Abstract
Background
Innate immune system stimuli, such as endotoxin, seem to affect allergy risk. Previously, we described gene-environment interactions between the endotoxin receptor polymorphism C-260T of the CD14 gene and endotoxin exposure on total serum IgE level; however, the mechanism of this interaction is not known.
Objective
To examine whether this gene-environment interaction affects early CD4+Foxp3− or CD4+Foxp3+ lymphocyte numbers.
Methods
Participating children were part of a birth cohort in the Detroit metropolitan area. Participants were genotyped for the CD14 C-260T polymorphism. Endotoxin exposure was estimated from dust measured in the home when children were 6 months old. Intracellular Foxp3 protein expression, a regulatory T-cell marker, was used to characterize CD4+ lymphocytes in blood samples collected at the age of 12 months; total serum IgE level was also measured at this time. Because race/ethnicity may confound or modify genetic associations, all analyses were stratified by race/ethnicity.
Results
We observed a significant gene-environment interaction between CD14 C-260T genotype and endotoxin exposure on CD4+ lymphocyte numbers, particularly CD4+Foxp3− lymphocytes. Stratified analyses suggest effect modification by race/ethnicity on CD4+Foxp3+ lymphocyte numbers, with a significant interaction in African American children but not in white children. The interaction between CD14 C-260T genotype and endotoxin exposure on total IgE levels was opposite that observed for CD4+ lymphocyte numbers, suggesting reciprocal relationships.
Conclusions
A gene-environment interaction between endotoxin and CD14 C-260T genotype on IgE levels may be the result of an upstream, opposing effect on CD4+Foxp3+ and CD4+Foxp3− lymphocyte numbers. Race/ethnicity may affect which of these cell populations is affected by this gene-environment interaction.
INTRODUCTION
Strong innate immune system stimuli may reduce the risk of atopic conditions. For example, our group1 previously showed that the frequency and intensity of fevers, a marker of innate immune stimulation, between 6 and 12 months old are associated with a lower likelihood of allergic sensitization, asthma, and asthma with allergic sensitization at 6 to 7 years old.
Endotoxin, a potent stimulator of inflammation through the innate immune system, has also been shown to have a protective association with allergic sensitization and asthma in some observational studies.2,3 As a component of gram-negative bacteria cell walls, endotoxin is ubiquitous in the environment, but levels are particularly high with farming and livestock contact.4 However, even in metropolitan settings endotoxin levels may be sufficient to affect atopy.5
The relationship between endotoxin exposure and IgE levels seems to be affected by a common single nucleotide polymorphism (SNP) in the promoter region of CD14, a receptor involved in endotoxin recognition.6 Although this apparent gene-environment interaction between a cytosine (C) to thymine (T) transition at base pair −260 (C-260T) of CD14 and endotoxin exposure has now been described for IgE levels6–8 and asthma severity,9 the immunologic mechanism of this interaction is not known.
Innate stimuli, such as endotoxin, may affect the expansion of regulatory T cells (Treg cells),10 which, in turn, regulate the expression of atopy through their immunosuppressive properties.11 Were endotoxin to affect Treg-cell development in vivo, we would also expect to see a gene-environment interaction between CD14 C-260T genotype and endotoxin exposure on Treg-cell expansion.
Studying children enrolled in the Wayne County Health, Environment, Atopy & Asthma Longitudinal Study (WHEALS), an ongoing longitudinal birth cohort, we examined the interactive relationship between endotoxin exposure at the age of 6 months and CD14 C-260T genotype on IgE levels and CD4+ lymphocyte numbers at the age of 1 year. This included the number of CD4+Foxp3+ lymphocytes, because Foxp3+ expression is considered to be specific for Treg cells.12,13 The interval was selected a priori based on previous observations of febrile infections.1 Because this is a racially diverse cohort, we stratified analyses by race/ethnicity to minimize confounding due to population stratification.14 Furthermore, race/ethnicity-specific gene-environment effects have previously been described for this polymorphism.15
METHODS
Study Population
This study was approved by the institutional review board at Henry Ford Health System and was compliant with its Health Insurance Portability and Accountability Act of 1996 policy. A full description of recruitment, data collection, and endotoxin measurements appears in an earlier publication.6 In brief, enrollment in the WHEALS birth cohort began in August 2003 and is planned to continue through 2007. To be eligible, pregnant women in their second or third trimester had to be at least 21 years old, live in a geographically defined area of urban and suburban Detroit, and attend 1 of 5 clinics for their prenatal care. A mother’s signed consent was required for both mother and child to participate. Compared with US census figures, the health system from which participants were recruited serves a population that is broadly representative of the Detroit metropolitan area in age, sex, and race/ethnicity. The intent of this longitudinal cohort is to followup newborns and parents to identify environmental and genetic factors associated with the development of atopy and associated conditions. The present analysis involves children enrolled in the study.
Data Collection
The mean ± SD age of the children included in this analysis at the “6-month” visit was 7.1 ± 1.2 months; the mean ± SD age at the “12-month” visit was 13.8 ± 2.0 months; and the mean ± SD time difference between the 2 visits was 6.7 ± 2.6 months. For the reasons mentioned in the “Introduction,” we were interested in endotoxin exposure at the age of 6 months; this exposure was estimated in dust collected from the home at this time. Blood collected from the child at approximately 1 year old was analyzed for CD4+ lymphocyte populations, total serum IgE levels, and total white blood cell (WBC) count. Genomic DNA was isolated from blood samples using a DNA kit (FlexiGene; Qiagen, Valencia, California).
Analysis of Total Serum IgE Levels
Plasma was separated, frozen, and shipped in batches for analysis of total IgE levels. Total IgE levels were measured using Pharmacia CAP (Pharmacia Diagnostics AB, Portage, Michigan) according to the manufacturer’s protocols.
CD14 C-260T Genotyping
Genotyping of children’s DNA for the CD14 C-260T polymorphism was performed by means of allelic discrimination using a SNP genotyping assay (TaqMan; Applied Biosystems, Foster City, California). Inconclusive results were adjudicated by direct sequencing. The CD14 SNP analyzed, rs2569190, has recently been redesignated C-260T16; it is also referred to as C-159T in several publications.15,17
Endotoxin Assay
The endotoxin activity in each house dust sample was measured using the fluorescent microplate assay based on recombinant Limulus factor C (PyroGene Recombinant Factor C; Cambrex Bio Science, Walkersville, Maryland) and an endotoxin standard (Cambrex Bio Science). A full description of the collection and assay procedure can be found in a previous publication.6
Quantifying CD4+Foxp3− and CD4+Foxp3+ Lymphoctyes
Blood samples were collected in heparinized tubes and were processed within 18 hours of initial collection following the Becton Dickinson FastImmune protocol (BD Biosciences, San Jose, California). Samples were then stored at −80°C until staining and analysis. Within 6 months of collection, frozen samples were analyzed for CD4+Foxp3− and CD4+Foxp3+ lymphocytes. We used mouse anti–human chlorophyll protein-cyanine (PerCP-Cy5.5)–conjugated anti-CD4 antibody (BD Biosciences) for surface molecule staining. After permeablizing the cells, phycoerythrin-conjugated rat anti–human monoclonal antibody against intracellular Foxp3 (eBioscience, San Diego) was added.
Cell populations were identified using a 3-laser benchtop flow cytometer (BD LSR; BD Biosciences). On each batch of samples run, quadrant markers were set according to the isotype control antibody results. CellQuest Pro v4.0.2 software (BD Biosciences) was used to gate on the CD4+ cell population. Among the CD4+ lymphocytes, scatterplots then quantified the proportion of Foxp3+ cells (ie, the proportion of CD4+ lymphocytes exhibiting the highest mean fluorescence intensity in the FL2 channel used to evaluate intracellular Foxp3 staining). We estimated the absolute number of CD4+Foxp3+ cells by multiplying the total WBC count by the proportion of CD4+ lymphocytes in all WBCs and the proportion of Foxp3+ cells in all CD4+ cells. Similar calculations were performed to quantify the number of CD4+Foxp3− cells. Additional information on the processing and staining of cells is available on request.
Statistical Analysis
We evaluated differences in the characteristics of children with vs without complete information at the age of 1 year using a 2-sample t test and χ2 tests. Endotoxin levels measured in dust collected from the floors of mothers’ bedrooms were used as a proxy for overall endotoxin exposure.6 Pearson correlation coefficients were used to evaluate correlations between dust endotoxin exposure at the age of 6 months and the following outcomes at the age of 1 year: total serum IgE levels, number of CD4+ lymphocytes, number of CD4+ Foxp3+ lymphocytes, and number of CD4+Foxp3− lymphocytes. In these analyses, dust endotoxin levels, serum IgE levels, and lymphocyte counts were loge transformed to normalize their distribution and reduce the effect of large observations. Analyses were stratified by CD14 C-260T genotype.
Linear regression was used to estimate the interactive relationship between CD14 C-260T genotype and endotoxin exposure on each of the following loge-transformed variables: total serum IgE levels and CD4+, CD4+Foxp3+, and CD4+Foxp3− lymphocyte numbers. Based on the observed correlations, we modeled CD14 genotype as an ordinal variable (ie, an additive genetic relationship). Adjusting for child’s age, child’s sex, being breastfed, number of siblings, pet exposure, parental smoke exposure, and maternal atopy did not substantively affect the relationships between CD14 C-260T genotype and endotoxin exposure on IgE levels or CD4+ lymphocyte numbers (data not shown); therefore, these variables were not included in the final models.
To our knowledge, this is the first effort to describe a gene-environment interaction effect on CD4+ lymphocyte numbers. As such, we did not have a precedent with which to estimate effect size. However, because we hypothesized that these changes occur upstream of effects on IgE, we estimated that the effect size for CD4+ lymphocyte numbers would be larger than that previously seen for IgE.6 With a sample size of 90, we had 90% power to detect a medium effect size of 0.15. Herein, effect size is defined as rall − rreduce/1 − rall, where rall is the squared correlation for the model with all the variables and rreduce is the squared correlation with the model variables excluding the interaction term.18 The power to detect interactions despite the small sample size was due, in part, to the cohort study design and the use of continuous outcome measures (ie, quantitative phenotypes).18
The frequency of the CD14 C-260T genotype has been shown to vary by race/ethnicity, and these differences could confound the results through population stratification.15 To address potential confounding and effect modification by race/ethnicity, we stratified the regression models for African American and white patients. Race/ethnicity categories were based on mothers’ reports. Finally, we reran the regression models substituting endotoxin measures from the 6-month visit with those from the 1-month visit. All statistical analyses were performed using a software program (SAS v9.1; SAS Institute Inc, Cary, North Carolina). P < .05 was considered statistically significant.
RESULTS
Study Population
At the time of this study, recruitment and follow-up in WHEALS were ongoing. Between August 1, 2003, and May 31, 2005, 476 children enrolled in WHEALS. However, data for the primary analysis, the relationship between CD14 C-260T genotype and dust endotoxin levels at the age of 6 months on CD4+ lymphocyte counts (Foxp3+ and Foxp3−) at the age of 1 year, were available on 90 children. These 90 children are compared with the 386 excluded children in Table 1. These groups did not differ significantly in the characteristics examined. In the analytic group, the geometric mean ± SD number of CD4+Foxp3− lymphocytes was 294.8 × 107 ± 1.6 × 107 cells/L and of CD4+Foxp3+ lymphocytes was 14.9 ± 2.1 × 107 cells/L (ie, approximately 4.8% of all CD4+ lymphocytes were Foxp3+). In the analytic group and in children not included, the CD14 C-260T genotype frequencies were in Hardy-Weinberg equilibrium. Complete data for the analysis between CD14 C-260T genotype and dust endotoxin levels at the age of 6 months on total IgE levels at the age of 1 year were available on 72 children. The geometric mean ± SD of the total serum IgE level in this group was 15.4 ± 4.0 IU/mL.
Table 1.
Characteristics of Patients Included and Not Included in the Analysisa
| Characteristic | Included (n = 90) | Not included (n = 386) | P value |
|---|---|---|---|
| Male sex | 48/90 (53.3) | 192/386 (49.7) | .54 |
| Race/ethnicity | .76 | ||
| African American | 48/90 (53.3) | 201/386 (52.1) | |
| White | 34/90 (37.8) | 132/386 (34.2) | |
| Other/unknown | 8/90 (8.9) | 53/386 (13.7) | |
| Household income, mean ± SD, $b | 61,039 ± 41,883 | 63,668 ± 41,337 | .61 |
| Parents reporting smoking | 15/78 (19.2) | 57/365 (15.6) | .50 |
| CD14 C-260T genotypec | .34 | ||
| CC | 28/90 (31.1) | 82/205 (40.0) | |
| CT | 44/90 (48.9) | 89/205 (43.4) | |
| TT | 18/90 (20.0) | 34/205 (16.6) | |
| Dust endotoxin level, geometric mean ± SD, EU/mgd | 18.2 ± 3.8 | 15.2 ± 3.3 | .26 |
| Total serum IgE level at the age of 1 y, geometric mean ± SD, IU/mLe | 15.4 ± 4.0 | 15.1 ± 3.9 | .93 |
Data are given as number/total number (percentage) except where indicated otherwise.
The numbers of children with data are 77 for included and 368 for not included.
CD14 C-260T denotes a single nucleotide polymorphism at position −260 resulting in a cytosine (C) to thymine (T) transition.
The numbers of children with data are 90 for included and 200 for not included.
The numbers of children with data are 72 for included and 51 for not included.
Gene-Environment Interactions on Total Serum IgE Levels
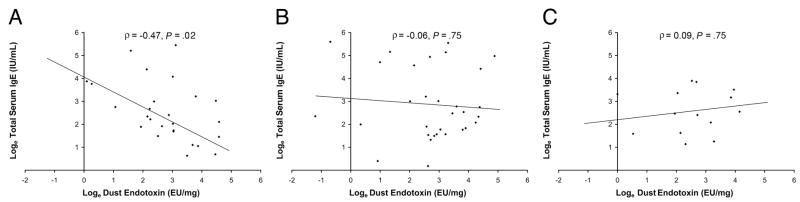
We observed a possible gene-environment interaction between CD14 C-260T genotype and endotoxin exposure at the age of 6 months on total IgE levels at the age of 1 year. Increasing endotoxin exposure at the age of 6 months was associated with significantly lower IgE levels at 1 year in children with the CD14 C-260T CC genotype (P = .02), nonsignificantly lower IgE levels in children with the CT genotype (P = .75), and nonsignificantly higher IgE levels at 1 year in children with the TT genotype (P = .75) (Fig 1). The interaction between CD14 C-260T genotype and endotoxin exposure was borderline significant (P = .08) (Table 2). To address potential confounding by race/ethnicity (ie, population stratification) or the possibility of a race/ethnicity-specific effect, we stratified these analyses for African American and white patients (Table 2). Parameter estimates were similar for both groups, suggesting that the observed relationships were due to neither population stratification nor an effect in only 1 race/ethnic group.
Figure 1.
Relationship between endotoxin exposure at the age of 6 months and total serum IgE levels at the age of 1 year stratified by CD14 C-260T genotype: CC (A), CT (B), and TT (C). CC denotes individuals homozygous for cytosine at both alleles (n = 26); CT, individuals heterozygous for the transition (n = 32); and TT, individuals homozygous for the cytosine (C) to thymine (T) transition at position −260 of CD14 (n = 14).
Table 2.
Gene-Environment Interaction Between Dust Endotoxin Exposure at the Age of 6 Months and CD14 C-260Ta Genotype on Total Serum IgE Levels and CD4+ Lymphocyte Numbers at the Age of 1 Yearb
| Outcome variable at 1 y of age | Independent variable | All patients (n = 90) |
African American patients (n = 48) |
White patients (n = 34) |
|||
|---|---|---|---|---|---|---|---|
| Independent variable parameter estimate | P value | Independent variable parameter estimate | P value | Independent variable parameter estimate | P value | ||
| Total IgEc | Endotoxin | −0.46 | .03 | −0.39 | .22 | −0.45 | .17 |
| CD14 C-260T genotype | −0.83 | .14 | −0.62 | .56 | −0.67 | .38 | |
| Endotoxin × CD14 interaction | 0.33 | .08 | 0.42 | .24 | 0.21 | .42 | |
| CD4+ cells (No.) | Endotoxin | 0.14 | .02 | 0.17 | .04 | 0.22 | .05 |
| CD14 C-260T genotype | 0.51 | .002 | 0.50 | .04 | 0.47 | .048 | |
| Endotoxin × CD14 interaction | −0.16 | .005 | −0.20 | .02 | −0.14 | .08 | |
| CD4+Foxp3+ cells (No.) | Endotoxin | 0.04 | .71 | 0.24 | .06 | −0.03 | .88 |
| CD14 C-260T genotype | 0.19 | .47 | 0.87 | .03 | −0.24 | .55 | |
| Endotoxin × CD14 interaction | −0.08 | .39 | −0.29 | .03 | 0.01 | .94 | |
| CD4+Foxp3− cells (No.) | Endotoxin | 0.15 | .02 | 0.16 | .04 | 0.23 | .04 |
| CD14 C-260T genotype | 0.52 | .002 | 0.48 | .05 | 0.51 | .04 | |
| Endotoxin × CD14 interaction | −0.16 | .005 | −0.20 | .02 | −0.15 | .07 | |
CD14 C-260T denotes a single nucleotide polymorphism at position −260 resulting in a cytosine (C) to thymine (T) transition.
Regression equations are of the form ln(outcome variable) = β1(endotoxin) + β2(CD14 genotype) + β3(endotoxin)(CD14 genotype) or outcome variable = eβ1(endotoxin) + β2(CD14 genotype) + β3(endotoxin)(CD14 genotype), where β represents the individual parameter estimates given in the table. Endotoxin is included as the loge transformation of measured value in endotoxin units per milligram of dust, and CD14 C-260T genotype is categorized into CC (=0, referent), CT (=1), and TT (=2).
For total IgE, the numbers of children with data are 72, 28, and 39, for all patients, African American patients, and white patients.
Gene-Environment Interactions on CD4+ Lymphocyte Numbers
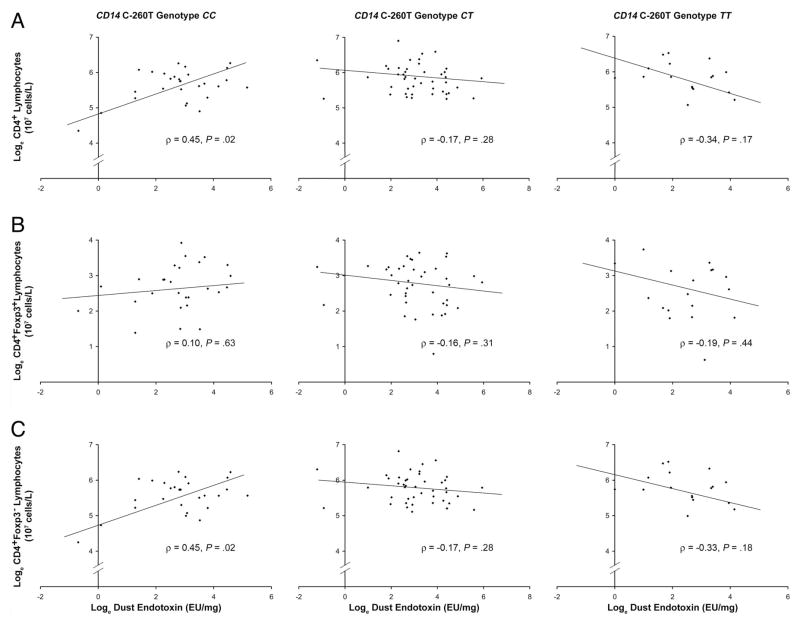
We also observed gene-environment interactions between children’s CD14 C-260T genotype and dust endotoxin exposure on total numbers of CD4+, CD4+Foxp3+, and CD4+Foxp3− lymphocytes at the age of 1 year. In general, these patterns were opposite those seen for total IgE levels. In children with the CC genotype at CD14 C-260T, increasing endotoxin exposure was associated with lower total serum IgE values at the age of 1 year (Fig 1) but higher numbers of CD4+Foxp3+ and CD4+Foxp3− cells (Fig 2). This relationship was statistically significant for CD4+Foxp3− lymphocytes, where endotoxin exposure seemed to account for 20% of the variation in these cell numbers at the age of 1 year.
Figure 2.
Relationship between endotoxin exposure at the age of 6 months and total CD4+ (A), CD4+Foxp3+(B), and CD4+Foxp3− (C) lymphocyte numbers at the age of 1 year stratified by CD14 C-260T genotype. TT denotes individuals homozygous for the cytosine (C) to thymine (T) transition at position −260 of CD14 (n = 18); CT, individuals heterozygous for the transition (n = 44); and CC, individuals homozygous for cytosine at both alleles (n = 28).
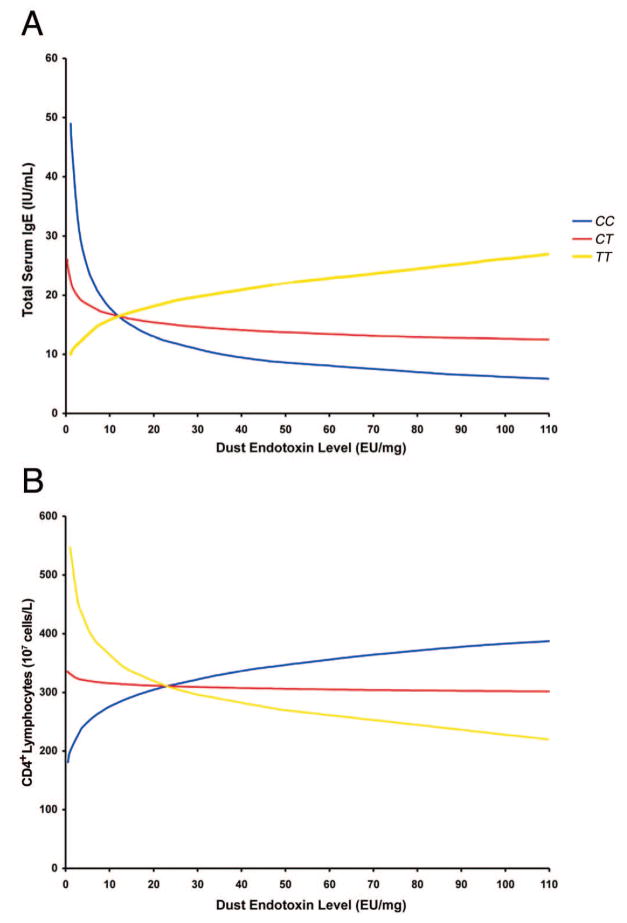
We explicitly tested an interaction between endotoxin exposure and CD14 C-260T genotype on CD4+ lymphocyte numbers in the regression models (Table 2). These suggested a significant gene-environment interaction on CD4+ lymphocyte numbers overall (P = .005) and, in particular, on CD4+Foxp3− lymphocyte numbers (P = .005). A significant gene-environment interaction was not evident for CD4+Foxp3+ lymphocyte numbers. The implications of these regression equations for the interaction between endotoxin exposure and CD14 C-260T genotype on total serum IgE levels and CD4+ lymphocyte numbers are shown in Figure 3.
Figure 3.
Interaction between CD14 C-260T genotype and dust endotoxin levels on total serum IgE levels (A) and CD4+ lymphocyte numbers (B) as implied by the regression models in Table 2, which included the variables CD14 C-260T genotype, endotoxin levels, and their interaction term for all study patients (n = 90). TT denotes individuals homozygous for the cytosine (C) to thymine (T) transition at position −260 of CD14; CT, individuals heterozygous for the transition; and CC, individuals homozygous for cytosine at both alleles.
To again address the possibility of confounding or effect modification by race/ethnicity, we stratified these analyses for African American and white patients (Table 2). Parameter estimates for both populations were similar for models predicting CD4+ lymphocyte numbers overall and CD4+Foxp3− lymphocyte numbers but were quite different for CD4+Foxp3+ lymphocyte numbers. Specifically, these models suggested that the gene-environment interaction between CD14 C-260T genotype and endotoxin exposure on CD4+Foxp3+ lymphocyte numbers was limited to African American patients (P = .03). The correlation coefficients between endotoxin exposure and CD4+Foxp3+ lymphocyte numbers in African American children were positive (ρ = 0.32; P = .18), neutral (ρ = −0.04; P = .84), and negative (ρ = −0.61; P = .20) for children with the CD14 C-260T CC genotype, CT genotype, and TT genotype, respectively.
Timing of Endotoxin Exposure on Outcomes
Given previous work on febrile infections,1 we hypothesized that endotoxin exposure as evaluated at 6 months would have the largest effect on study outcomes at the age of 1 year compared with earlier exposure. When we reanalyzed the data looking at an earlier time point for endotoxin exposure (ie, age of 1 month), we did not find a significant gene-environment interaction between CD14 C-260T genotype and endotoxin exposure on total IgE levels or CD4+ lymphocyte numbers (data not shown). This suggested that outcomes were the result of recent endotoxin exposure (ie, approximately 6 months of age) and not earlier exposures. This is in keeping with the hypothesis of an important window for innate immune system stimuli in the later half of the first year of life.1
DISCUSSION
We show for the first time a gene-environment interaction between CD14 C-260T genotype and endotoxin exposure on CD4+ lymphocytes numbers, including putative Treg cells. In children with the CD14 C-260T CC genotype, increasing endotoxin exposure at the age of 6 months was associated with lower IgE levels and higher numbers of CD4+ lymphocytes, particularly CD4+Foxp3− lymphocytes, for which endotoxin exposure accounted for 20% of the variance. We also show potential effect modification by race/ethnicity whereby CD14 C-260T genotype and endotoxin exposure seem to interact to affect Treg-cell numbers in African American children but not in white children.
Consistent with previous work looking at the timing of innate stimuli, such as febrile infections,1 we also found that endotoxin exposure at the age of 6 months, but not at the age of 1 month, was associated with IgE levels and CD4+ lymphocyte numbers at the age of 1 year. Together, these findings provide evidence of the importance of innate immune system stimuli and their timing on adaptive immune system responses (ie, CD4+ lymphocyte numbers and IgE levels). Although the present study does not settle the question as to whether the effect of innate stimuli on allergy risk results from Treg-cell expansion or immune deviation (ie, TH1-cell expansion),19,20 both processes may be involved, and the degree to which each process is involved may be genotype and race/ethnicity specific. For example, we observed that the numbers of Treg cells (ie, CD4+Foxp3+ lymphocytes) and effector T-helper cells (ie, CD4+Foxp3− lymphocytes) rose and fell in parallel, particularly in African American children. This finding is consistent with others that Treg-cell number expansion requires interleukin 2 (IL-2), and this cytokine indexes the Treg-cell population to the number of IL-2–producing CD4+ T cells.21,22
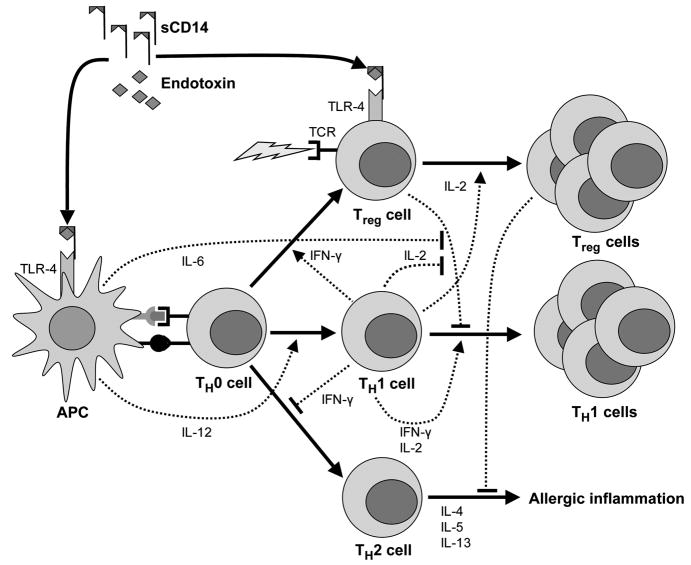
Recently, a mechanism relating innate immune system stimulation to adaptive immune responses has become better understood (Fig 4). Innate stimuli, such as endotoxin, can induce antigen-presenting cells (APCs) to up-regulate co-stimulatory molecules (eg, CD80 and CD86) and at high levels can produce proinflammatory cytokines (eg, IL-6 and IL-12).23,24 In the context of antigen presentation, these stimuli may promote TH2-type responses at low levels of endotoxin exposure25 but TH1-cell differentiation at high levels of exposure.26 Production of IL-6 by APCs can also promote effector cell proliferation by blocking Treg-cell suppression27; APCs matured through toll-like receptor (TLR) stimulation may also induce the conversion of naïve CD4+ cells into Treg cells. Similarly, interferon-γ production by TH1 cells may induce Foxp3 production in CD4+CD25− T cells.28 Last, direct stimulation of TLRs located on Treg cells can result in regulatory cell proliferation and affect suppressive function.10,29–32 Although this mechanism explains the observed relationship between endotoxin exposure and increasing CD4+ lymphocyte numbers in children with the CD14 C-260T CC genotype, it does not explain the seemingly opposite relationship observed for children with the TT genotype.
Figure 4.
Hypothetical model for the relationships among endotoxin exposure, regulatory and effector T-cell expansion, and allergic disease. Dashed lines ending in arrows indicate stimulation, whereas dashed lines ending in a vertical line indicate inhibition. Endotoxin binds to soluble CD14 (sCD14) and other binding proteins. Endotoxin then interacts with the toll-like receptor 4 (TLR-4) receptor complex, which stimulates antigen-presenting cells (APCs) to express co-stimulatory molecules at the time of antigen presentation. Interleukin 6 (IL-6) and IL-12 produced by APCs inhibit regulatory T-cell (Treg-cell) suppression and promote the differentiation of naïve T cells (TH0 cells) into TH1 cells, respectively. Interferon-γ (IFN-γ) and IL-2 promote the expansion of Treg cells and TH1 cells. The TLR-4 stimulation may also promote TH0-cell differentiation into Treg cells or their expansion. Both Treg cells and TH1 cells suppress allergy, promoting TH2 cells through cell-to-cell contact or cytokine production. Low levels of endotoxin exposure in the setting of antigen presentation may also promote TH2 responses if the former is sufficient to promote APC maturation but insufficient to induce inflammatory cytokine production. TCR indicates T-cell receptor.
CD14, an accessory receptor that facilitates the binding of endotoxin to TLR-4,33 exists as a membrane bound receptor and in soluble form (sCD14). The C to T transition at CD14 position −260 has been associated with increased transcriptional activity34 and increased levels of sCD14.17,35–37 These differences in sCD14 levels by CD14 C-260T genotype may be seen as early as the age of 3 months.38 However, the disparate effects of increasing endotoxin exposure by CD14 C-260T genotype suggest that the effect of this polymorphism may also differ by level of endotoxin exposure.
Unlike earlier work that showed a race/ethnicity-specific interaction between CD14 C-260T genotype and environmental tobacco exposure on total IgE levels in Puerto Rican patients but not in Mexican patients,15 we did not find a race/ethnicity-specific interaction between CD14 C-260T genotype and endotoxin exposure on total IgE levels in African American and white patients. However, we observed potential effect modification by race/ethnicity on Treg-cell numbers. African American children, but not white children, demonstrated a significant interaction between CD14 C-260T genotype and endotoxin exposure on Treg-cell numbers. These findings need to be confirmed in other diverse cohorts.
This study has several limitations. First, there were limited numbers of children with data available for both 6 months and 1 year for outcomes of interest. Although this prohibited us from looking in detail at changes across time, we examined the a priori hypothesis between exposures at the age of 6 months and outcomes at the age of 1 year. Despite limited numbers, the findings were consistent with the CD14 C-260T genotype and endotoxin interaction that has been described for total IgE,6 allergen specific IgE,7 and asthma severity9 in other populations. In these studies, the CD14 C-260T CC and TT genotypes seemed to have opposite effects on outcomes, and these relationships were reversed at low and high levels of endotoxin exposure. Second, we identified Treg cells by intracellular Foxp3 expression and not by surface CD25 expression because Foxp3 expression seems to better delineate CD4+ Treg cells, at least in animal models, compared with CD25 expression.13,39 In addition to the limited specificity of CD25, we could not further distinguish between thymus-generated natural Treg cells and allergen-specific adaptive Treg cells, because both may be characterized by Foxp3 expression.40 We also did not attempt to identify other Treg cells, such as those in CD8+ and natural killer T-cell populations or those characterized by IL-10 secretion.41 Third, although we enumerated CD4+Foxp3+ lymphocytes, we did not evaluate Treg-cell function. As mentioned already, endotoxin may affect the suppressive function of Treg cells, 10 which may be relevant to allergic diseases, because individuals with atopy have been shown to have Treg cells with diminished suppressive function.42 Next, we used endotoxin measured on mothers’ bedroom floors as a proxy of exposure in the first year of life. It is unknown whether this is the most biologically relevant location or method of measuring exposure in infants. However, these findings were consistent with previous observations,6 suggesting that this location was a reasonable proxy for biologically relevant exposure. The absence of an association between earlier endotoxin levels and study outcomes suggested that the relationships shown herein were related to recent exposure, supporting the a priori hypothesis of a postnatal period of increased susceptibility.1 Last, we did not specifically delineate whether CD4+Foxp3− lymphocytes had a TH1 or TH2 phenotype. This is important because it could help clarify the relative importance of endotoxin exposure on TH1/TH2 differentiation vs Treg-cell expansion and its subsequent effects on IgE levels. We hope to include this in future studies.
Although this study was small, it provides some interesting directions for further research. We identified that the gene-environment interaction between CD14 C-260T genotype and endotoxin exposure may have reciprocal effects on total serum IgE levels and CD4+ lymphocyte numbers (ie, CD4+Foxp3+ and CD4+Foxp3−). Although this may provide a simple immunologic explanation for the observed gene-environment interaction on IgE levels, research is needed to determine the upstream effects of this polymorphism on the innate immune response and antigen presentation and why this promoter SNP seems to have disparate effects at high and low endotoxin levels. Given well-described disparities in atopic conditions by race/ethnicity,43–45 it will be important to clarify whether the early race/ethnicity differences observed herein for Treg cells contribute to later disparities. These findings provide a rationale for further research into the early determinants of these disparities.
Acknowledgments
We acknowledge the contributions of all the WHEALS staff, whose dedication to the study made this article possible.
Funding Sources: This work was supported by grants from the Fund for Henry Ford Hospital and the National Institute of Allergy and Infectious Diseases (AI61774, AI50681, AI59415) and the National Heart, Lung, and Blood Institute (HL79055), National Institutes of Health.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Williams LK, Peterson EL, Pladevall M, Tunceli K, Ownby DR, Johnson CC. Timing and intensity of early fevers and the development of allergies and asthma. J Allergy Clin Immunol. 2005;116:102–108. doi: 10.1016/j.jaci.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002;166:939–944. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]
- 3.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 4.von Mutius E, Braun-Fahrlander C, Schierl R, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 5.Gereda JE, Leung DY, Thatayatikom A, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–1683. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 6.Williams LK, McPhee RA, Ownby DR, et al. Gene-environment interactions with CD14 C-260T and their relationship to total serum IgE levels in adults. J Allergy Clin Immunol. 2006;118:851–857. doi: 10.1016/j.jaci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Eder W, Klimecki W, Yu L, et al. Allergy and Endotoxin Alex Study Team. Opposite effects of CD14/−260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005;116:601–607. doi: 10.1016/j.jaci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Simpson A, John SL, Jury F, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 9.Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/−260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol. 2005;115:1203–1209. doi: 10.1016/j.jaci.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 14.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4:58–68. doi: 10.1513/pats.200607-146JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhry S, Avila PC, Nazario S, et al. CD14 tobacco gene-environment interaction modifies asthma severity and immunoglobulin E levels in Latinos with asthma. Am J Respir Crit Care Med. 2005;172:173–182. doi: 10.1164/rccm.200409-1232OC. [DOI] [PubMed] [Google Scholar]
- 16.Litonjua AA, Belanger K, Celedon JC, et al. Polymorphisms in the 5′ region of the CD14 gene are associated with eczema in young children. J Allergy Clin Immunol. 2005;115:1056–1062. doi: 10.1016/j.jaci.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism* in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. AmJ Respir Cell Mol Biol. 1999;20:976–983. doi: 10.1165/ajrcmb.20.5.3494. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Rev. New York, NY: Academic Press; 1977. p. 412. [Google Scholar]
- 19.Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Scheffold A, Huhn J, Hofer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol. 2005;35:1336–1341. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 22.Almeida AR, Zaragoza B, Freitas AA. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J Immunol. 2006;177:192–200. doi: 10.4049/jimmunol.177.1.192. [DOI] [PubMed] [Google Scholar]
- 23.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 25.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4–dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Hong J, Sun W, et al. Role of IFN-γ in induction of Foxp3 and conversion of CD4+ J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 30.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.da Silva CJ, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 34.Vercelli D. The functional genomics of CD14 and its role in IgE responses: an integrated view. J Allergy Clin Immunol. 2002;109:14–21. doi: 10.1067/mai.2002.121015. [DOI] [PubMed] [Google Scholar]
- 35.Koenig W, Khuseyinova N, Hoffmann MM, et al. CD14 C (−260)–>T polymorphism, plasma levels of the soluble endotoxin receptor CD14, their association with chronic infections and risk of stable coronary artery disease. J Am Coll Cardiol. 2002;40:34–42. doi: 10.1016/s0735-1097(02)01937-x. [DOI] [PubMed] [Google Scholar]
- 36.Amar J, Ruidavets JB, Bal dit Sollier C, et al. CD14 C(−260)T gene polymorphism, circulating soluble CD14 levels and arteriosclerosis. J Hypertens. 2004;22:1523–1528. doi: 10.1097/01.hjh.0000133724.16947.a3. [DOI] [PubMed] [Google Scholar]
- 37.Morange PE, Tiret L, Saut N, et al. TLR4/Asp299Gly, CD14/C-260T, plasma levels of the soluble receptor CD14 and the risk of coronary heart disease: the PRIME Study. Eur J Hum Genet. 2004;12:1041–1049. doi: 10.1038/sj.ejhg.5201277. [DOI] [PubMed] [Google Scholar]
- 38.LeVan TD, Guerra S, Klimecki W, et al. The impact of CD14 polymorphisms on the development of soluble CD14 levels during infancy. Genes Immun. 2006;7:77–80. doi: 10.1038/sj.gene.6364276. [DOI] [PubMed] [Google Scholar]
- 39.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102:4091–4096. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10–secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 42.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 43.Joseph CL, Williams LK, Ownby DR, Saltzgaber J, Johnson CC. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. J Allergy Clin Immunol. 2006;117:233–240. doi: 10.1016/j.jaci.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litonjua AA, Celedon JC, Hausmann J, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115:751–757. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 45.Lester LA, Rich SS, Blumenthal MN, et al. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]