Abstract
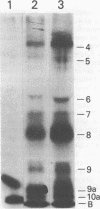
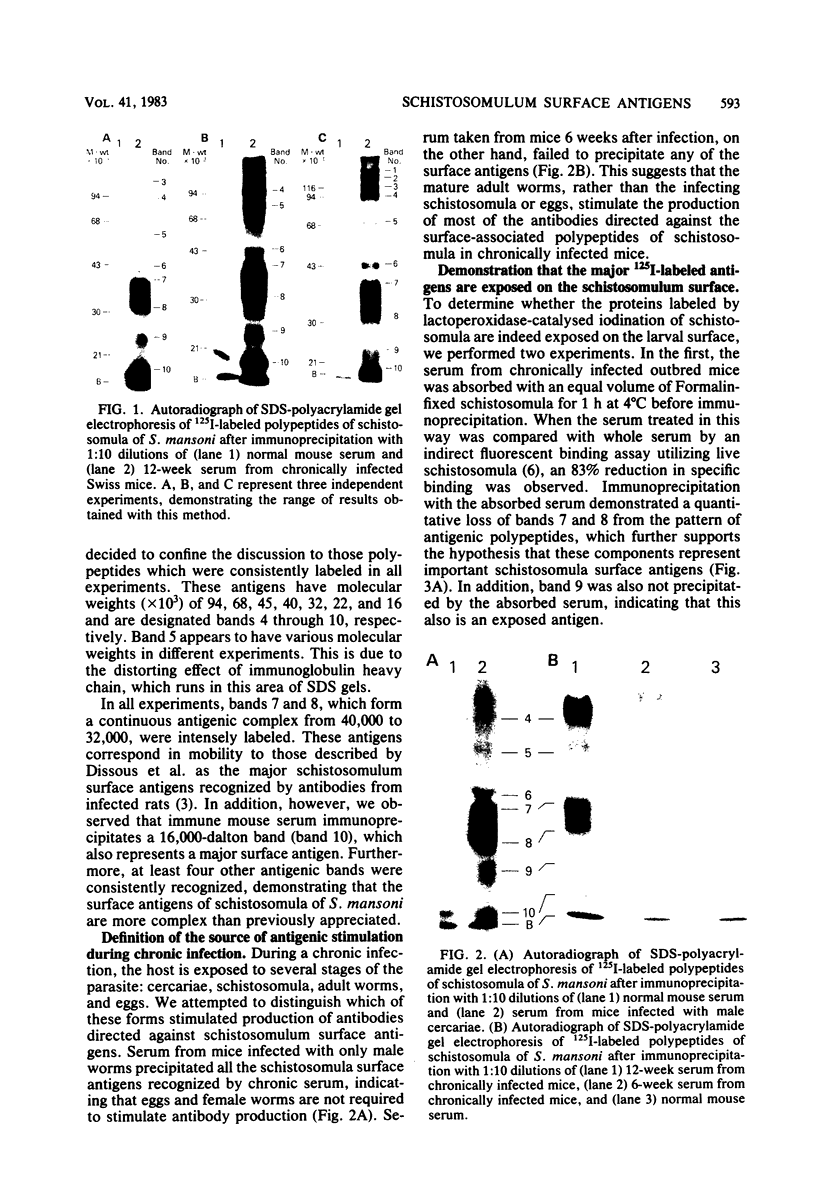
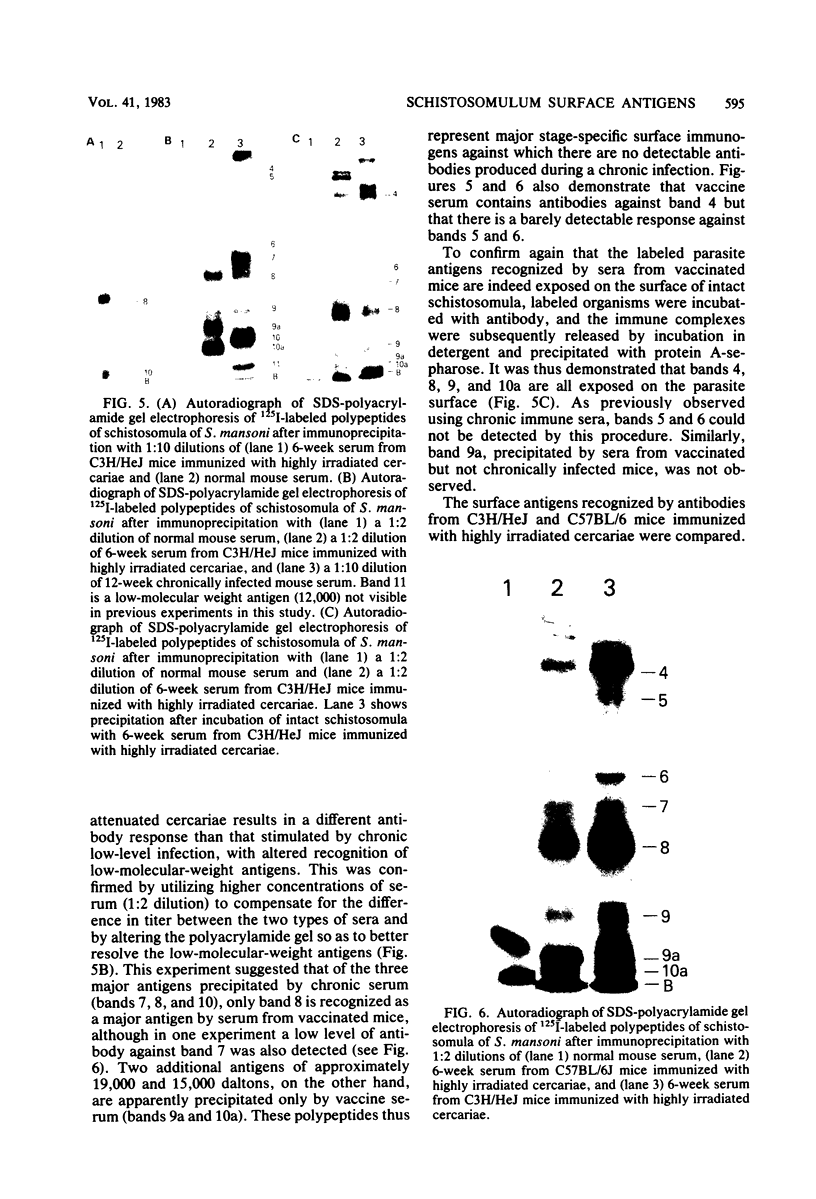
Surface components of mechanically transformed schistosomula of Schistosoma mansoni were labeled by lactoperoxidase-catalyzed iodination. After solubilization with Triton X-100, antigens were identified by immunoprecipitation. Serum from chronically infected Swiss mice reproducibly precipitated seven major polypeptides with approximate molecular weights (X 10(3] of 94, 68, 45, 40 to 32, 22, and 16. The antigens of molecular weights (X 10(3] of 94, 40 to 32, 22, and 16 were shown to be exposed on the parasite surface by interaction of the antibodies with intact labeled schistosomula. Sera from several strains of infected inbred mice precipitated the same polypeptides. The antibodies produced during chronic infection were found to be stimulated by adult worms since sera from 6-week-infected animals precipitated none of the surface antigens, and the pattern produced by precipitation with antibodies from a mouse infected with male worms only was indistinguishable from the pattern obtained with sera from mice with bisexual infections. Antibodies from mice immunized with highly irradiated cercariae reproducibly precipitated major polypeptides of approximately (X 10(3] 94, 68, 45, 32, 22, 19, and 15 daltons. The antigens of (X 10(3] 94, 43, 32, 22, and 15 daltons were shown to be exposed on the parasite surface by interaction of the antibodies with intact labeled schistosomula. The 15 X 10(3)-dalton surface protein was recognized by sera from vaccinated, but not chronically infected, mice, suggesting that it represents a stage-specific immunogen present on schistosomula but not on adult worms. Sera from two inbred strains of mice which develop different degrees of immunity recognized the same antigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickle Q., Long E., James E., Doenhoff M., Festing M. Schistosoma mansoni: influence of the mouse host's sex, age, and strain on resistance to reinfection. Exp Parasitol. 1980 Oct;50(2):222–232. doi: 10.1016/0014-4894(80)90023-5. [DOI] [PubMed] [Google Scholar]
- Dissous C., Dissous C., Capron A. Isolation and characterization of surface antigens from Schistosoma mansoni schistosomula. Mol Biochem Parasitol. 1981 Aug;3(4):215–225. doi: 10.1016/0166-6851(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Dissous C., Grzych J. M., Capron A. Schistosoma mansoni surface antigen defined by a rat monoclonal IgG2a. J Immunol. 1982 Nov;129(5):2232–2234. [PubMed] [Google Scholar]
- Eveland L. K., Morse S. I. Schistosoma mansoni: infectivity and immunizing effects of in vitro derived schistosomula attenuated by X irradiation. Exp Parasitol. 1978 Jun;45(1):19–25. doi: 10.1016/0014-4894(78)90040-1. [DOI] [PubMed] [Google Scholar]
- James S. L., Labine M., Sher A. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. I. Analysis of antibody and T-lymphocyte responses in mouse strains developing differing levels of immunity. Cell Immunol. 1981 Nov 15;65(1):75–83. doi: 10.1016/0008-8749(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard P., Dean D. A., Jacobson R. H., Vannier W. E., Murrell K. D. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- Philipp M., Parkhouse R. M., Ogilvie B. M. Changing proteins on the surface of a parasitic nematode. Nature. 1980 Oct 9;287(5782):538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- Ramasamy R. Surface proteins on schistosomula and cercariae of Schistosoma mansoni. Int J Parasitol. 1979 Dec;9(6):491–493. doi: 10.1016/0020-7519(79)90003-1. [DOI] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P. Loss of covalently labeled glycoproteins and glycolipids from the surface of newly transformed schistosomula of Schistosoma mansoni. J Cell Biol. 1982 Aug;94(2):363–369. doi: 10.1083/jcb.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Hieny S., James S. L., Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982 Apr;128(4):1880–1884. [PubMed] [Google Scholar]
- Sher A., Smithers S. R., Mackenzie P. Passive transfer of acquired resistance to Schistosoma mansoni in laboratory mice. Parasitology. 1975 Jun;70(Pt 3):347–357. doi: 10.1017/s0031182000052124. [DOI] [PubMed] [Google Scholar]
- Simpson A. J., Schryer M. D., Cesari I. M., Evans W. H., Smithers S. R. Isolation and partial characterization of the tegumental outer membrane of adult Schistosoma mansoni. Parasitology. 1981 Aug;83(Pt 1):163–177. doi: 10.1017/s0031182000050137. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Clegg J. A., Snary D., Trejdosiewicz A. J. Passive immunization of mice against Schistosoma mansoni with an IgM monoclonal antibody. Parasitology. 1982 Feb;84(1):83–91. doi: 10.1017/s0031182000051684. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. Resistance to experimental infection with Schistosoma mansoni in rhesus monkeys induced by the transfer of adult worms. Trans R Soc Trop Med Hyg. 1967;61(4):517–533. doi: 10.1016/0035-9203(67)90102-2. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The immunology of schistosomiasis. Adv Parasitol. 1976;14:399–422. doi: 10.1016/s0065-308x(08)60518-7. [DOI] [PubMed] [Google Scholar]
- Snary D., Smith M. A., Clegg J. A. Surface proteins of Schistosoma mansoni and their expression during morphogenesis. Eur J Immunol. 1980 Jul;10(7):573–575. doi: 10.1002/eji.1830100716. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Hayunga E. G., Vannier W. E. Surface antigens of Schistosoma mansoni. Mol Biochem Parasitol. 1981 Jul;3(3):157–168. doi: 10.1016/0166-6851(81)90046-3. [DOI] [PubMed] [Google Scholar]
- Zodda D. M., Phillips S. M. Monoclonal antibody-mediated protection against Schistosoma mansoni infection in mice. J Immunol. 1982 Dec;129(6):2326–2328. [PubMed] [Google Scholar]