalization.
Table 1.
Baseline Characteristics of the Study Population Comprising Patients With Asthma
| Characteristic | Study population, No. (%)(N = 2,056)a |
|---|---|
| Age, mean (SD), y | 30.4 (17.3) |
| Age, y 5–11 | 430 (20.9) |
| Age, y 12–17 | 348 (16.9) |
| Age, y 18–56 | 1,278 (62.2) |
| Female | 1,179 (57.3) |
| Race | |
| African American | 841 (40.9) |
| White | 1,097 (53.4) |
| Other/unknown | 118 (5.7) |
| Patients without short-acting β-agonist use in the year preceding index dateb | 487 (23.7) |
| Patients with short-acting β-agonist metered-dose inhaler fill(s) alone in the year preceding index dateb | 1,138 (55.4) |
| Patients with short-acting β-agonist nebulizer fill(s) alone in the year preceding index dateb | 85 (4.1) |
| Patients with both short-acting β-agonist metered-dose inhaler and nebulizer fills in the year preceding index dateb | 346 (16.8) |
| Patients with ≥1 oral corticosteroid fill in the year preceding index dateb | 780 (37.9) |
| Patients with ≥1 asthma-related emergency department visit in the year preceding index dateb | 387 (18.8) |
| Patients with ≥1 asthma-related hospitalization in the year preceding index dateb | 102 (5.0) |
Data are number (percentage) of patient unless otherwise indicated.
The index date was defined as the date of the first electronic prescription for an inhaled corticosteroid during the interval January 1, 2005, to December 31, 2006.
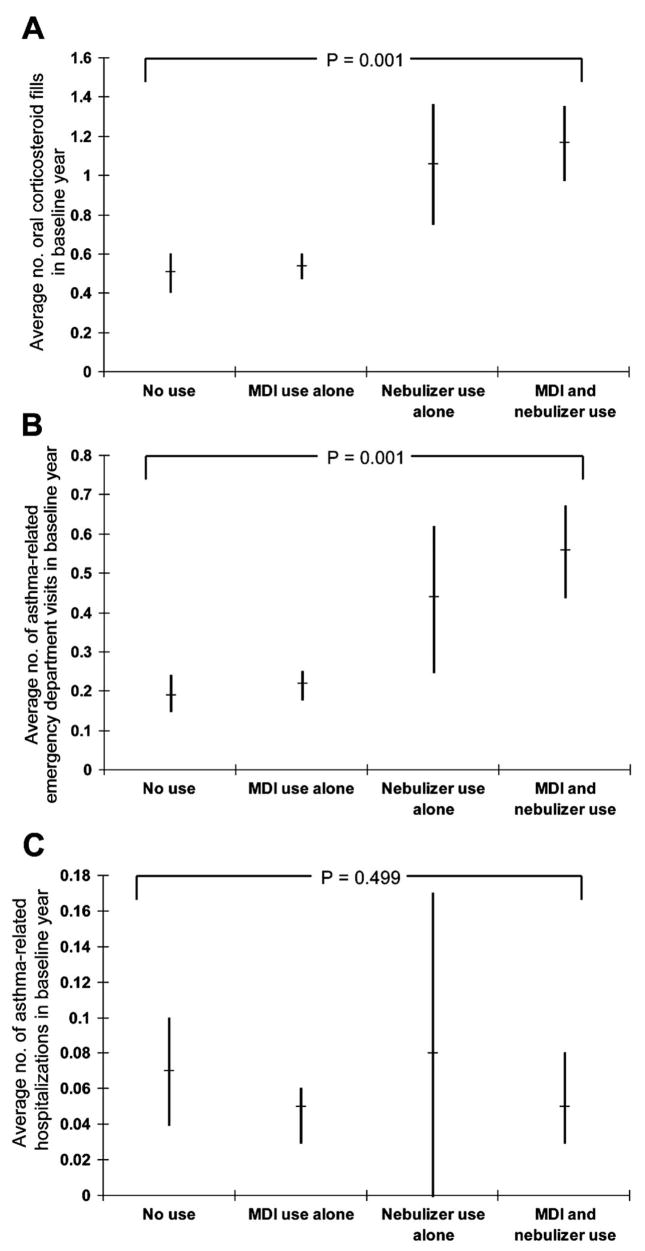
In the baseline year, there was a relationship between the type of SABA used and the frequency of both oral corticosteroid use and asthma-related emergency department visits (Fig 1, A and B). The average number of corticosteroid fills and the average number of asthma-related emergency department visits was increasingly higher among no-SABA users, individuals who used SABA MDI alone, individuals who used SABA nebulizer medication alone, and individuals who used both SABA MDI and SABA nebulizer medication. However, the likelihood of these events appeared to be much higher for those who had used nebulizers when compared with those who did not. In the baseline year, no relationship was seen between the type of SABA used and the frequency of asthma-related hospitalizations (Fig 1C).
Figure 1.
Relationship in the baseline year between the type of short-acting acting β-agonist used and the average number of the following: oral corticosteroid fills (A), asthma-related emergency department visits (B), and asthma-related hospitalizations (C). Bars represent 95% confidence intervals for category means and P values represent the test for trend across short-acting β-agonist categories. Tests for overall significance across categories were similar, with P = .001, .001, and .64 for A, B, and C, respectively.
Beginning with the index date, we prospectively evaluated the relationship between SABA use and asthma outcomes (Table 2). We adjusted for potential confounders, including the following in the year before the index date: oral steroid use, asthma-related emergency department visits, asthma-related hospitalizations, historic short-acting MDI use, and historic short-acting nebulizer use. These models also adjusted for patient age, sex, and race-ethnicity. For the outcome of oral corticosteroid use, only baseline oral corticosteroid use (adjusted hazard ratio [aHR], 1.19; 95% CI, 1.13 to 1.25) and historic SABA nebulizer use per day (aHR, 1.22; 95% CI, 1.10 to 1.35) were associated with oral corticosteroid use in follow-up. However, for the outcomes asthma-related emergency department visits and asthma-related hospitalizations, SABA nebulizer was strongly associated with these events, whereas SABA MDI use was not. For asthma-related emergency department visits, only current SABA nebulizer use and baseline year asthma-related emergency department visits were associated with subsequent asthma-related emergency department visits. Each increasing SABA nebulizer use per day was associated with an approximate 6-fold increase in the likelihood of asthma-related emergency department visit (aHR, 6.32; 95% CI, 2.38 to 16.80), whereas SABA MDI use was not significantly associated with this outcome (aHR, 1.05; 95% CI, 0.97 to 1.14).
Table 2.
Relationship Between the Frequency of Short-Acting β-Agonist Use Via Metered-Dose Inhaler and/or Nebulizer and Asthma-Related Outcomes
| Exposure variable(s) | Outcome variable |
||
|---|---|---|---|
| Oral corticosteroid use |
Asthma-related emergency department visit |
Asthma-related hospitalization |
|
| aHR (95% CI)a | aHR (95% CI)a | aHR (95% CI)a | |
| Current short-acting β-agonist nebulizer use per day | 1.37 (0.63–2.97) | 6.32 (2.38–16.80)e | 21.62 (3.17–147.57)d |
| Current short-acting β-agonist metered-dose inhaler use per day | 1.04 (0.99–1.09) | 1.05 (0.97–1.14) | 1.17 (0.98–1.40) |
| Oral corticosteroid fill in the year preceding index date | 1.19 (1.13–1.25)e | 1.02 (0.88–1.19) | 1.21 (0.96–1.52) |
| Asthma-related emergency department visits in the year preceding index date | 1.07 (0.96–1.19) | 1.53 (1.32–1.78)e | 1.49 (1.08–2.05)c |
| Asthma-related hospitalizations in the year preceding index date | 1.04 (0.73–1.47) | 1.20 (0.71–2.03) | 1.61 (0.57–4.58) |
| Historic short-acting β-agonist nebulizer use per dayb | 1.22 (1.10–1.35)e | 1.12 (0.92–1.37) | 1.21 (0.72–2.03) |
| Historic short-acting β-agonist metered-dose inhaler use per dayb | 1.06 (0.99–1.13) | 1.21 (1.04–1.40)c | 0.91 (0.67–1.25) |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval.
Relationship between current short-acting metered-dose inhaler use and/or nebulizer use and outcome, adjusting for the following in the year before the index date: oral steroid use, asthma-related emergency department visits, asthma-related hospitalizations, historic short-acting metered-dose inhaler use, and historic short-acting nebulizer use. The model also adjusts for patient age, sex, and race-ethnicity. Because this population was drawn from a larger study of inhaled corticosteroid use, the index date was defined as the first electronic prescription for an inhaled corticosteroid between January 1, 2005, and December 31, 2006. Current short-acting β-agonist use is defined as the calculated number of uses per day in the 3 months preceding the outcome; therefore, the hazard ratio for these variables represents the increased risk associated with one more use (ie, puff or nebulizer dose) per day.
Variables are natural log-transformed.
P <.05.
P <.01.
P <.001.
When compared with asthma-related emergency department visits, a similar pattern was observed with asthma-related hospitalizations as the outcome (Table 2). After adjusting for baseline variables, current SABA nebulizer use (aHR, 21.62; 95% CI, 3.17 to 147.57) and baseline asthma-related emergency department visits (aHR, 1.49; 95% CI, 1.08 to 2.05) were associated with asthma-related hospitalization. In other words, each increasing use per day of a SABA nebulizer medication was associated with a nearly 20-fold increase in the likelihood of asthma-related hospitalization, whereas SABA MDI use was not significantly associated with this outcome.
As a post hoc analysis, we analyzed whether using historic number of SABA fills, rather than average use per day, would have affected our results. Adjusting for the number of SABA MDI fills and the number of SABA nebulizer fills in the baseline year did not substantively change our results.
To exclude the possibility of including medication use resulting from an event (eg, after an emergency department visit or hospitalization), we also implemented a lag in our evaluation of β-agonist exposure such that we examined 3-month windows of use ending 1 week before outcomes. Implementing this lag had no substantive effect on the observed associations, which further supports the reported relationship between recent β-agonist medication use and asthma outcomes.
DISCUSSION
Short-acting β-agonists are an important treatment modality for asthmatic patients because they are the most potent and fast-acting bronchodilators clinically available.16 Their effects include relaxing smooth muscle, decreasing inflammatory cellular responses, increasing mucociliary clearance, and decreasing edema formation by altering vascular tone.16,17 Because of their “as needed” use for incompletely controlled asthma, SABA rescue medicine use has been proposed as a tool to identify patients at risk for an exacerbation.6–8,18
In this study, we found significant associations between SABA use and outcomes related to poor asthma control, particularly asthma-related emergency department visits and hospitalizations. Our study is unique in that we evaluated outcomes prospectively and SABA use was evaluated proximate (ie, within the preceding 3 months) to these events. We also evaluated SABA nebulizer and SABA MDI use separately and found that SABA nebulizer use in particular was associated with outcomes. These latter relationships remained significant even after accounting for baseline variables, including historic SABA use and outcomes.
Although approximately 20% of our study population used SABA nebulizer medication at baseline, our data suggest that use of these medications may identify a group at particular risk of having a serious asthma event (ie, emergency department visit or hospitalization) and that this risk increases with increasing use. Our findings are supported by those of Mullen et al,19 who combined existing case-control studies to examine the relationship between SABA use and asthma-related death.19 Although weak (mean correlation = 0.103), the relationship between SABA use and asthma-related death was seen only for nebulizer use. The relationship was not seen among oral or MDI SABA use.
Perhaps the relationship between SABA use and serious asthma-related outcomes reflects selection bias, such that physicians prescribe nebulizers to those severe patients who appear not to respond to SABA administered via MDI. Although this study suggested such a relationship between SABA nebulizer use and baseline asthma severity, we also showed nebulizer use to be prospectively associated with poor outcomes after accounting for these baseline events. Indeed, despite evidence of equivalent efficacy between SABA medication delivered by MDI with spacer and that delivered by nebulizer,20 some clinicians believe that SABA nebulizers are more effective during acute events.21 It is possible that these perceptions and patterns of use by clinicians may indoctrinate their patients to also use SABA nebulizers preferentially during exacerbations.
The lack of a consistent association between SABA MDI use and poor outcomes is surprising but may have multiple explanations. For example, patients can underestimate the amount of remaining medication in a MDI. In a study by Rubin and Durotoye,22 patients typically used their inhaler until it no longer produced a sound, yet in most circumstances the number of actuations far exceeded the nominal dose. By relying on prescription refills to estimate daily use, we may have misestimated the frequency of SABA MDI use. This may be less of an issue in estimating nebulizer use because this medication is dispensed in vials, and the availability of medication is less likely to be mistaken. Overuse23 and differences in physiologic responsiveness24 may also diminish the predictive utility of SABA MDI use for impending exacerbations, although these limitations do not appear to extend to SABA nebulizer use.
This study must be interpreted in light of its other limitations. As mentioned above, we used prescription refills to estimate daily SABA use. Although this method allowed us to calculate SABA use for many patients simultaneously and to estimate each individual’s daily use, we did not measure inhaler actuation. Therefore, we may have missed important patterns of use predictive of asthma outcomes. In addition because this was an observational study, we cannot comment about a cause-and-effect relationship between SABA use and outcomes. For example, other studies have reported an association between SABA use and worsening asthma morbidity,25,26 including some specific for SABA nebulizer use.27 However, the goal of this study was to demonstrate a predictive relationship useful in identifying patients at risk for an exacerbation rather than comment on the safety of these medications. Because our study was performed at a single health care institution, it is possible that our results may not apply to patients seen elsewhere. However, our large patient population is broadly representative of southeast Michigan population in age, sex, and race-ethnicity (data not shown). Last, we restricted our sample to patients who had a prior outpatient electronic prescription for an ICS, thereby implicitly limiting our population to patients deemed to have persistent asthma by their outpatient physicians. Therefore, patterns of β-agonist use may have different predictive importance in patients with intrinsically milder disease who were not studied.
This study has obvious relevance to the current national asthma guidelines, which recommend reviewing patterns of SABA use to place patients in asthma severity and control categories.6 In particular, our study suggests that the type of SABA use, namely nebulizer use, may be relevant in assessing risk of a severe asthma exacerbation. How and to what extent SABA MDI use is predictive of impending asthma exacerbations is yet to be determined. Future studies are needed to evaluate patterns of SABA MDI use in finer detail, perhaps through the use of electronic monitoring devices, to better evaluate its relationship with outcomes.
Acknowledgments
This work was supported by grants from the Fund for Henry Ford Hospital, the Sandler Program for Asthma Research, and the National Institute of Allergy and Infectious Diseases (AI61774) and the National Heart, Lung, and Blood Institute (HL79055), National Institutes of Health.
Footnotes
Disclosures: Dr Paris owns shares of Merck & Co. The other authors have nothing to disclose.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130 (Suppl 1):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Redd SC. Asthma in the United States: burden and current theories. Environ Health Perspect. 2002;110(Suppl 4):557–560. doi: 10.1289/ehp.02110s4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangan JM, Wittich AR, Gerald LB. The potential for reducing asthma disparities through improved family and social function and modified health behaviors. Chest. 2007;132 (Suppl 5):789S–801S. doi: 10.1378/chest.07-1908. [DOI] [PubMed] [Google Scholar]
- 4.Watson L, Kerstjens HA, Rabe KF, Kiri V, Visick GT, Postma DS. Obtaining optimal control in mild asthma: theory and practice. Fam Pract. 2005;22:305–310. doi: 10.1093/fampra/cmi013. [DOI] [PubMed] [Google Scholar]
- 5.Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur Respir J. 1994;7:1602–1609. doi: 10.1183/09031936.94.07091602. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120 (Suppl 5):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Development and validation of a medication intensity scale derived from computerized pharmacy data that predicts emergency hospital utilization for persistent asthma. Am J Manag Care. 2006;12:478–484. [PubMed] [Google Scholar]
- 8.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006;117:995–1000. doi: 10.1016/j.jaci.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50:367–382. [PubMed] [Google Scholar]
- 10.Colacone A, Afilalo M, Wolkove N, Kreisman H. A comparison of albuterol administered by metered dose inhaler (and holding chamber) or wet nebulizer in acute asthma. Chest. 1993;104:835–841. doi: 10.1378/chest.104.3.835. [DOI] [PubMed] [Google Scholar]
- 11.Idris AH, McDermott MF, Raucci JC, Morrabel A, McGorray S, Hendeles L. Emergency department treatment of severe asthma: metered-dose inhaler plus holding chamber is equivalent in effectiveness to nebulizer. Chest. 1993;103:665–672. doi: 10.1378/chest.103.3.665. [DOI] [PubMed] [Google Scholar]
- 12.Simpkins J, Divine G, Wang M, Holmboe E, Pladevall M, Williams LK. Improving asthma care through recertification: a cluster randomized trial. Arch Intern Med. 2007;167:2240–2248. doi: 10.1001/archinte.167.20.2240. [DOI] [PubMed] [Google Scholar]
- 13.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2. New York, NY: Wiley; 1999. [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 15.SAS Institute Inc. SAS/STAT Users Guide Version 9.1. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 16.Nelson HS. Beta-adrenergic bronchodilators. N Engl J Med. 1995;333:499–506. doi: 10.1056/NEJM199508243330807. [DOI] [PubMed] [Google Scholar]
- 17.Lemanske RF, Jr, Joad J. Beta-2 receptor agonists in asthma: a comparison. J Asthma. 1990;27:101–109. doi: 10.3109/02770909009073304. [DOI] [PubMed] [Google Scholar]
- 18.British guideline on the management of asthma. Thorax. 2003;58 (Suppl 1):i1–i94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen M, Mullen B, Carey M. The association between beta-agonist use and death from asthma: a meta-analytic integration of case-control studies. JAMA. 1993;270:1842–1845. [PubMed] [Google Scholar]
- 20.Radzik D, Peroni DG, Pescollderungg L, Piacentini GL, Chatzimichail A, Boner AL. Nebulizers or pressurized metered-dose inhalers in the treatment of asthma exacerbations. Allergy Asthma Proc. 2005;26:207–209. [PubMed] [Google Scholar]
- 21.Hurley KF, Sargeant J, Duffy J, Sketris I, Sinclair D, Ducharme J. Perceptual reasons for resistance to change in the emergency department use of holding chambers for children with asthma. Ann Emerg Med. 2008;51:70–77. doi: 10.1016/j.annemergmed.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Rubin BK, Durotoye L. How do patients determine that their metered-dose inhaler is empty? Chest. 2004;126:1134–1137. doi: 10.1378/chest.126.4.1134. [DOI] [PubMed] [Google Scholar]
- 23.Diette GB, Wu AW, Skinner EA, Markson L, Clark RD, McDonald RC, et al. Treatment patterns among adult patients with asthma: factors associated with overuse of inhaled beta-agonists and underuse of inhaled corticosteroids. Arch Intern Med. 1999;159:2697–2704. doi: 10.1001/archinte.159.22.2697. [DOI] [PubMed] [Google Scholar]
- 24.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 26.Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149 (3 Pt 1):604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 27.Suissa S, Hemmelgarn B, Blais L, Ernst P. Bronchodilators and acute cardiac death. Am J Respir Crit Care Med. 1996;154 (6 Pt 1):1598–1602. doi: 10.1164/ajrccm.154.6.8970341. [DOI] [PubMed] [Google Scholar]