Abstract
Background
In older adults, changes in speed and stability during walking are associated with impaired balance and increased fall risk. Narrow-base walking requires increased frontal plane stability and can be used to assess postural control while walking. Performance of a concurrent cognitive task (dual task) may further increase the complexity of walking, potentially allowing identification of individuals with instability that is not detected under single-task conditions. The purpose of this study was to examine age-associated effects of a cognitive task on speed and frontal plane stability during narrow-base walking.
Methods
Thirty-four healthy adults participated, categorized by age: <65, 65–74, and ≥75 years. Participants walked at a comfortable pace within a narrow path under both single- and dual-task conditions. We examined spatiotemporal variables and frontal plane center of mass (CoM) parameters using a 13-segment biomechanical model.
Results
Increasing age (p < .001) and the performance of a concurrent cognitive task (p < .001) were both associated with decreased speed, with no interaction between these factors. Frontal plane CoM displacement and velocity increased with increasing age (both p < .001), but dual-task performance had no effect on these variables (both p > .450).
Conclusions
Age-associated changes in both speed and stability are observed during narrow-base walking. Among this sample of healthy older adults, the addition of a concurrent cognitive task resulted in reduced speed, with no effect on frontal plane stability. Further research is needed to determine if dual-task, narrow-base walking is a sensitive and specific approach to identifying older adults at risk for falls.
Keywords: Dual task, Gait, Frontal plane stability, Falls, Aging
Falls are common among community-dwelling older adults (1), and in this population, the incidence of falls increases with age (2). The majority of falls occur during walking (3), and unsteady gait is a leading cause of falls among elderly persons (4). Age-related declines in gait speed and stability have been associated with increased fall risk in older adults. Gait speed during functional walking tasks is slower in older adults with a history of falls compared to those without a history of falls (5,6). Similarly, decreased frontal plane stability when walking over obstacles and increased stride-to-stride variability during level walking have been associated with increased fall risk in older adults (7,8). Thus, the assessment of gait speed and stability among older adults may be useful in understanding the cause of falls when walking and in identifying individuals with an increased risk for falls.
For clinicians, the ability to identify gait instability and associated fall risk is critical. Although the cause of falls is typically multifactorial, performance-based measures are often used to assess the contribution of balance and gait impairments to fall risk. However, among community-dwelling older adults, performance-based measures are not always accurate in identifying individuals at risk for falls, even when used in combination (9). Mobility during daily life often requires the performance of concurrent cognitive or motor tasks, such as talking or carrying objects. Because of the increasingly recognized role of cognition in postural control and gait, many researchers have used dual-task paradigms incorporating a concurrent cognitive task to improve fall risk assessment (10). For example, the use of a concurrent cognitive task has been shown to distinguish between older adults with and without a history of falls during standing postural control tasks (11). In addition, complex walking tasks such as dual-task walking may be more sensitive than simple walking tasks for identifying early declines in postural control among nondisabled older adults without frank mobility limitations (12).
During walking, the primary direction of instability is in the frontal plane (13,14), and impairments in frontal plane stability during stance and gait have been identified as a major risk factor for falls in older adults (14). Tandem and narrow-base walking are commonly used to assess postural control during walking (15), because the center of mass (CoM) must be tightly controlled within a narrowed base of support. Adding a concurrent cognitive task (i.e., dual task) to narrow-base walking may further challenge stability, allowing identification of early balance deficits not detected under single task conditions. The purpose of this study was to examine age-associated effects of a concurrent cognitive task on gait speed and frontal plane stability during narrow-base walking in a population of healthy older adults. We hypothesized that both speed and frontal plane stability during narrow-base walking would decrease with increasing age. We also hypothesized that the performance of a concurrent cognitive task would result in further decreases in gait speed and stability, and that this effect would be greatest among the oldest participants.
Methods
Participants
Thirty-four healthy, community-dwelling adults between 54 and 95 years of age were enrolled in this study based on the following age categories: <65 years (n = 9), 65–74 years (n = 13), and ≥75 years (n = 12). All participants were enrolled in the Baltimore Longitudinal Study of Aging (BLSA), an ongoing, multidisciplinary, observational study initiated in 1958. Details of the BLSA recruitment methodology have been outlined previously (16). As part of the BLSA visit, all participants received a complete medical history and physical examination, including history of falls over the previous 12 months. A simple count of comorbid medical diagnoses was used to examine disease burden for each participant. The Blessed Dementia Scale (BDS) was used to assess the impact of any cognitive impairment on activities of daily living (17). Exclusion criteria for the gait evaluation included the need for assistance (from an assistive device or another person) when walking, legal blindness, an inability to follow instructions because of cognitive impairment, and the report of one or more falls in the previous 12 months. An independent institutional review board approved the BLSA study protocol, and participants provided written informed consent for all procedures.
Experimental Protocol
Participants were asked to walk at a comfortable pace within a narrow path (6.7 m long) both without (single task) and with (dual task) a concurrent cognitive task. Participants performed 3–4 trials in each condition. The width of the path was normalized to 50% of the distance between the participant’s anterior superior iliac spines, to produce a similar challenge for individuals with different body morphologies. The narrow path was outlined by tape on the walking surface, and participants were instructed to walk within the taped path. Retroreflective markers were used to define the narrow-base path in the motion capture and analysis software and to detect step errors. For the cognitive task, participants were asked to say the days of the week backwards. This task was selected to provide a moderate cognitive load, similar to that required in functional situations (e.g., walking while conversing). Under the dual-task condition, no specific attentional focus was instructed, and participants were asked to perform both tasks as best they could.
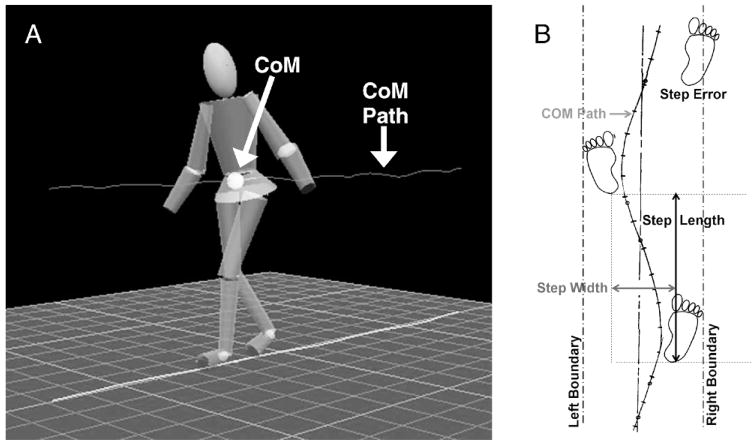
All participants were tested while walking barefoot. A set of 37 retroreflective markers was placed on bony landmarks of the feet, legs, pelvis, trunk, arms, and head. A six-camera VICON motion analysis system (VICON Motion Systems, Inc., Lake Forest, CA) was used to collect three-dimensional (3-D) marker position data at 60 Hz. Visual 3-D software (C-Motion, Rockville, MD) was then used to create a 13-segment biomechanical model, and the location of the whole-body CoM was calculated as the weighted sum of each body segment’s CoM. This model included six segments for the lower extremities (bilateral feet, shanks, and thighs), four segments for the upper extremities (bilateral upper and lower arms), one for the pelvis, one for the trunk, and one for the head (Figure 1A). The trajectory of the CoM during each trial was then computed using Visual 3-D software.
Figure 1.
Schematic of (A) 13-segment model used for center of mass (CoM) calculations and (B) narrow-base walking task, showing the method for calculating step width, step length, and step errors.
Custom software was used to calculate step and stride characteristics for spatiotemporal and CoM variables. Only steps taken independently were analyzed; one step and the associated stride were excluded from the analysis because the participant touched the laboratory wall for support. Stride velocity was the primary measure of gait speed and was calculated as the distance between one heel strike and the next heel strike on the same foot divided by the time between these heel strike events. Stride time was defined as the time between one heel strike and the next heel strike on the same foot. Step width and step length were calculated as the distance (width and length) between the heel marker of one foot and the heel marker of the opposite foot. A step error was defined as any step in which the ankle marker was detected outside of the corridor at heel strike. Step error rates were calculated for both single- and dual-task conditions as the number of step errors divided by the total number of steps in that condition. Inaccurate steps were those in which a step error occurred, and accurate steps were within the narrow base path. Frontal plane stability was assessed using mediolateral (M-L) CoM displacement and peak velocity. The M-L CoM displacement was defined as the maximum minus the minimum value of the CoM in the frontal plane during a single stride, and the M-L CoM peak velocity was defined as the maximum M-L velocity of the CoM during a single stride (Figure 1B).
Statistical Analysis
For each participant, the mean for each parameter was calculated using all valid steps or strides within a given walking condition. For each age group, means, standard errors (SE), and 95% confidence intervals (CI) were then calculated. Data were analyzed in SPSS (version 11.5; SPSS Inc., Chicago, IL) using a general linear model with 1 within-subjects factor (task: single or dual) and 1 between-subjects factor (age group: <65, 65–74, and ≥75 years). Post hoc analyses of significant age effects (α level set at p = .05) were performed using the least significant difference (LSD) test.
Results
Participant Demographics
There were no significant differences in height (p = .267), weight (p = .098), or body mass index (p = .221) between age groups (Table 1). Participants in the <65 group had fewer comorbidities (0.7 comorbidities [SE: 0.2; 95% CI: 0.1–1.2]) than did participants in the two older groups (65–74 group: 1.9 comorbidities [SE: 0.3; 95% CI: 1.3–2.6]; p = .008; ≥75 group: 2.0 comorbidities [SE: 0.3; 95% CI: 1.3–2.7]; p = .006). Scores on the BDS did not differ across groups (p = .424), and the mean BDS score was 1.7 (SE: 0.3; 95% CI: 1.2–2.2).
Table 1.
Participant Demographics for Each Group
| Group | Age, y | Gender | Height, m | Weight, kg | BMI, kg/m2 |
|---|---|---|---|---|---|
| <65 | 60.1 (1.1) | 4 M/5 F | 1.68 (0.08) | 78.4 (19.0) | 27.5 (4.6) |
| 54–64 | 1.56–1.83 | 51.1–109.4 | 21.0–34.0 | ||
| 65–74 | 69.0 (0.9) | 6 M/7 F | 1.70 (0.07) | 78.8 (10.7) | 27.3 (3.3) |
| 65–74 | 1.59–1.82 | 58.5–95.7 | 22.5–34.5 | ||
| ≥75 | 85.2 (6.0) | 8 M/4 F | 1.64 (0.10) | 67.5 (12.4) | 24.9 (3.7) |
| 75–95 | 1.49–1.81 | 48.3–93.4 | 19.4–33.9 |
Notes: Values shown are mean (standard error) and range.
BMI = body mass index; M = male; F = female.
Step Error Rates
Across all participants, the mean width of the narrow base path was 16.7 cm (SE: 0.6; 95% CI: 15.5–17.9), and the width of the path did not differ between age groups (p = .963). Collapsed across groups, the mean number of steps analyzed was 10.6 steps (SE: 1.3; 95% CI: 7.9–13.2) in the single task condition and 11.9 steps (SE: 1.1; 95% CI: 9.6–14.3) in the dual-task condition. Overall, more steps were available for the ≥75 group (16.0 steps [SE: 1.8; 95% CI: 12.2–19.8]) compared to the two younger groups (<65 group: 9.3 steps [SE: 0.9; 95% CI: 7.4–11.1], p = .013; 65–74 group: 8.2 steps [SE: 0.9; 95% CI: 6.4–10.0]; p = .002). The mean step error rate for all participants was 16.6% (SE: 2.4; 95% CI: 11.9–21.2). There was a trend toward increased step error rates with increasing age, although this difference was not significant (p = .082). Mean (SE) step error rates were 6.6% (3.1) for the <65 group, 17.1% (3.7) for the 65–74 group, and 23.4% (4.4) for the ≥75 group. Collapsed across age groups, step error rates were not affected by performance of a cognitive task (p = .783). The mean step error rate was 17.2% (SE: 3.3; 95% CI: 10.5–24.0) for the single-task condition and 15.9% (SE: 3.4; 95% CI: 9.0–22.8) for the dual-task condition. In comparing accurate and inaccurate steps, only step width differed (accurate: 5.1 cm [SE: 0.5; 95% CI: 4.1–6.1]; inaccurate: 6.1 cm [SE: 0.6; 95% CI: 4.9–7.2]; p = .008). There were no differences between accurate and inaccurate steps in terms of the remaining spatiotemporal and CoM parameters (all p > .160). For all results presented below, similar results were found when analyzing accurate steps alone or accurate and inaccurate steps together. Therefore, both accurate and inaccurate steps were included in the remaining analyses.
Spatiotemporal Variables
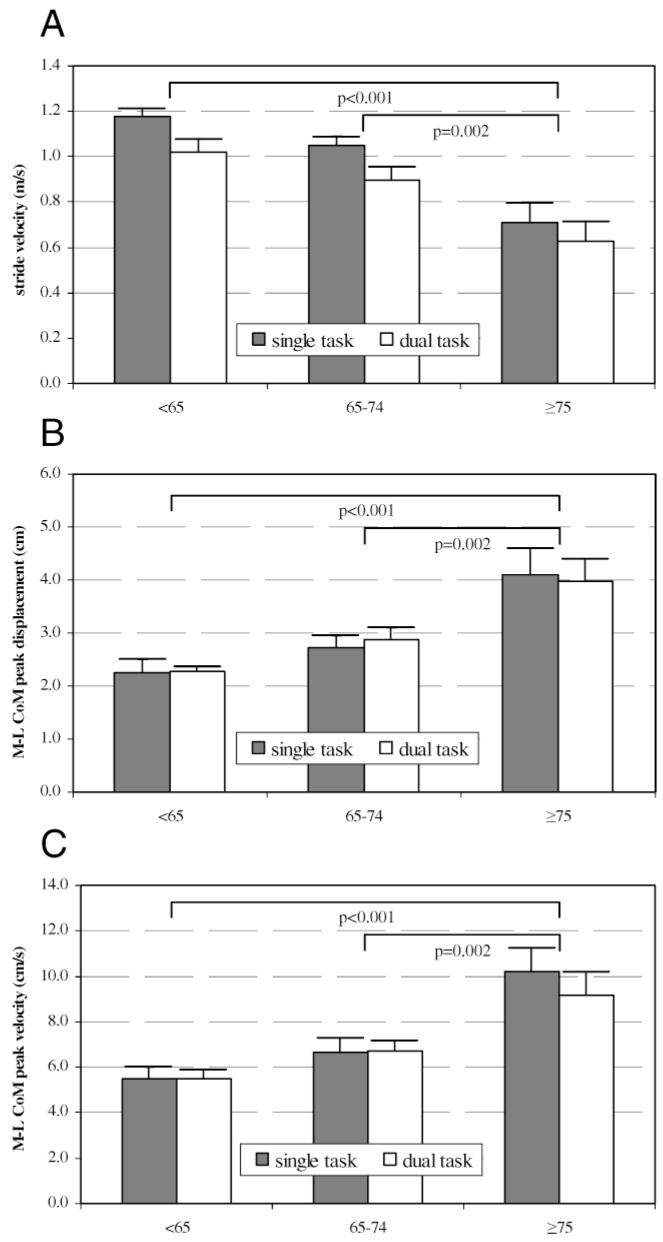
Stride velocity decreased with increasing age (p < .001) and with the performance of a cognitive task (p < .001), with no interaction effect (p = .316; Figure 2A). Mean stride velocity, collapsed across task, was 1.10 m/s for participants <65 years (SE: 0.04; 95% CI: 1.01–1.18), 0.97 m/s for participants 65–74 years (SE: 0.04; 95% CI: 0.89–1.05), and 0.67 m/s for participants ≥75 years (SE: 0.06; 95% CI: 0.54–0.80). Participants in the ≥75 group walked significantly more slowly than did those in the <65 group (p < .001) and those in the 65–74 group (p = .002). Collapsed across groups, mean stride velocity was 0.96 m/s (SE: 0.05; 95% CI 0.86–1.06) under single-task conditions and 0.83 m/s (SE: 0.05; 95% CI: 0.73–0.93) under dual-task conditions. The effect of a cognitive task on stride velocity was similar across all age groups (single-task – dual-task difference: <65 group = 0.16 m/s; 65–74 group = 0.15 m/s; ≥75 group = 0.08 m/s).
Figure 2.
Age-associated effects of a concurrent cognitive task on (A) stride velocity (m/s), (B) mediolateral center of mass (M-L CoM) displacement (cm), and (C) M-L CoM peak velocity (cm/s). For all graphs, bars represent means and whiskers represent standard errors. Values for the single task condition are represented by gray bars and values for the dual-task condition are represented by white bars. Age groups are shown on the x-axis of all graphs: <65 years, 65–74 years, and ≥75 years. Values of p are shown for significant between-group differences.
There was a trend toward increased stride time with increasing age (mean [SE]: <65 group = 1.12 seconds [0.04]; 65–74 group = 1.20 seconds [0.04]; ≥75 group = 1.31 seconds [0.05]), but this change did not reach significance (p = .068). Collapsing across groups, stride time increased when walking with a concurrent cognitive task, from 1.14 seconds (SE: 0.03; 95% CI: 1.07–1.21) under single-task conditions to 1.29 seconds (SE: 0.04; 95% CI: 1.21–1.37) under dual-task conditions (p < .001). Stride time increases in response to a concurrent cognitive task were similar between groups (single-task – dual-task difference: <65 group = −0.17 seconds; 65–74 group = −0.15 seconds; ≥75 group =−0.15 seconds), and there was no interaction between age and the performance of a cognitive task on stride time (p = .913).
Step length decreased with increasing age (p < .001) and with performance of a cognitive task (p = .013), with no interaction effect (p = .251). Collapsed across task condition, mean step length was longer in the <65 group (0.59 m [SE: 0.01; 95% CI: 0.58–0.60]; p < .001) and the 65–74 group (0.56 m [SE: 0.01; 95% CI: 0.54–0.59]; p < .001) compared to the ≥75 group (0.41 m [SE: 0.02; 95% CI: 0.36–0.54]). Mean step length decreased by 0.02 m in the dual-task compared to the single-task condition (single-task – dual-task difference: <65 group = 0.00 m; 65–74 group = 0.03 m; ≥75 group = 0.02 m). In contrast, step width increased with increasing age (p = .013), but there was no effect of a cognitive task on step width (p = .273) and no interaction between age and task conditions (p = .759). Collapsing across single- and dual-task conditions, mean step width was narrower in the <65 group (3.1 cm [SE: 0.5; 95% CI: 2.1–4.0]; p = .005) and the 65–74 group (4.2 cm [SE: 0.4; 95% CI: 3.3–5.0]; p = .030) compared to the ≥75 group (6.5 cm [SE: 0.7; 95% CI: 5.0–7.9]). Collapsing across age groups, mean step width was 4.9 cm (SE: 0.5; 95% CI: 3.9–5.9) in the single-task condition and 4.5 cm (SE: 0.5; 95% CI: 3.4–5.6) in the dual-task condition (single-task – dual-task difference: <65 group = 0.0 cm; 65–74 group = 0.5 cm; ≥75 group = 0.5 cm).
Frontal Plane Stability
Frontal plane stability was assessed using M-L CoM displacement and peak velocity (Figure 2B and C). Mediolateral CoM displacement increased with increasing age (p < .001), but the performance of a concurrent cognitive task did not affect this variable (p = .946). There was no interaction of task condition and age (p = .870). Collapsing across tasks, mean M-L CoM displacement was smaller in the <65 group (2.3 cm [SE: 0.1; 95% CI: 2.0–2.5]; p < .001) and the 65–74 group (2.8 cm [SE: 0.2; 95% CI: 2.5–3.1]; p =.002) compared to the ≥75 group (4.0 cm [SE: 0.3; 95% CI: 3.4–4.7]). Mean M-L CoM displacement was 3.1 cm in both the single-task and dual-task conditions (SE: 0.2; 95% CI: single, 2.6–3.6; dual, 2.7–3.5), with no significant group effect (single-task – dual-task difference: <65 group = −0.1 cm; 65–74 group = −0.2 cm; ≥75 group = 0.1 cm).
Mediolateral CoM peak velocity also increased with increasing age (p < .001), with no effect of a concurrent task (p = .458). Across task conditions, mean M-L CoM peak velocity was smaller in the <65 group (5.5 cm/s [SE: 0.3; 95% CI: 4.8–6.1]; p < .001) and the 65–74 group (6.7 cm/s [SE: 0.4; 95% CI: 5.9–7.5]; p = .002) compared to the ≥75 group (9.7 cm/s [SE: 0.7; 95% CI: 8.1–11.2]). Across age groups, mean M-L CoM peak velocity was 7.6 cm/s (SE: 0.6; 95% CI: 6.5–8.7) in the single-task condition and 7.2 cm/s (SE: 0.5; 95% CI: 6.2–8.2) in the dual-task condition. The difference between single-task and dual-task M-L CoM peak velocity was 1.1 cm/s in the ≥75 group, whereas the two younger groups did not differ (single-task – dual-task difference for both groups = 0.0 cm/s).
Discussion
The purpose of this study was to examine age-associated effects of a concurrent cognitive task on gait speed and frontal plane stability during narrow-base walking in a population of healthy older adults. We anticipated a decline in the performance of narrow-base walking, as indicated by decreased gait speed and frontal plane stability, with increasing age. We also hypothesized that the performance of a concurrent cognitive task during narrow-base walking would result in further decreases in gait speed and stability, with the greatest performance decrements found in the oldest participants. The primary findings of this research are: i) age-associated changes in both gait speed and frontal plane stability are evident during narrow-base walking; and ii) performance of a concurrent cognitive task resulted in decreased gait speed for all age groups but did not affect frontal plane stability in this sample of healthy older adults.
Performance during a tandem or narrow-base walk has previously been shown to identify recurrent fallers among a population of older adults with balance impairments (18). In addition, prior research has identified age-associated declines in the ability to walk with a narrow base of support (12,19), similar to the age-associated decrease in speed and stability reported in this study. For example, Shumway-Cook and colleagues (12) reported an age-associated decline in gait speed during narrow-base walking tasks among a population of community-dwelling adults. These results also confirmed reports of decreasing frontal plane stability with increasing age (13,14,20,21). Reduced frontal plane stability may largely explain impaired performance on narrow-base walking tasks, which require precise control of the M-L CoM in response to a restricted base of support.
In this study, the addition of a cognitive task to narrow-base walking resulted in decreased stride velocity for all age groups. Contrary to our hypothesis, the addition of a concurrent cognitive task did not affect M-L CoM displacement or velocity during narrow-base walking. Thus, the performance of a cognitive task, although reducing gait speed, had no effect on frontal plane stability in healthy older adults without a history of falls. Reduced gait speed is often interpreted as a sign of impaired postural control during walking. However, based on the results of our study, an alternative explanation is that reduced gait speed is a compensatory mechanism used to maintain frontal plane stability in healthy older adults.
Previous research has examined whether the addition of a concurrent cognitive task to balance or gait measures improves the ability to predict falls, and the results of these studies are mixed. Shumway-Cook and colleagues (6) found that the addition of a concurrent task to the Timed Up & Go test did not improve the sensitivity and specificity of this test for identifying fall-prone community-dwelling elders. In contrast, other research has found that the addition of a concurrent task (talking) to walking does predict future falls in a population that included institutionalized older adults (22). Inconsistencies in the reported predictive ability of dual-task walking tests may be related in part to differences in the difficulty of the mobility task performed, differences in the challenge presented by the concurrent cognitive task, and differences in the age and postural control capabilities of the populations being examined (10).
In the current study, healthy older adults without a history of falls demonstrated reduced gait speed but maintained frontal plane stability when walking and performing a concurrent cognitive task. It is not clear whether performance on this combination of tasks can be used to discriminate among older adults with versus without a history of falls or to predict individuals with early postural control impairments (not apparent on simpler walking tasks) that could increase the risk for falls. Based on previous research (7,18), we expect that this combination of tasks could discriminate between older adults with versus without a history of falls caused by impaired postural control. Furthermore, we anticipate that this combination of tasks could be predictive of early declines in postural control, thus identifying older adults at risk for future falls. Among older individuals who perform within normal limits on simple walking tasks, complex walking tasks such as this may be useful in identifying early stages of mobility limitations. Further research is needed to test these hypotheses.
One of the limitations of the current study is the relatively small sample size. The assessment of age-associated effects of a concurrent cognitive task on gait speed and stability would be improved both by increasing the number of participants and by increasing the age range of the population examined. Similarly, equal gender distributions in each age group would eliminate the possibility that gender-based differences in body morphology or postural control influenced the outcomes observed. In addition, performance of the cognitive task was not characterized in this case. It is not clear how differences in task prioritization and performance under dual-task conditions might impact the observed effects of a cognitive task on narrow-base walking. Finally, this study would be strengthened by the addition of neuropsychological data characterizing executive function and attentional capabilities in these individuals. These data could provide a better understanding of the potential contributions of cognitive deficits to reduced speed and stability during walking.
Summary
These data demonstrate age-associated decreases in gait speed and stability during narrow-base walking. Among healthy older adults, performance of a concurrent cognitive task resulted in decreased gait speed, but frontal plane stability was not affected by a concurrent task. Thus, healthy older adults without a history of falls reduced gait speed but maintained frontal plane stability during dual-task narrow-base walking. Further research is needed to determine if narrow-base walking with a concurrent cognitive task can discriminate between older adults with versus without a history of falls or predict older adults at risk for falls.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging contract number Z01AG000015.
References
- 1.AGS Panel on Falls Prevention. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 2.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141–158. doi: 10.1016/s0749-0690(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 5.Gunter KB, White KN, Hayes WC, Snow CM. Functional mobility discriminates nonfallers from one-time and frequent fallers. J Gerontol A Biol Sci Med Sci. 2000;55A:M672–M676. doi: 10.1093/gerona/55.11.m672. [DOI] [PubMed] [Google Scholar]
- 6.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 7.Chou LS, Kaufman KR, Brey RH, Draganich LF. Motion of the whole body’s center of mass when stepping over obstacles of different heights. Gait Posture. 2001;13:17–26. doi: 10.1016/s0966-6362(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 9.Boulgarides LK, McGinty SM, Willett JA, Barnes CW. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys Ther. 2003;83:328–339. [PubMed] [Google Scholar]
- 10.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci. 1997;52A:M232–M240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- 12.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judge JO, Ounpuu S, Davis RB., 3rd Effects of age on the biomechanics and physiology of gait. Clin Geriatr Med. 1996;12:659–678. [PubMed] [Google Scholar]
- 14.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 15.Gill J, Allum JH, Carpenter MG, et al. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol Med Sci. 2001;56A:M438–M447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 16.Shock NW, Greulich RC, Andres R. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Government Printing Office; 1984. [Google Scholar]
- 17.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral gray matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 18.Cho BL, Scarpace D, Alexander NB. Tests of stepping as indicators of mobility, balance, and fall risk in balance-impaired older adults. J Am Geriatr Soc. 2004;52:1168–1173. doi: 10.1111/j.1532-5415.2004.52317.x. [DOI] [PubMed] [Google Scholar]
- 19.Speers RA, Ashton-Miller JA, Schultz AB, Alexander NB. Age differences in abilities to perform tandem stand and walk tasks of graded difficulty. Gait Posture. 1998;7:207–213. doi: 10.1016/s0966-6362(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 20.Rogers MW, Hedman LD, Johnson ME, Cain TD, Hanke TA. Lateral stability during forward-induced stepping for dynamic balance recovery in young and older adults. J Gerontol Med Sci. 2001;56A:M589–M594. doi: 10.1093/gerona/56.9.m589. [DOI] [PubMed] [Google Scholar]
- 21.Chou LS, Kaufman KR, Hahn ME, Brey RH. Medio-lateral motion of the center of mass during obstacle crossing distinguishes elderly individuals with imbalance. Gait Posture. 2003;18:125–133. doi: 10.1016/s0966-6362(02)00067-x. [DOI] [PubMed] [Google Scholar]
- 22.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]