Abstract
Background
Treatment studies are lacking for patients with bipolar II disorder (BDII). The objective of this study was to compare lamotrigine (LTG) and lithium (Li) monotherapy for the treatment of BDII depression.
Methods
Patients with BDII acute depression were randomized to open-label monotherapy with LTG or Li, and evaluated by trained raters blinded to treatment. Patients were titrated to 200 mg/day of LTG over 8 weeks or at least 900 mg/day of Li over 2 weeks (serum level 0.6–1.2 mEq/L), and seen biweekly for 16 weeks. The primary outcome variable was change in the Hamilton Depression Rating Scale 17-item (Ham-D17), evaluated using mixed effects random regression.
Results
Both groups showed significant improvement from baseline to endpoint on the Ham-D17 (p<0.0001), with no between group differences (p=0.95). Seventy-two percent of the population was rapid cycling by DSM-IV criteria. No differences in response were noted between rapid cyclers and non-rapid cyclers. Early termination for any cause was 42%. The Li group reported significantly more side effects, although drop-out due to side effects did not differ between groups.
Limitations
This study was limited by an open treatment design, a lack of placebo arm, and uneven treatment groups.
Conclusions
Lamotrigine and lithium were effective monotherapy for BDII depression, with comparable response and remission rates. Naturalistic design and lack of placebo limit conclusions, though patient history indicated long standing depression unlikely to be alleviated by time. Patients who received Li reported more side effects, but this did not appear to impact drop out rates.
Keywords: Bipolar II Disorder, Bipolar Depression, lithium, lamotrigine
Introduction
Bipolar disorder is a severe, persistent psychiatric disorder. The prevalence of bipolar II disorder (BDII) is estimated in the U.S. at 1.1% (Merikangas et al, 2007) and in Europe estimates of 3–6% have been reported when varying definitions of hypomania duration and symptoms are used (Angst, 1998; Akiskal, 1996). It has recently been recognized that for patients with BDII, a significantly greater proportion of time over the course of the illness is spent in the depressive vs. hypomanic phase of the illness, with subjects spending up to 50.3% of their time depressed (Judd et al, 2003; Kupka et al, 2007). Despite the prevalence, severity, and persistence of depression in individuals with BD II, research in bipolar depression has been primarily in bipolar I disorder (BDI) depression (Bowden, 2005). Only two medications are approved by the Food and Drug Administration (FDA) for the treatment of BDI depression, and for BDII depression, only quetiapine has been specifically studied or approved by the FDA. With so little data to support treatment decisions, including efficacy and tolerability data, clinicians rely on past experience and clinical judgment when choosing medication therapy for BDII patients.
Lithium (Li) and lamotrigine (LTG) both have evidence supporting their use in maintenance treatment of bipolar I depression, with negative or conflicting evidence supporting their use in the acute treatment of BDI depression. Because of their potential usefulness in the treatment of BDI depression, these medications may have a role in the treatment of BDII depression.
Although no randomized acute trials of Li for the treatment of BDII have been published, early studies of Li for the acute and maintenance treatment of bipolar disorder included BDII patients in their samples. These controlled trials, which included patients with BDI, BDII, BD NOS, or unipolar depression, found that that Li was superior to placebo for the acute (up to 7 weeks) treatment of bipolar depression (Zornberg and Pope, 1993) and that Li significantly prolonged time to mood relapse as well as shortened the duration of current depressive episodes (Kane et al, 1982; Greil and Kleindienst, 1999). An uncontrolled, prospective clinical evaluation of patients with BDI and BDII on Li maintenance treatment followed patients for an average of 6.35 years and found that the average time to relapse for patients with BDI was 17 months while the average time to relapse for patients with BDII was 100 months (Tondo et al, 1998). This study supports previous findings of efficacy of Li in the treatment of BDII, and suggests that patients with BDII may be more responsive to Li treatment than patients with BDI.
There have been a number of negative controlled trials with LTG in BDI alone and in trials which included BDI and BDII, and one negative trial including only patients with BDII (Calabrese et al, 2007; gsk.com). However, three recent meta-analyses of LTG trials found an overall positive effect, though this wasn’t observable on a per study basis (Geddes et al, 2007; Smith et al, 2007; Soares-Weiser et al, 2007). Four double-blind studies assessing LTG for the treatment of BDII have been published to date. The two larger trials included patients with BDI, BDII, or major depressive disorder. One study failed to separate on the primary outcome measure, but overall, patients treated with LTG showed significantly greater response rates, significant improvement in depression symptoms, and significantly longer median time to additional pharmacology (Frye et al, 2000; Calabrese et al, 2000). Two additional small double blind studies also support a potential role for LTG in the treatment of BDII depression (Schaffer et al, 2006; Barbosa et al, 2003).
We report here on 102 patients who were enrolled into this single-blind, 16-week, randomized comparison of open-label Li and LTG in the acute treatment of patients with BDII depression.
Methods
Study Design
This study consisted of two randomized, 16-week trials designed a priori with the same design for combined analysis. Patients were recruited from two sites in Texas (Dallas and Houston) utilizing newspaper ads, radio ads, flyers, and referrals from other physicians. The studies were approved by the UT Southwestern Medical Center Institutional Review Board and the Baylor College of Medicine Institutional Review Board. After complete description of the study to the subjects, written informed consent was obtained.
Patients
Outpatients between the ages of 18–65 were eligible for study entry if they had a clear history of BDII confirmed by SCID-research version interview (First et al, 2002) and met criteria for DSM-IV bipolar II disorder (hypomanic episode duration of 4 or more days), current episode depressed, and currently had a score of 18 or greater on the Hamilton Rating Scale for Depression – 17 item (Ham-D17) or Montgomery Asberg Depression Rating Scale (MADRS). Patients were excluded if they had a history of clinically relevant intolerance or nonresponse to lithium (Li) or lamotrigine (LTG), an unstable medical illness, psychotic symptoms, active suicidal ideation or intent, or substance abuse or dependency within the last month. Women who were pregnant, planning to conceive, or breastfeeding were excluded; all other women of child-bearing potential were enrolled only if they had a negative pregnancy test at screening and agreed to use an effective contraceptive method.
Patients taking other medications but who wished to change due to intolerance or lack of efficacy were eligible. Due to ethical and safety concerns, patients that were taking ineffective medications were tapered off these medications, and randomization scheduled to coincide with the last day of the taper to avoid a medication free period. Patients taking fluoxetine within 2 weeks of randomization were excluded due to the long half-life of this medication. Similarly, patients were excluded if they took valproate or carbamazepine within 3 days of randomization to avoid drug interactions. Short-term use of limited benzodiazepines/hypnotics for a maximum of 5 consecutive days, on no more than one occasion over the course of the 16-week trial, was permitted.
Treatments
Patients were randomized 1:1 to receive either Li or LTG. In order to minimize the risk of developing a rash, LTG was titrated over 8 weeks in the following manner: 25 mg/day for 2 weeks, 50 mg/day for two weeks, then 75 mg/day for one week, 100 mg/day for one week, 150 mg/day for one week, until patients reached 200 mg/day at week 8. Li was titrated starting at 450 mg/day for a week and then increased to 900 mg/day for a week. Further increases or decreases were dictated by consideration of clinical symptoms, maximum dosing, and tolerability. LTG could be increased by 100 mg/day per week to a maximum dose of 400 mg/day, and Li dosing was dictated by clinical response, tolerability, and blood levels (minimum 0.8 to maximum 1.2 mEq/L).
At every visit, vital signs (weight, blood pressure, and pulse) were measured and a side effect assessment completed. For patients on Li, blood levels were drawn at week 2, then as needed based on dose changes and Li serum levels. The target LTG dose was 200 mg/day, but if tolerability was an issue and patient symptoms were improving, the minimum dose permitted was 100 mg/day. The target Li level was 0.8 mEq/L, but if tolerability was an issue and patient symptoms were improving, the minimum serum level allowed was 0.6 mEq/L.
Outcome Measures
As patients in the LTG group did not reach usual therapeutic dose until week 8, the study was designed for 16 weeks to provide ample opportunity to assess the effects of therapeutic dosing of both study medications and to assess tolerability and continued response. Patients and physicians knew their group assignment, but personnel completing symptom ratings were blind to group assignment. All patients met with a psychiatrist or mental health nurse practitioner and symptom severity was assessed by a blinded research assistant at biweekly visits for the duration of the 16-week study. During the initial titration period (8 weeks), an unblinded research clinician contacted patients in between visits via phone to provide support and screen for potential serious side effects.
Mood was rated using the Ham-D17 (Hamilton, 1960) Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), Young Mania Rating Scale (YMRS) (Young et al, 1978), and the Clinical Global Impression Scale for Bipolar Disorder (CGI-BP) (Spearing et al, 1997). Overall functioning was rated using the Global Assessment of Functioning (GAF) (DSM-IV-TR).
The primary efficacy variable was change in depression symptoms from baseline to week 16, as measured by the Ham-D17. Secondary efficacy measures include incidence and severity of hypomanic and/or depressive symptoms, medication tolerability, response (defined as a 50% reduction on the Ham-D17), remission (defined as Ham-D17 or MADRS ≤ 12), and switch into hypomania (defined as CGI-BP Mania severity score of 4 or greater).
Statistics
Ham-D17, MADRS, YMRS, CGI-BP, and GAF assessments were analyzed using mixed effects random regression (Gibbons et al, 1993). The random regression model contained terms for the baseline score (covariate), treatment group, week, and treatment group by week interaction. Due to the large percentage of rapid cycling patients enrolled in this study, separate analyses were done on this subgroup of patients. A power analysis was performed using the primary outcome measure, the HAMD-17. The residual error and estimated means from the random regression were used as input to SAS Proc Power with an alpha of .05 and a power of .80.
A logistic regression was used to model the probability of response at exit. The model included terms for treatment group and whether the patient was a rapid cycler. The logistic regression model provided an estimate of the odds of exit response for Li versus LTG after adjusting for covariates.
The SAS lifetest was used to calculate survival probability, and a t-test was used to compare duration of treatment between groups. A chi-square test of treatment group by adverse event status was done to determine if specific adverse events differed between groups.
In a planned interim analysis it was noted that patients with rapid cycling were unevenly distributed between the LTG and Li groups. To correct this, an Efron procedure was used to force randomization for additional patients entered into the study (Efron, 1971; Kraemer, 1981). This technique uses a rank order method to help ensure equal group sizes in small populations.
Results
Demographics
One hundred two patients were randomized to receive study medication. One patient never received medication and 3 patients failed to meet entry criteria at randomization. These patients were excluded from all analyses, resulting in a safety population of 98 patients. An additional 8 patients had only a baseline visit (lost to follow-up at week 2) and these patients were excluded from efficacy analyses, but were included in baseline demographic analyses. There were no significant differences in baseline characteristics of the two groups (Table 1).
Table 1.
Patient Demographics (n=98)
| Lamotrigine | Lithium | |
|---|---|---|
| Characteristics | (n=44) | (n=54) |
| Gender, N (%) | ||
| Female | 30 (68.2%) | 31 (57.4%) |
| Age, mean ± sd | 36.9 ± 12.3 | 36.2 ± 11.4 |
| Race, N (%) | ||
| Caucasian | 33 (75.0%) | 42 (77.8%) |
| DSM-IV diagnosis, N (%) | ||
| Rapid Cycling | 35 (79.6%) | 36 (66.7%) |
| Non-rapid Cycling | 9 (20.4%) | 18 (33.3%) |
| Age at Diagnosis* | 29.5 ± 11.56 | 28.2 ± 11.18 |
| Mean Age of First Depressive Episode | 15.8 ± 8.06 | 15.7 ± 6.03 |
| Mean Age of First Manic Episode | 18.2 ± 10.21 | 17.6 ± 7.08 |
Initial diagnosis of major depressive disorder or bipolar disorder
The evaluable number of patients for efficacy analyses was 90; 41 for the LTG group and 49 for the Li group. Of the 90 patients evaluated, 76% (n=68) were rapid cycling; 83% of the LTG group and 69% of the Li group. A total of 40 patients (44%) completed the study, 21 (51%) in the LTG group and 19 (39%) in the Li group (p=0.29).
The first quartile, median, and third quartile peak dose of LTG were 100, 250, and 300 mg, respectively; the first quartile, median, and third quartile peak dose of Li were 1200, 1200, and 1500 mg; and the first quartile, median, and third quartile serum levels were 0.7, 0.8, and 1.1 mEq/L.
Patients with a history of rapid cycling were compared to patients without a history of rapid cycling on age, sex, and ethnicity to determine if there were any differences between these groups of patients. As in the full sample, there were no significant between group differences on baseline characteristics.
Depressive Symptoms
Hamilton Depression Rating Scale
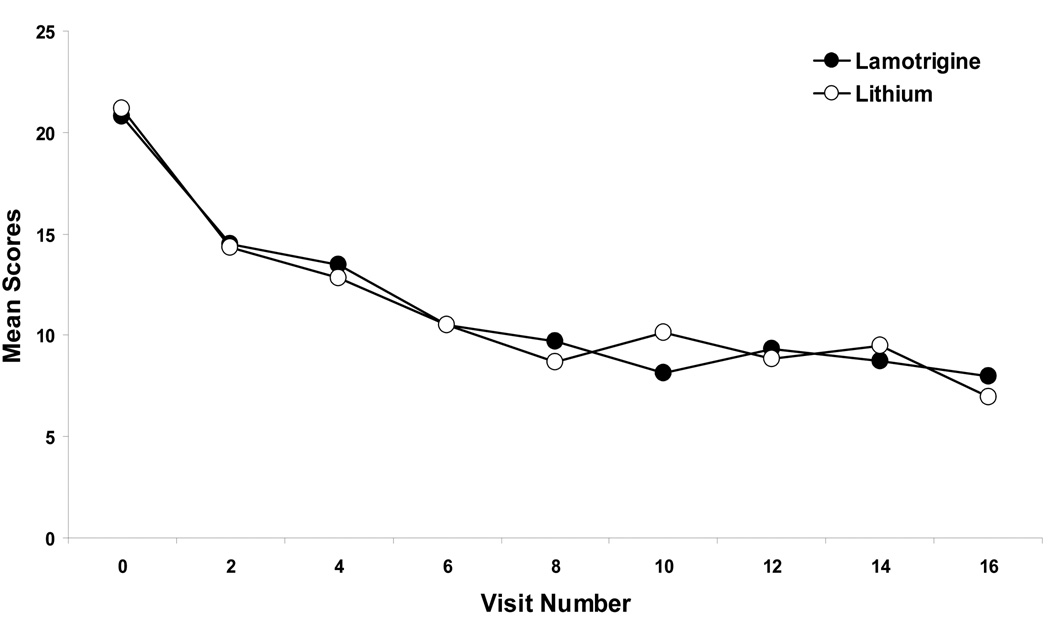
Figure 1 shows the mean scores over time on the primary outcome – the Ham-D17. Using the baseline value as a covariate, a random regression analysis showed that the overall difference between baseline and endpoint Ham-D17 scores was significant for both LTG and Li groups (F(7, 402)=12.92, p<0.0001). There were non-significant between group differences for the Ham-D17 total score (F(7,402)=0.54, p = 0.95). Mean Ham-D17 scores and standard deviations at baseline were 20.8 ± 4.27 and 21.2 ± 4.15 for the LTG and the Li groups (p=0.73), respectively. Mean Ham-D17 scores and standard errors at endpoint were 8.00 ± 1.28 and 6.97 ± 1.33 for the LTG and the Li groups, respectively. A random regression analysis was run to determine improvement at week 8. Degrees of freedom reflect the number of visits during the 8 weeks (n=4). Results from this analysis were similar to those for week 16; both group showed significant improvement from baseline to week 8 (F(3, 214)=15.27, p<0.001), and there were no significant between group differences for the Ham-D17 total score (F(3, 214)=0.21, p = 0.89). For the subset of patients with a history of rapid cycling, both groups showed significant improvement on the Ham-D17 (p<0.001) at week 16, with no between group differences in improvement (p=0.39).
Figure 1.
Mean Ham-D17 scores over time for monotherapy lithium and lamotrigine in patients with bipolar II acute depression. Based on mixed effects random regression.
The results of the power analysis suggest that the sample size needed to find a significant difference between groups was over 10,000 subjects. This is a reflection of the very small effect size (0.01 for the Ham-D17).
Montgomery-Asberg Depression Rating Scale
Both groups experienced significant baseline to endpoint improvement on the MADRS (F(7, 403)=11.68; p<0.001). There were no between group differences for mean change from baseline (F(7, 403)=0.28; p=0.96). Mean baseline MADRS scores and standard deviations were 29.7 ± 6.30 and 30.2 ± 6.30 for the LTG and the Li groups (p=0.97), respectively, and mean endpoint scores and standard errors were 9.48 ± 2.08 and 8.59 ± 2.15 for the LTG and Li groups. A random regression analysis was performed to determine improvement at week 8. Results from this analysis were similar to those for week 16; both groups showed significant improvement from baseline to week 8 on the MADRS (F(3, 214)=12.01, p<0.001). There were no significant between group differences for the MADRS total score (F(3, 214)=0.18, p = 0.91). For the patients with a history of rapid cycling, both groups demonstrated significant improvement on the MADRS (p<0.001) at week 16, with no between group differences in improvement (p=0.96).
Hypomanic Symptoms
Young Mania Rating Scale
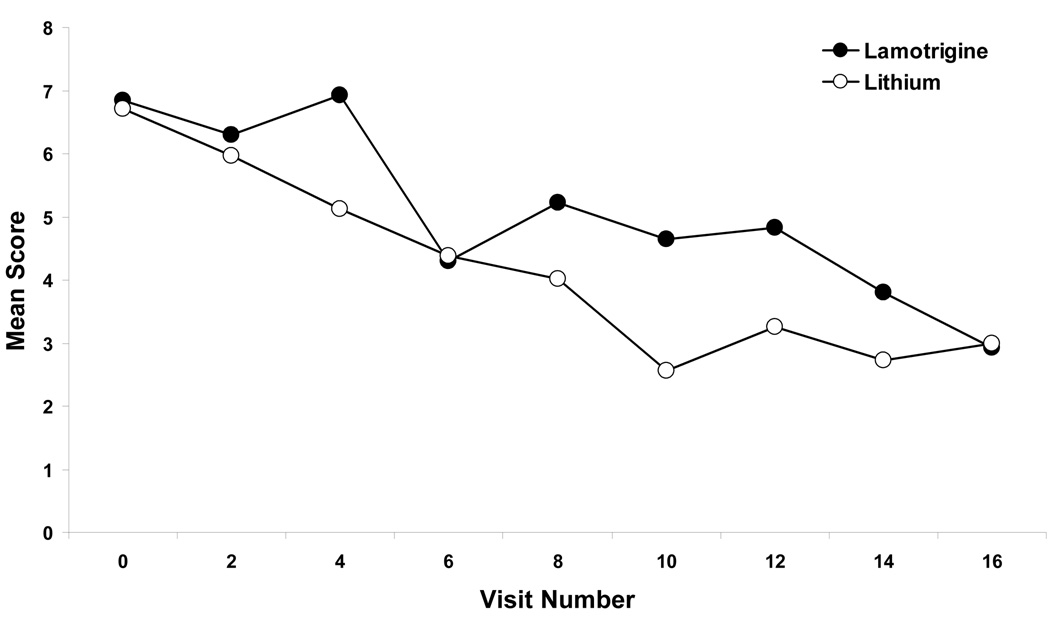
Patients experienced significant improvement on mean YMRS scores (F(7, 406)=5.44; p<0.001), although there were no between group differences (F(7, 406)=0.69; p=0.68) (Figure 2). Mean baseline YMRS scores and standard deviations were 6.84 ± 4.34 and 6.71 ± 3.82 for the LTG and the Li groups (p=0.63) and mean endpoint scores and standard errors were 2.92 ± 0.93 and 3.00 ± 0.97 for the LTG and Li groups. Patients with a history of rapid cycling experienced significant improvement on the YMRS (p<0.001), with no significant differences between groups (p=0.74).
Figure 2.
Mean YMRS Scores over time for monotherapy lithium and lamotrigine in patients with bipolar II depression. Based on mixed effects random regression.
Overall Functioning
Clinical Global Impression Scale for Bipolar Disorder, Overall Severity
For the CGI-BP (overall illness severity), both groups showed significant improvement from baseline to endpoint (F(7, 402)=11.23; p<0.001) with no between group differences in improvement (F(7, 402)=0.77; p=.61). Mean baseline CGI-BP severity scores were 4.5 ± 0.7 and 4.6 ± 0.6 for the LTG and the Li groups and mean endpoint scores and standard errors were 1.9 ± 0.31 and 2.2 ± 0.32 for the LTG and Li groups. Patients with a history of rapid cycling showed significant improvement in overall mood severity (p<0.001), with no between group differences (p=0.43).
Global Assessment of Functioning
Both LTG and Li groups showed significant improvement over time on the GAF (F(7, 408)=14.43; p<0.001), with a trend for between group differences favoring LTG (F(7, 408)=2.00; p=0.054). Mean baseline scores on the GAF were 52.2 ± 6.69 and 53.8 ± 5.66 for the LTG and the Li groups (p=0.63) and mean endpoint scores and standard errors were 73.1 ± 2.05 and 71.1 ± 2.12 for the LTG and Li groups. Patients experiencing rapid cycling showed significant improvement on GAF scores (p<0.001). There was also a significant group by visit interaction for the GAF (p=0.019), with the LTG rapid cycling group showing a greater improvement on the GAF.
Response and Survival Analyses
Patients in both groups experienced relatively similar rates of response and remission, and limited switch into hypomania was noted. For the Ham-D17, 67.5% of the LTG group and 55.1% of the Li group met response criteria (50% or greater reduction score), while 65.9% of the LTG group and 55.1% of the Li group met remission criteria (Ham-D17 or MADRS ≤ 12). On the CGI-BP (mania) item, 0% of the LTG and 2% (n=1) of the Li group met criteria for switch. A trend was noted for response without switch into hypomania; 77.5% of the LTG group and 59.2% of the Li group met criteria for response on either the Ham-D17 or the MADRS without meeting switch criteria. There were no significant differences between groups in remission rates without switch; 75.6% of the LTG group and 59.2% of the Li group met criteria for remission on either the Ham-D17 or the MADRS without switch into hypomania.
There were no between group differences in survival probability or in mean duration of treatment. Patients in the LTG group were treated for a mean of 88.8 (35.4) days and in the Li group, for 78.0 (35.2) days.
Item Analyses
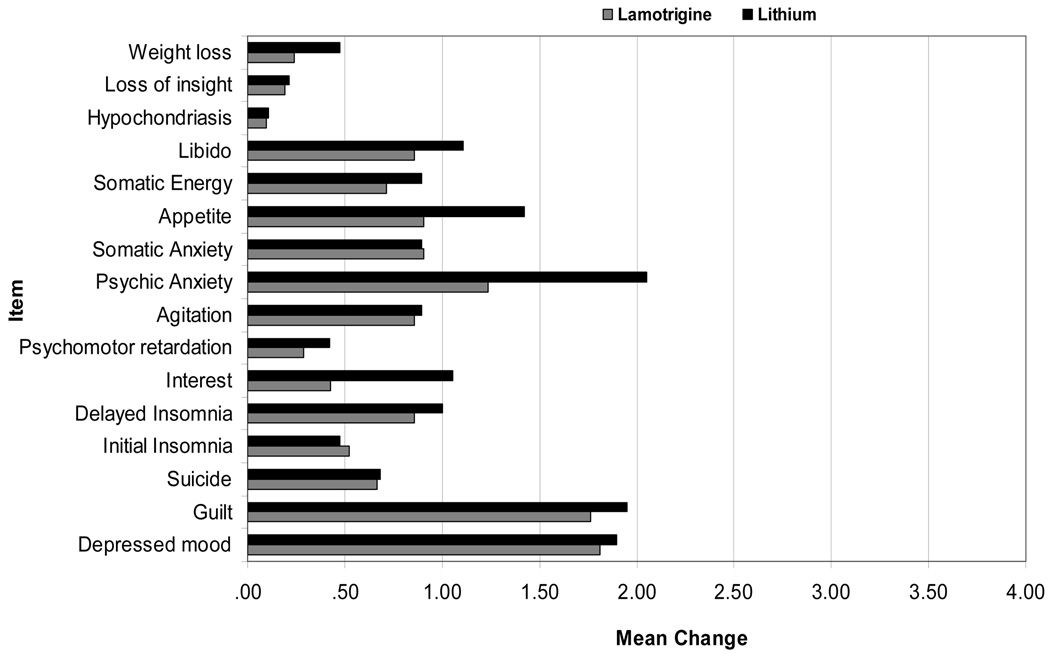
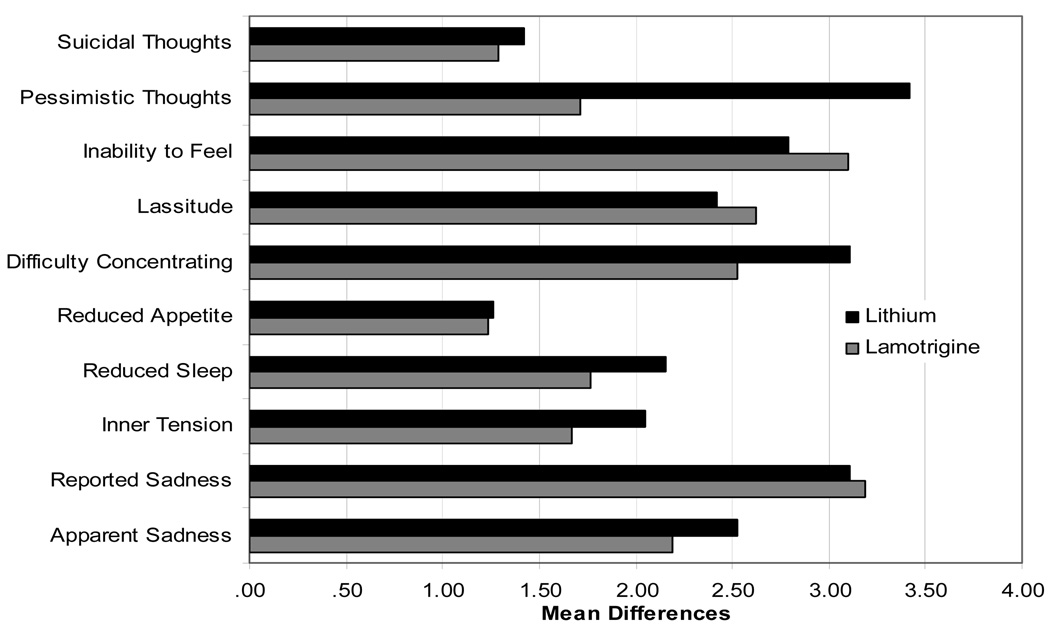
In order to determine if LTG and Li impacted different aspects of depression, item analyses were run on the Ham-D17 and the MADRS at weeks 8 and 16. Results for mean differences at week 8 were significant on the majority of items for both the Ham-D17 and the MADRS, and at week 16, continued improvement was evident as a greater number of item differences became significant from baseline (Figure 3).
Figure 3.
Figure 3a. Ham-D Item Differences by group at week 16
For LTG, all items significant at p<0.05 or better except items psychomotor retardation, hypochondriasis, loss of insight, and weight loss. For Li, all items significant at p<0.05 or better except items hypochondriasis and loss of insight.
Figure 3b. MADRS Item Differences by group at week 16
All items significantly different from baseline for both groups.
There were few between group differences on item improvement for the Ham-D17 or the MADRS. On the Ham-D17, the mean difference on item 10, psychic anxiety, for patients taking Li was significantly greater than that for patients taking LTG. There were no other significant differences, although improvement on interest and appetite trended in favor of Li. On the MADRS, patients receiving Li had a significantly greater mean difference than patients taking LTG on the item “pessimistic thoughts”. No other significant between group differences were seen.
Side Effects
The mean number of side effects (SD) reported by patients receiving LTG was 4.2 (3.2) and the mean number of side effects reported by patients receiving Li was 9.2 (6.4) (p<0.001). Patients receiving Li reported experiencing a number of side effects more frequently than those in the LTG group. The overall withdrawal rate for side effects was 17% (n=15). See Table 2 for a complete breakdown of patient disposition. Withdrawal due to reported side effects was non-significant between groups, although between group differences were reported for individual side effects (Table 3).
Table 2.
Patient Disposition
| Lamotrigine (n=41) |
Lithium (n=49) |
Total (n=90) |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Completed | 21 | 51% | 19 | 39% | 40 | 44% |
| Early Termination | 20 | 49% | 30 | 61% | 50 | 56% |
| Side Effects | 8 | 20% | 10 | 20% | 15 | 17% |
| Lost to follow up | 7 | 17% | 8 | 16% | 15 | 17% |
| Withdrew consent | 2 | 5% | 3 | 6% | 5 | 6% |
| Noncompliance with protocol | 1 | 2% | 4 | 8% | 5 | 6% |
| Lack of improvement | 1 | 2% | 3 | 6% | 4 | 4% |
| Worsening mood (depression) | 0 | 2 | 4% | 2 | 2% | |
| Worsening mood (hypomania) | 1 | 2% | 0 | 1 | 1% | |
Table 3.
Percentage of patients reporting side effects (n=90)
| Name | Lamotrigine | Lithium |
|---|---|---|
| Cognitive Slowing | 7.3% | 26.5% |
| Decreased Sexual Interest | 2.4% | 16.3% |
| Dizziness/Lightheadedness | 7.3% | 30.6% |
| Drowsiness/Panic | 9.8% | 30.6% |
| Dry Mouth | 19.5% | 53.1% |
| Feeling Dull | 2.4% | 18.4% |
| Impaired Memory | .0% | 20.4% |
| Increase Thirst | 7.3% | 49.0% |
| Increased Appetite | 4.9% | 28.6% |
| Increased Urinary | ||
| Frequency | 2.4% | 32.7% |
| Increased Weight | 4.9% | 22.5% |
| Nausea/Vomiting | 24.4% | 46.9% |
| Ringing in Ears | .0% | 12.2% |
| Tremor | 9.8% | 40.8% |
| Upset Stomach | 19.5% | 42.9% |
| Word Finding | 4.9% | 24.5% |
Only side effects with significant between group differences shown.
The side effects experienced most frequently by patients taking LTG were nausea/vomiting, upset stomach, dry mouth, tremors, and drowsiness/panic. The side effects experienced most frequently by patients taking Li were dry mouth, increased thirst, nausea/vomiting, upset stomach, and tremors.
During the course of the study, 16 patients required rescue medications, 8 from each group (20% of the LTG group, 16% of the Li group). There were no between group differences in the number of patients requiring rescue medications. Patients who required rescue medications in the LTG group used them for a mean of 3.75 days, and patients in the Li group used them for a mean of 3.88 days.
Discussion
In this 16-week, single-blind monotherapy study of lamotrigine versus lithium for the acute treatment of bipolar II depression, both groups showed significant improvement on depression and hypomania scores while maintaining a low switch rate. This trial is one of the largest bipolar II disorder, acute phase depression studies ever completed. The baseline to endpoint improvement was substantial across both groups, though between group differences were not noted on the primary outcome or secondary measure symptom assessments. Clinical results did not differ between those patients with or without rapid cycling. A striking number of patients met response and remission criteria, with more than 65% of the LTG group and more than 55% of the Li group meeting criteria on both. A very small number of patients in either group showed evidence of switch in this four month study. As statistical improvement was noted as well on the manic symptom assessment, it is reasonable to consider that patients demonstrated overall improvement in stability and not only a decrease of depressive symptoms.
In order to provide more direct comparison of our findings from those in shorter duration studies, we assessed the changes in symptoms at week 8 of the study. Both item analyses and assessment of clinical symptoms at week 8 showed significant improvement with continued gains over the remaining 8 weeks of the study. This is also pertinent in that it suggests improvements observed were not simply the natural course of illness but reflective of response to medication.
The completion rate for the study was at 44%. Patients showed reasonable tolerability, with stated dropout from study about the same across groups. Patients in the Li group reported a greater number of side effects, and significant differences were noted for the development of side effects with Li relative to LTG. Side effect symptoms were consistent with our understanding of each of these medications and what other studies have reported.
Due to the lack of a placebo arm, definitive statements cannot be made regarding treatment efficacy, though results support the efficacy of both lithium and lamotrigine in the treatment of BDII depression. Patients who received lithium showed significant improvement over the course of the study. Although there are no published randomized acute or maintenance treatment trials of BDII only using Li, these findings support early acute and maintenance findings for a role for Li in the treatment of bipolar depression (Kane et al, 1982; Zornberg and Pope, 1993; Greil and Kleindienst, 1999), suggesting an early acute efficacy maintained into the continuation treatment of bipolar II depression.
These results also support that lamotrigine may be effective in the treatment of BDII acute depression. It is possible that the long titration schedule required for lamotrigine limits the time for response in more usual shorter duration acute depression trials. In this study, the 16-week design provided 8 weeks for the titration of lamotrigine followed by 8 weeks of therapeutic dosing. Patients receiving LTG showed improvement consistent with positive findings in shorter acute trials (Frye et al, 2000). The continuous improvement over the course of the full 16 week trial is also consistent with a longer (26-week) trial (Calabrese et al, 2000).
This study is limited by its single-blind design and lack of a placebo-controlled arm. Of note, the relative degree of improvement on either Li or LTG was consistent with that seen on drug for the recently reported placebo-controlled, acute depression studies of quetiapine in BDI and BDII (Calabrese et al, 2005; Thase et al, 2006). In particular, the degree of improvement is similar to that observed in the pooled BDII data from quetiapine studies (n=351, Suppes et al, 2007). While supportive that overall improvement in mood stability noted was not a placebo response, further studies, including a placebo-controlled arm, are needed to rule out the possibility results reflect course of illness. Without a placebo control results cannot be absolutely ascribed to medication effects. However, as with other BDII populations, the history of depression in this population studied was one of persistent symptoms, regardless of cycling status. Thus while conclusions must be cautionary, the overall course of illness in a well-defined BDII population supports that results reflected a medication effect.
In the analyses of patients with rapid cycling bipolar II disorder, similar outcomes were seen. These results are interesting as previous lithium research has suggested that lithium is less effective in rapid cycling populations. In a recent double-blind study of Li monotherapy and divalproex (DVP) monotherapy in patients with rapid-cycling bipolar disorder, Li performed as well as DVP suggesting that overall, rapid-cycling is difficult to successfully stabilize (Calabrese et al, 2000). The results from this study also support that Li has a place in treatment consideration, as well as other agents, with patients recently depressed or rapid cycling.
The percentage of the population reporting a course of illness consistent with DSM-IV-TR rapid cycling criteria was high. As this was not an exclusion or inclusion criteria we do not know why this percentage was higher than usual BDI trials. Possible reasons include that a higher percentage of BDII are rapid cycling at any given time, patients with BDII may show more monthly variations in mood, patients with BDII may be more susceptible to seasonal variations and changes in photoperiod, and most subjects volunteered in early winter or late summer, seasons associated with the fastest changes in photoperiod (Friedman et al, 2006). Regardless of cycling status, the patients reported long term and persistent depressive symptoms over the years preceding the trial.
The findings from this research support the use of both lithium and lamotrigine in the treatment of BDII depression. Significant improvement was observed from baseline depression and hypomanic symptoms, though limited between group differences were noted. Patients receiving Li reported significantly more side effects, but discontinuation from side effects was not significant between groups. Further studies are needed to confirm results in a placebo controlled trial and elucidate relative differences between treatments including the role of atypical antipsychotics, antidepressants and the relative role of lithium, lamotrigine, and other anticonvulsants in BDII depression.
The number of individuals estimated to have bipolar II disorder is significant. Our knowledge base of appropriate acute and longer term treatments is extremely limited and is a critical area of needed future study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiskal HS. The prevalent clinical spectrum of bipolar disorders: beyond DSM-IV. J. Clin. Psychopharmacol. 1996;16(2 Suppl 1):4–14. doi: 10.1097/00004714-199604001-00002. [DOI] [PubMed] [Google Scholar]
- Angst J. The emerging epidemiology of hypomania and bipolar II disorder. J. Affect. Disord. 1998;50:143–151. doi: 10.1016/s0165-0327(98)00142-6. [DOI] [PubMed] [Google Scholar]
- Barbosa L, Berk M, Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J. Clin. Psychiatry. 2003;64(4):403–407. doi: 10.4088/jcp.v64n0407. [DOI] [PubMed] [Google Scholar]
- Bowden C. Treatment options for bipolar depression. J. Clin. Psychiatry. 2005;66 Suppl 1:3–6. [PubMed] [Google Scholar]
- Calabrese JR, Suppes T, Bowden CL, Sachs GS, Swann AC, McElroy SL, Kusumakar V, Ascher JA, Earl NL, Greene PL, Monaghan ET. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 Study Group. J. Clin. Psychiatry. 2000;61:841–850. doi: 10.4088/jcp.v61n1106. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE, Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, Cutler AJ, McCoy R, Wilson E, Mullen J. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am. J. Psychiatry. 2005;162(7):1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- Data on file, available on GSK Clinical Trial Register. www.GSK.com
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58(3):403–417. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. (SCID-I/P) [Google Scholar]
- Friedman E, Gyulai L, Bhargava M, Landen M, Wisniewski S, Foris J, Ostacher M, Medina R, Thase M. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta Psychiatr. Scand. 2006;113:510–517. doi: 10.1111/j.1600-0447.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, Luckenbaugh DA, Cora-Ocatelli G, Leverich GS, Post RM. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J. Clin. Psychopharmacol. 2000;20:607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- Geddes J, Huffman R, Paska W, Evoniuk G, Thompson T. Lamotrigine for acute treatment of bipolar depression: individual patient data meta-analysis of five randomized, placebo-controlled trials. Bipolar Disord. 2007;9 supple. 1:42–43. [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch. Gen. Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Greil W, Kleindienst N. Lithium versus carbamazepine in the maintenance treatment of bipolar II disorder and bipolar disorder not otherwise specified. Int. Clin. Psychopharmacol. 1999;14:283–285. [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch. Gen. Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- Kane JM, Quitkin FM, Rifkin A, Ramos-Lorenzi JR, Nayak DD, Howard A. Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar II illness: a prospective, placebo-controlled comparison. Arch. Gen. Psychiatry. 1982;39:1065–1069. doi: 10.1001/archpsyc.1982.04290090053011. [DOI] [PubMed] [Google Scholar]
- Kraemer HC. Coping strategies in psychiatric clinical research. J. Consult. Clin. Psychol. 1981;49(3):309–319. doi: 10.1037//0022-006x.49.3.309. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382-38. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Schaffer A, Zuker P, Levitt A. Randomized, double-blind pilot trial comparing lamotrigine versus citalopram for the treatment of bipolar depression. J. Affect. Disorders. 2006;96:95–99. doi: 10.1016/j.jad.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Smith LA, Cornelius V, Warnock A, Bell A, Young AH. Effectiveness of mood stabilizers and antipsychotics in the maintenance phase of bipolar disorder: a systematic review of randomized controlled trials. Bipolar Disord. 2007;9:394–412. doi: 10.1111/j.1399-5618.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, Geddes J, Gilbody S, Palmer S, Woolacott N. A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder. Health Technol. Assess. 2007;11(39):1–226. doi: 10.3310/hta11390. [DOI] [PubMed] [Google Scholar]
- Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the clinical global Impression scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Suppes T, Hirschfeld RM, Vieta E, Raines S, Paulsson B. Quetiapine for the treatment of bipolar II depression: Analysis of data from two randomized, double-blind, placebo-controlled studies. World J. Biol. Psychiatry. 2007 May 11;:1–14. doi: 10.1080/15622970701317265. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR BOLDER II Study Group. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study) J. Clin. Psychopharmacol. 2006;26(6):600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ, Hennen J, Floris G. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am. J. Psychiatry. 1998;155:638–645. doi: 10.1176/ajp.155.5.638. [DOI] [PubMed] [Google Scholar]
- Weisler RH, Calabrese JR, Bowden CL, Ascher JA, DeVeaugh-Geiss J, Evoniuk G. Discovery and development of lamotrigine for bipolar disorder: a story of serendipity, clinical observations, risk taking, and persistence. J. Affect. Disord. 2007 doi: 10.1016/j.jad.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VT, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zornberg GL, Pope HG., Jr Treatment of depression in bipolar disorder: new directions for research. J. Clin. Psychopharmacol. 1993;13:397–408. [PubMed] [Google Scholar]