Abstract
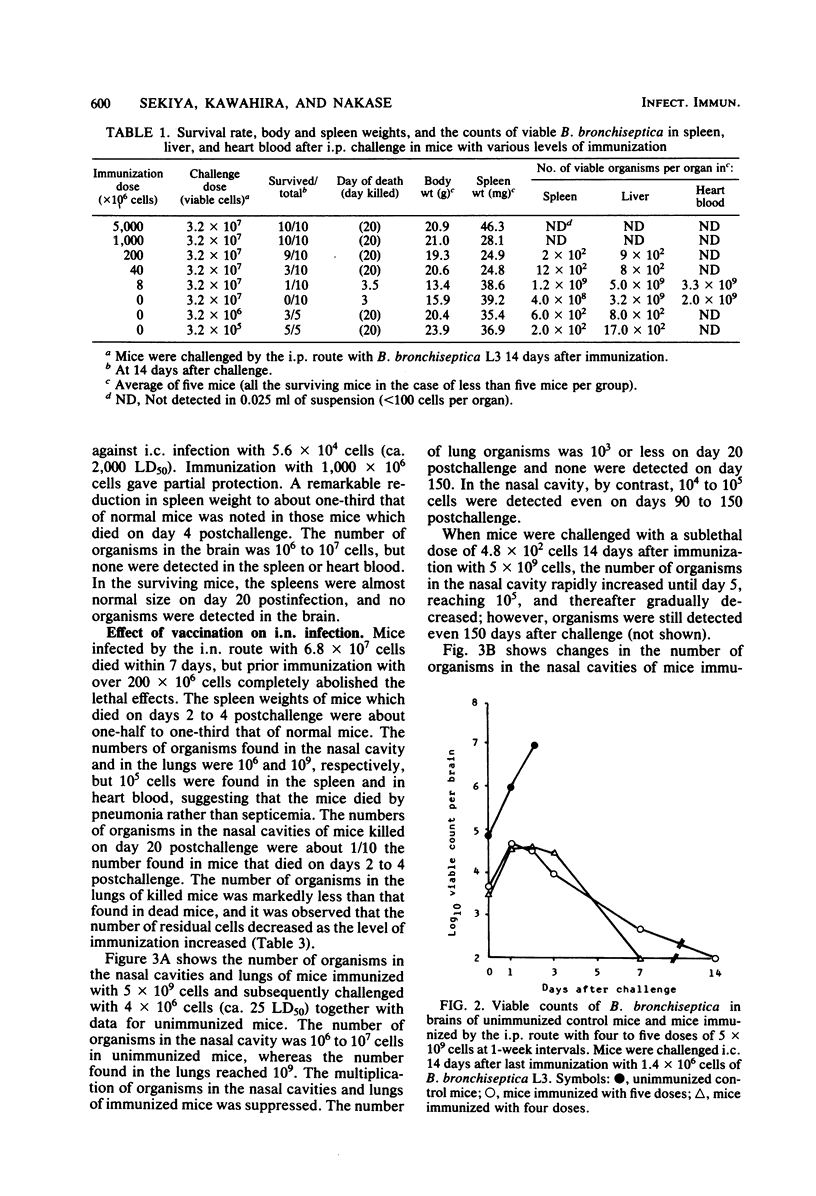
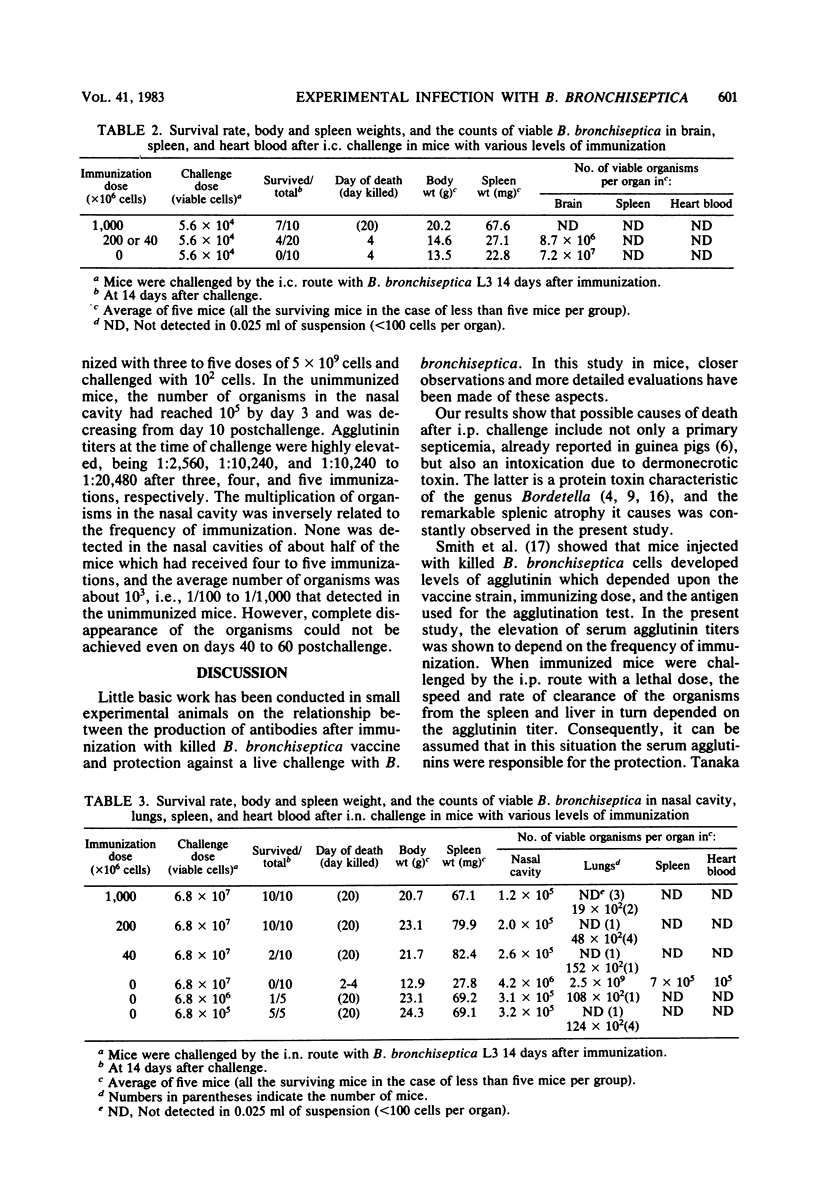
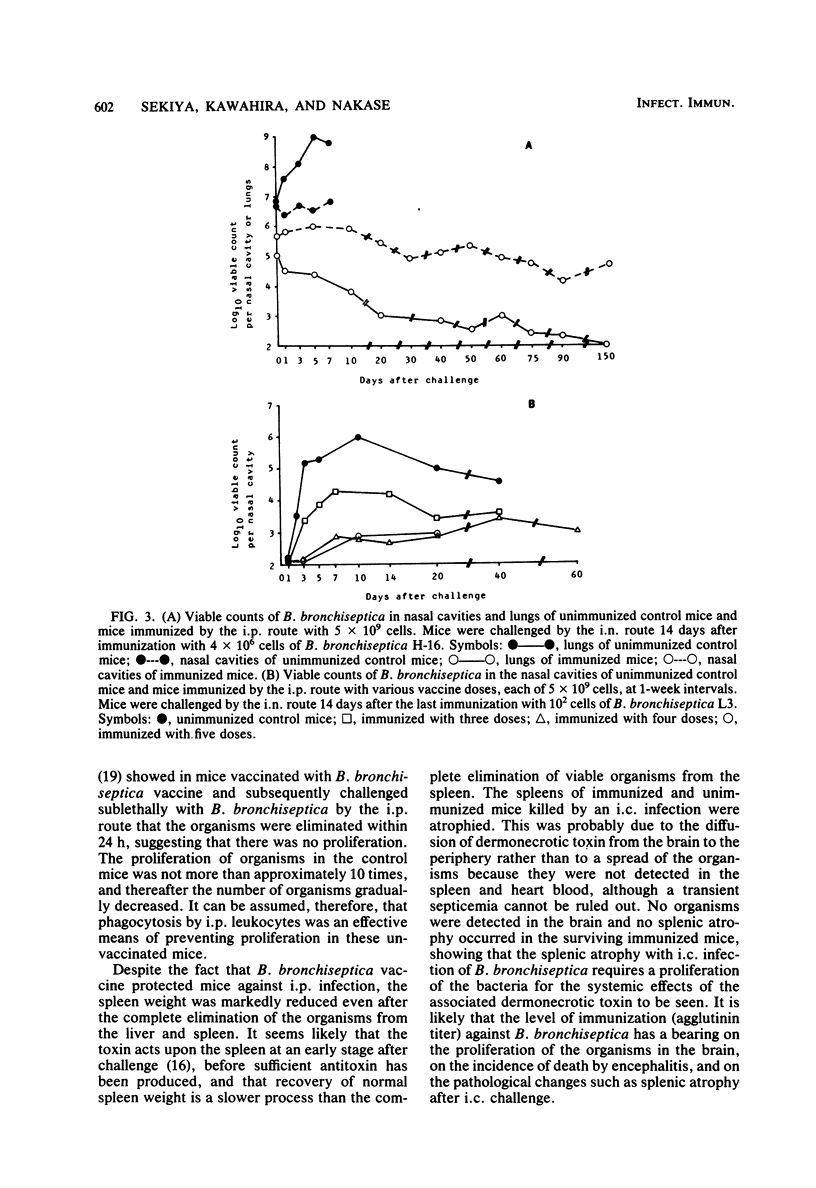
Mice immunized with a killed vaccine of phase I Bordetella bronchiseptica were challenged with various numbers of virulent B. bronchiseptica by intraperitoneal, intracerebral, or intranasal routes. The course of infection was compared among these routes, and the protective effect of vaccination was quantitatively analyzed. In ddN mice infected intraperitoneally with 1.8 X 10(8) cells (ca. 80 times the 50% lethal dose [LD50]) the organisms rapidly increased in the intraperitoneal fluid, spleen, and liver within few days and caused splenic atrophy, septicemia, and death. However, immunizations with 5 X 10(9) cells gave the mice a high agglutinin titer and suppressed the increase in the number of organisms. With four immunizations, the lungs and livers were clear within 3 days, and with one or two immunizations, they were clear within 7 days. These immunizations effectively protected the mice from death but did not protect them from splenic atrophy. In the intracerebral infection with 1.4 X 10(6) cells (ca. 1.2 X 10(5) LD50), the number of organisms rapidly increased in the brain and caused encephalitis, splenic atrophy, and death. However, four or five immunizations completely suppressed the increase in the brain and protected the mice from death and splenic atrophy. After intranasal infection with 4 X 10(6) cells (ca. 25 LD50), the organisms rapidly increased in the nasal cavity and lungs and caused pneumonia and death. Immunization with 5 X 10(9) cells was effective in clearing the organisms from the lungs and in protecting against death and splenic atrophy. However, the organisms were not cleared from the nasal cavity for 60 to 150 days after the challenge with as little as 10(2) cells, even in the mice with an agglutinin titer as high as 1:10,000.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. M., Wheeler M. W. Pertussis Vaccine Prepared with Phase-I Cultures Grown in Fluid Medium. Am J Public Health Nations Health. 1946 Apr;36(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- ELDERING G., HORNBECK C., BAKER J. Serological study of Bordetella pertussis and related species. J Bacteriol. 1957 Aug;74(2):133–136. doi: 10.1128/jb.74.2.133-136.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANAWAY J. R., ALLEN A. M., MCPHERSON C. W. PREVENTION OF ACUTE BORDETELLA BRONCHISEPTICA PNEUMONIA IN A GUINEA PIG COLONY. Lab Anim Care. 1965 Apr;15:156–162. [PubMed] [Google Scholar]
- Goodnow R. A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980 Dec;44(4):722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow R. A., Lehr C. D., Shade F. J., Wisecarver J. L. Mouse potency assay for Bordetella bronchiseptica bacterins. J Clin Microbiol. 1977 Oct;6(4):337–339. doi: 10.1128/jcm.6.4.337-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDRICK P. A., NADOLSKI E. B., ELDERING G., BAKER J. Antigenic relationships of Haemophilus pertussis, the parapertussis bacillus, and Brucella bronchiseptica as shown by cross protection tests in mice. J Bacteriol. 1953 Aug;66(2):166–169. doi: 10.1128/jb.66.2.166-169.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Muto T., Yoda H., Nakano T., Imaizumi K. Experimental Bordetella bronchiseptica infection in guinea pigs. Nihon Juigaku Zasshi. 1971 Apr;33(2):53–60. doi: 10.1292/jvms1939.33.53. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Yoda H., Muto T., Imaizumi K. Prophylaxis of Bordetella bronchiseptica infection in guinea pigs by vaccination. Nihon Juigaku Zasshi. 1974 Feb;36(1):33–42. doi: 10.1292/jvms1939.36.33. [DOI] [PubMed] [Google Scholar]
- POWELL H. M., CULBERTSON C. G., ENSMINGER P. W. Charcoal agar culture medium for preparing Hemophilus pertussis vaccine. Public Health Rep. 1951 Mar 16;66(11):346–348. [PubMed] [Google Scholar]
- Sekiya K., Munoz J. J., Nakase Y. Effect of dermonecrotic toxin of Bordetella pertussis on the spleen of CFW and C57BL/10ScN mice. Microbiol Immunol. 1982;26(10):971–977. doi: 10.1111/j.1348-0421.1982.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Smith I. M., Baskerville A. J., Brothwell E., Oliphant J. Immunogenicity of killed Bordetella bronchiseptica vaccines in the mouse. Res Vet Sci. 1982 Mar;32(2):248–252. [PubMed] [Google Scholar]



