Abstract
Since the establishment of the 1998 folate recommended dietary allowance (RDA), the methylenetetrahydrofolate reductase (MTHFR) 677C→T variant has emerged as a strong modifier of folate status. This controlled feeding study investigated the adequacy of the RDA, 400 μg/d as dietary folate equivalents (DFE), for Mexican American men with the MTHFR 677CC or TT genotype. Because of the interdependency between folate and choline, the influence of choline intake on folate status was also assessed. Mexican American men (n = 60; 18–55 y) with the MTHFR 677CC (n = 31) or TT (n = 29) genotype consumed 438 μg DFE/d and total choline intakes of 300, 550 (choline adequate intake), 1100, or 2200 mg/d for 12 wk. Folate status response was assessed via serum folate (SF), RBC folate, plasma total homocysteine (tHcy), and urinary folate. SF decreased (P < 0.001) 66% to 7.9 ± 0.7 nmol/L (means ± SEM) in men with the 677TT genotype and 62% to 11.3 ± 0.9 nmol/L in the 677CC genotype. Plasma tHcy increased (P < 0.0001) 170% to 31 ± 3 μmol/L in men with the 677TT genotype and 18% to 11.6 ± 0.3 μmol/L in the 677CC genotype. At the end of the study, 34% (677TT) and 16% (677CC) had SF concentrations <6.8 nmol/L and 79% (677TT) and 7% (677CC) had tHcy concentrations >14 μmol/L. Choline intake did not influence the response of the measured variables. These data showed that the folate RDA is not adequate for men of Mexican descent, particularly for those with the MTHFR 677TT genotype, and demonstrated a lack of influence of choline intake on the folate status variables measured in this study.
Introduction
Folate is a water-soluble B vitamin that acquires and provides 1-carbon derivatives that may be used for nucleotide synthesis or for methylation reactions. Methylenetetrahydrofolate reductase (MTHFR),9 an important regulated enzyme, directs 1-carbon units toward methylneogenesis (at the expense of nucleotide formation) by converting 5,10-methylenetetrahydrofolate (THF) to 5-methyl-THF. The folate-derived methyl group is used for homocysteine remethylation to methionine and for numerous transmethylation reactions, following the activation of methionine to S-adenosylmethionine. A common genetic variant involving a cytosine (C) to thymine (T) transition at nucleotide 677 (677C→T) exists in the MTHFR gene (1) and is associated with reduced enzyme activity, lower serum 5-methyl-THF concentrations, higher plasma total homocysteine (tHcy) concentrations, and increased requirements for folate (1,2).
Betaine, the oxidized form of choline, is an alternative to 5-methyl-THF as a source of methyl groups for homocysteine remethylation and transmethylation reactions. Support for an interdependency between folate and choline comes from data showing an influence of folate intake on choline status (i.e., plasma phosphatidylcholine and/or betaine) in humans (3,4) and from an influence of betaine and/or phosphatidylcholine supplementation on homocysteine concentrations (5,6). In addition, results from a large-scale epidemiological study showed a small decrease in betaine according to the number of T alleles of the MTHFR C677T genotype (7). Whether choline intake modulates folate status is unclear, although data from animal studies support a reciprocal relationship (8).
Folate inadequacy is associated with several chronic diseases, including certain cancers, cardiovascular disease, and cognitive impairments (reviewed in 9,10), as well as gestational complications (reviewed in 11). Not surprisingly, the MTHFR C677T genotype modulates the risk of several of these conditions (reviewed in 12). Although fewer studies have been done that examined the association between choline intake/status and disease risk, existing data suggest that suboptimal choline intake/status increases the risk of organ dysfunction (13,14), hyper-homocysteinemia (15), DNA methylation abnormalities (16,17), and neural tube defects (18).
The 1998 folate recommended dietary allowance (RDA) is 400 μg/d as dietary folate equivalents (DFE) for adult women and men (19). Several controlled feeding studies support the adequacy of the RDA for premenopausal women differing in ethnicity and MTHFR C677T genotype (2,20–22). However, the adequacy of the 1998 folate RDA for men is unknown, particularly with regards to those with the MTHFR 677TT genotype. Thus, the main objective of this study was to investigate the adequacy of the folate RDA in men differing in the MTHFR C677T genotype. A secondary objective was to examine the influence of differing choline intakes (300, 550 [choline adequate intake (AI) level], 1100, or 2200 mg/d) on homocysteine and folate metabolism. Men of Mexican descent were chosen as the study population due to the high prevalence (~20%) of the MTHFR 677TT genotype in this ethnic group (23).
Subjects and Methods
Subjects
Men of Mexican descent, defined as having 2 Mexican parents, were recruited from the Pomona, California, community between June 2005 and September 2006. Inclusion was contingent on good health status, which was ascertained by a medical history questionnaire and standard clinical laboratory tests, such as complete blood count and fasting blood chemistries. Nineteen of the subjects admitted to the study had minor elevations in blood lipids that the study physician deemed not to have clinical importance. Specific inclusion criteria were 1) age 18–55 y, 2) MTHFR 677CC or TT genotype; 3) no use of tobacco products, 4) no use of medications known to interfere with folate metabolism/measurements, 5) no history of chronic disease, 6) no anemia (hemoglobin >120 g/L), 7) normal functioning liver and kidney, and 8) BMI <38 kg/m2. The screening and experimental procedures were reviewed and approved by the Cal Poly Pomona Institutional Review Board for Human Subjects, and informed consent was obtained from all participants. Of the ~1200 men recruited for the study, 249 participated in the initial screening process, which included 1 fasting blood draw and completion of the medical history questionnaire, 118 qualified for participation, and 73 started the study. Of the 73 men who started the study, 10 withdrew from the study, 2 were dropped from the study for not adhering to the dietary protocol, and 61 finished the study (32 with the MTHFR 677CC genotype and 29 with the MTHFR 677TT genotype). Of the 61 men finishing the study, 1 subject was later deemed to be noncompliant (based on his serum folate [SF] response) and was excluded from the statistical analysis.
Study design
This was a 12-wk controlled feeding study that provided folate intakes (i.e., 438 μg DFE/d) approximating the 1998 folate RDA and total choline intakes of 300, 550 (the choline AI), 1100, or 2200 mg/d throughout the study duration. The 438 μg DFE/d were composed of 319 μg food folate and 70 μg supplemental folic acid. The choline intakes of 300, 550, 1100, or 2200 mg/d consisted of 300 mg/d food choline and 0, 250, 800, or 1900 mg/d supplemental choline, respectively. The feeding phase was conducted continuously over a course of 18 mo until target numbers were reached.
All meals and snacks were prepared in the Human Nutrition’s metabolic kitchen at Cal Poly Pomona University. Subjects consumed at least 1 meal/d in the metabolic kitchen (e.g., breakfast or dinner) and were provided the other meals/snacks as takeout. Subjects were instructed to consume all of the foods/beverages provided and not to consume any foods or beverages (except water) outside of those provided. Weight was monitored weekly and any deviation of ±5% from baseline was addressed by modifying energy intake with folate- and choline-free items, such as diet or regular sodas, lemonade, Gatorade, select juices (i.e., apple, cranberry, grape, cranapple, crangrape), corn chips, popcorn, potato chips, apple sauce, and gelatin. The principal investigator and/or trained graduate students had daily contact with the subjects to help ensure compliance to the study protocol.
Diet and supplements
A relatively low choline, betaine, and folate 5-d menu (Supplemental Table 1) was designed. The mean folate content of the diet, as determined by a trienzyme methodology on 3 separate occasions, was 319 μg/d and varied ±26 μg among days. Folic acid–free flour (Kansas State University, Manhattan, KS) was used to prepare menu items that would normally contain folic acid if purchased commercially (i.e., biscuits, waffles, and pizza dough). The mean total choline and betaine contents of the diet, as determined by liquid chromatography-MS/MS after methanol chloroform extraction on 3 separate occasions, were 300 and 173 mg/d, respectively, and varied ±53 and 49 mg among d, respectively. To ensure that all subjects received the same amount of food, menu items were weighed to the nearest 1.0 g.
The nutrient content of the diet for all other nutrients was estimated using the ESHA Food Processor Nutrient Data Base (version 7.81). The diet provided a mean energy intake of 2570 kcal/d (10,760 kJ/d) and varied ±58 kcal (±243 kJ) among days. Totals of 57, 13, and 30% of the energy was obtained from carbohydrate, protein, and fat, respectively. Supplements were given to subjects to provide at least 90% of the dietary reference intakes (19,24–26) for essential nutrients not met by the diet, with the exception of potassium; the 1989 RDA was used (27). Supplements given to subjects included a multimineral supplement (Trader Joe’s), given everyday; vitamin K (Solgar Vitamin and Herb), given everyday; vitamin A and D (Target Corporation), given every fourth day; and iron (Nature Made Nutritional Products), given as needed (based on weekly hematocrit readings).
The folic acid supplement was prepared from commercially available folic acid (Sigma Chemical), as previously described (2), and was consumed in the morning with breakfast. The folic acid stock solution was prepared once before the beginning of the study, dispensed into multiple 50-mL conical tubes, covered with aluminum foil, and stored at −80°C. Approximately every 2 mo, appropriate volumes of the folic acid stock solution were dispensed into 50-mL conical tubes, mixed with crangrape juice, and stored at −20°C. The folic acid–crangrape cocktail was thawed 1–2 d prior to consumption by the subjects.
The choline stock solution was prepared every 3 mo by dissolving choline chloride (USP, BCP Ingredients) in autoclaved Millipore water, filtering the solution through 0.22-μm filters, dispensing it into amber glass bottles, and storing it at 4°C. The molarity of the choline stock solution was determined by LC/MS-MS. The variability between the choline stock solutions was <5%. Periodic testing of the choline stock solution during the 3-mo period indicated no degradation. Appropriate volumes of the choline stock solution were dispensed into 50-mL conical tubes, mixed with crangrape juice, and stored at −20°C. The choline-crangrape cocktail was thawed 1–2 d prior to consumption by the subjects. The 250-mg/d dose for the 550-mg/d treatment group was consumed at breakfast; the 800-mg/d dose for the 1100-mg/d treatment group was consumed at breakfast (250 mg/d) and dinner (550 mg/d); and the 1900-mg/d dose for the 2200-mg/d treatment group was consumed at breakfast (800 mg/d), lunch (550 mg/d), and dinner (550 mg/d).
During the last 3 wk of the study (wk 10–12), subjects in the 550- and 1100-mg/d (10 CC and 10 TT) group consumed 15% of their total choline intake as D9-choline (Cambridge Isotope Laboratories). The administration of labeled choline will allow for future investigations of choline metabolism; the results of which will be presented separately. The D9-choline stock solution was prepared the same way as the unlabeled choline stock solution, described previously, and stored at 4°C in amber glass bottles. Appropriate volumes of the deuterium-labeled choline were dispensed into 50-mL conical tubes, mixed with crangrape juice, and stored at −20°C until used for consumption.
Sample collection and blood processing
Baseline and weekly fasting (10-h) venous blood samples were collected from subjects in serum separator gel and clot-activator tubes (Vacutainer; Becton Dickinson) and EDTA tubes (Vacutainer), then processed and stored at −80°C, as previously detailed (2). Urine collections (24-h) were obtained at baseline (wk 0), wk 6, and wk 12 of the study in 2-L brown plastic bottles containing 3 g of sodium ascorbate, then processed and stored at −20°C, as described previously (2).
Analytical methods
Folate and choline content of diet
The folate and choline content of the diet was determined before starting the study and twice during the study. Each meal (i.e., breakfast, lunch and snack, or dinner), including beverage, was prepared as for the subject’s consumption, blended with 150 mL of cold 0.1 mol potassium phosphate buffer/L (pH 6.3) that contained 57 mmol ascorbic acid/L. This mixture was dispensed into 50-mL conical tubes and stored at −20°C. Food folate was determined by the method of Tamura et al. (28) and double extraction (29). Duplicates of the blended diet aliquots (1–2 g) were thawed, homogenized in a hot extraction buffer (15 mL of 0.05 mol/L HEPES, 0.05 mol/L CHES, 0.1 mol/L sodium ascorbate, 0.2 mol/L 2-mercaptoethanol, pH 7.85) and subjected to a trienzyme treatment and double extraction. Total food folate was measured microbiologically (30) using Lactobacillus Rhamnosis (ATCC). The total folate content of the 3 replicates was 319 ± 8 μg/d. Total choline and betaine contents were determined by the method developed by Koc et al. (31) and Choudhary et al. (32), with modifications based on our instrumentation (4). Total choline and betaine contents of the 3 replicates were 300 ± 5.5 and 173 ± 6 mg/d, respectively.
MTHFR C677T genotype
DNA for genotyping was extracted from leukocytes by using a commercially available kit (DNeasy Tissue kit, Qiagen Science). Determination of the C677T MTHFR genotype involved PCR and Hinf1 restriction enzyme digestion (1). PCR products were separated by gel electrophoresis on a 2% agarose gel and viewed under UV light.
Plasma tHcy
Total homocysteine concentrations were measured in duplicate by the use of a modified HPLC method with fluorometric detection (33,34). The samples were run in batches because of the large sample number. Each batch contained all 4 choline treatments and both the MTHFR CC and TT genotypes, as well as a positive control. Based on the positive control, the intra- and interassay CV were <8%.
Blood and urinary folate
Folate concentrations of serum, erythrocytes, and urine were determined microbiologically by the use of the microtiter plate adaptation with Lactobacillus rhamnosis (30). The intra- and interassay CV were <12% based on the positive control.
Clinical measurements
The clinical laboratory analysis (i.e., creatinine, blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase, LDL cholesterol) was conducted by Cedars-Sinai Medical Center, Department of Pathology and Laboratory Medicine (Los Angeles, CA), by routine clinical assays using commercial kits on an automated analyzer (Roche Hitachi pModular). The Department of Pathology and Laboratory Medicine at Cedars-Sinai Medical Center is licensed by the state of California’s Department of Health Services and is Clinical Laboratory Improvement Act (CLIA)–certified by the Centers of Medicare and Medicaid Services and by the College of American Pathologists. Measurements were performed at baseline and biweekly thereafter for each subject.
Statistical analysis
Each dependent variable of interest (i.e., plasma tHcy) was tested for normality with the Shapiro-Wilk test (SAS PROC UNIVARIATE). Variables that were not normally distributed were transformed to normal (or near normal) by log transform or the Box-Cox method (SAS PROC TRANSREG). Transformed values were used in all ANOVA procedures. To test for baseline differences (wk 0) in the dependent variables among the various groups, a 2-factor ANOVA (choline treatment and MTHFR C677T genotype) was performed. To test for an effect of MTHFR C677T genotype and/or dietary treatment on the dependent variables, a repeated measures ANOVA (SAS PROC GLM) with 1 within factor (time) and 2 between factors (choline treatment and MTHFR C677T genotype) was performed. All possible 2-way and 3-way interactions among factors were included in the model. In each analysis, the assumption of sphericity was assessed with a test of the orthogonal components. When the assumptions were not met, the Huynh-Feldt correction was applied to all tests involving the within-subjects effect. For variables showing a significant time × genotype interaction, the data were stratified by genotype and the analysis was repeated to allow for further exploration of the time effect within a genotype. The data were analyzed using SAS/STAT software (version 9.1.3 for Windows). Differences were considered to be significant at P < 0.05. Data were presented as means ± SEM for all dependent variables with the exception of urinary folate (UF) excretion. For this variable, medians were given due to the extreme variation at baseline.
Results
Subject characteristics and blood indexes at baseline
Sixty-one men completed the study. However, measurements of SF indicated that 1 of the subjects with the MTHFR 677CC genotype was noncompliant with the dietary protocol (i.e., marked fluctuations in SF throughout the study period), and he was not included in the statistical analysis. Thus, the final study group was comprised of 60 men, 29 with the MTHFR 677TT genotype and 31 with the MTHFR 677CC genotype. The BMI and age of the research participants were 26 ± 0.54 kg/m2 (range: 20–37 kg/m2) and 25 ± 1 y (range:18–55 y), respectively. BMI and age did not differ between the MTHFR C677T genotypes or among the choline treatment groups at baseline (Table 1). Throughout the study, subject weights were maintained within 5% of initial values for all but 5 men who experienced weight changes ranging from −8.3 to 6.8%. Indicators of kidney function (serum creatine and blood urea nitrogen) and liver function (alanine aminotransferase and aspartate aminotransferase) were within normal range at baseline and throughout the study duration for all study participants. Minor elevations in blood lipids that the study physician deemed not to have clinical importance were observed in 18 of the 60 men (i.e., 30%) at baseline. The measured variables, including SF, did not differ (P > 0.05) among the choline treatment groups or between the MTHFR C677T genotypes (Table 1).
TABLE 1.
Baseline (wk 0) characteristics of Mexican American men at the time of random assignment to choline treatment1,2
| Choline intake, mg/d |
||||
|---|---|---|---|---|
| Variable | 300 | 550 | 1100 | 2200 |
| Age, y | ||||
| 677CC | 20 (18–45) | 22 (19–42) | 29 (19–35) | 21 (19–31) |
| 677TT | 23 (19–55) | 22(18–27) | 22(19–28) | 21 (18–48) |
| BMI, kg/m2 | ||||
| 677CC | 25(23–32) | 23(20–30) | 27(20–36) | 26(20–32) |
| 677TT | 25(23–32) | 27(22–37) | 25(21–30) | 26(21–37) |
| SF, nmol/L | ||||
| 677CC | 30.4 ± 6.1 | 31.0 ± 4.5 | 34.0 ± 3.4 | 22.0 ± 4.3 |
| 677TT | 23.8 ± 5.2 | 23.8 ± 3.2 | 23.8 ± 2.9 | 22.2 ± 5.2 |
| RBCF, nmol/L | ||||
| 677CC | 1704 ± 143 | 1928 ± 95 | 1922 ± 122 | 1586 ± 104 |
| 677TT | 1622 ± 113 | 1731 ± 120 | 1681 ± 120 | 1586 ± 134 |
| tHcy, μmol/L | ||||
| 677CC | 10.5 ± 2.7 | 9.9 ± 0.7 | 10.1 ± 1.0 | 8.5 ± 0.8 |
| 677TT | 9.6 ± 1.6 | 12.7 ± 4.0 | 12.0 ± 2.6 | 11.3 ± 2.5 |
| UF, nmol/L | ||||
| 677CC | 48 (36–612) | 52 (5–125) | 66 (25–480) | 34 (14–91) |
| 677TT | 70 (23–943) | 88 (<2–1133) | 45 (<2–1360) | 39 (25–52) |
Values are means ± SEM or median (min–max); n = 6–9/group.
MTHFR C677T genotypes and choline treatment groups did not differ at the baseline, P > 0.05 (2-way ANOVA).
Serum folate
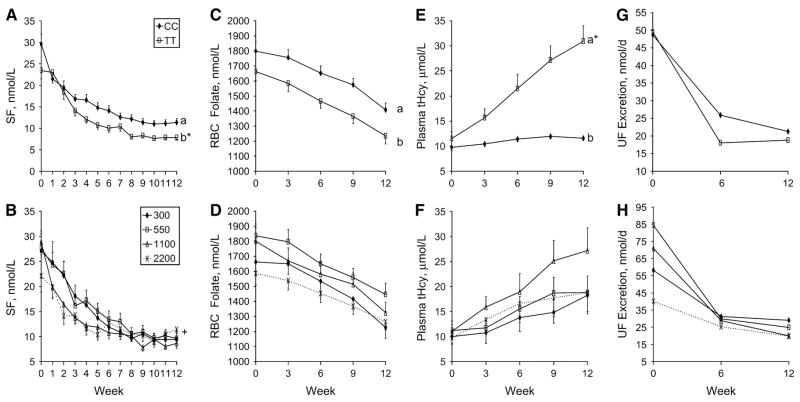
Men with the MTHFR 677TT genotype had lower SF concentrations throughout the study period relative to men with the 677CC genotype (P = 0.002; Fig. 1A). SF decreased (P < 0.0001) 66% (from 23.6 ± 2.0 to 7.9 ± 0.7 nmol/L) in men with the 677TT genotype, and it decreased (P < 0.0001) 62% (from 30.0 ± 2.3 to 11.3 ± 0.9 nmol/L) in men with the 677CC genotype. Although both genotypes experienced a marked decline in SF throughout the study, SF declined faster in men with the 677TT genotype (time × genotype, P = 0.001; Fig. 1A). At the end of the study, 27 of 29 men (93%) with the 677TT genotype had SF concentrations in the suboptimal (59%; 6.8–13.6 nmol/L) or deficient (34%; <6.8 nmol/L) range, and 24 of 31 men (77%) with the MTHFR 677CC genotype had SF concentrations in the suboptimal (61%) or deficient (16%) range. Choline intake did not affect the SF concentration (P = 0.63); however, it modified the response of SF to the study protocol (time × choline, P < 0.0001; Fig. 1B). This interaction transpired mainly at the end of the study whereby a relative increase occurred in SF in the men consuming 2200 mg/d choline (i.e., ~4.5 nmol/L) and not in the other groups.
FIGURE 1.
SF (A, B), RBCF (C, D), tHcy (E, F), and UF (G, H) in Mexican American men differing in MTHFR C677T genotype (upper panels: n = 29 TT and 31 CC) that consumed different levels of choline (mg/d, lower panels: n = 6–9/intake group) and 438 μg DFE/d for 12 wk. Data were analyzed using a repeated measures ANOVA with 1 within-group factor (time) and 2 between-group factors (genotype and choline intake). Data are presented as means ± SEM (SF, RBCF, tHcy) or as medians (UF). Time affected all variables, P < 0.05. Choline intake did not affect any of the variables. Different letters indicate an effect of genotype, P < 0.05. Symbols indicate significant interactions: *, week × genotype; +, week × choline.
RBC folate
Men with the MTHFR 677TT genotype had lower RBC folate (RBCF) concentrations throughout the study period relative to men with the 677CC genotype (P = 0.015; Fig. 1C). RBCF decreased (P < 0.0001) 26% (from 1661 ± 59 to 1233 ± 52 nmol/L) in men with the 677TT genotype, and 22% (from 1799 ± 61 to 1409 ± 45 nmol/L) in men with the 677CC genotype. The response to the dietary regimen was not modified by the MTHFR C677T genotype (time × genotype, P = 0.50). No main effect of choline intake was detected on RBCF (P = 0.14) nor did choline intake modify the response of RBCF to the study protocol (time × choline, P = 0.38; Fig. 1D).
Plasma tHcy
Men with the MTHFR 677TT genotype had higher plasma tHcy concentrations throughout the study period relative to men with the 677CC genotype (P < 0.0001; Fig.1E). Although both genotypes experienced an increase (P < 0.0001) in plasma tHcy, this increase was far greater in men with the 677TT genotype relative to those with the 677CC genotype (time × genotype, P < 0.0001; Fig. 1E). Specifically, plasma tHcy increased (P <0.0001) 170% (from 11.5 ± 1.4 to 31 ± 3.1 μmol/L) in men with the 677TT genotype compared with an increase (P < 0.0001) of 18% (from 9.8 ± 0.7 to 11.6 ± 0.3 μmol/L) in men with the 677CC genotype. At the end of the study, 23 of 29 men (79%) with the 677TT genotype had plasma tHcy concentrations >14 μmol/L compared with 2 of 31 men (~7%) with the 677CC genotype. No main effect of choline intake was detected (P = 0.10) nor did choline intake modify the response of plasma tHcy to the study protocol (time × choline, P = 0.42; Fig. 1F).
UF excretion
Throughout the study period, no differences (P = 0.73) in UF excretion were detected among the MTHFR 677TT or CC genotypes (Fig. 1G). UF excretion declined (P < 0.001) 65% (from 50 [range: not detectable to 1366] to 17 [range: 3.4 to 36] nmol/d) in men with the 677TT genotype and 57% (from 50 [range 4.5–612] to 9.4 [not detectable to 41] nmol/d) in men with the 677CC genotype. No effects of the MTHFR C677T genotype were detected on the response of UF excretion throughout the study period (time × genotype, P = 0.85). In addition, no main effect of choline intake was detected (P = 0.30) nor did choline intake modify the response of UF excretion to the study protocol (time × choline, P = 0.71; Fig.1H).
Discussion
By definition, an RDA should meet the requirements of 97–98% of the individuals for which it was designed. Since the establishment of the 1998 folate RDA for men and women, the MTHFR 677TT genotype has emerged as a strong modifier of folate status and 1-carbon metabolism, particularly when folate intake is suboptimal. Of the U.S. population, ~10% has the MTHFR 677TT genotype; however, its prevalence is approximately double in people of Mexican descent, the fastest growing population in the United States. Our research group (2,22) and others (20) have demonstrated that 400 μg DFE/d is enough to maintain normal folate status in premenopausal women homozygous for the variant T allele. However, prior to this study, limited data were available upon which to assess the adequacy of 400 μg DFE/d in men with the MTHFR 677TT genotype. This controlled feeding study was designed in part to examine the adequacy of the 1998 folate RDA, 400 μg DFE/d, in men of Mexican descent having the MTHFR 677CC or TT genotype. Compliance to the dietary protocol was monitored through the use of weekly SF concentration, which is sensitive to recent dietary folate intakes. Under conditions of steady folate intake, SF should not fluctuate (i.e., rise and fall) from week to week. SF declined steadily throughout this study in all but 1 subject who experienced marked fluctuations in his SF and was thus excluded from the statistical analysis.
The folate status variables used to evaluate folate adequacy during the establishment of the 1998 folate RDA included RBCF, an indicator of tissue stores; tHcy, a functional biomarker; and SF, a sensitive marker of changes in folate status, particularly under conditions of controlled folate intake (19). Cutoff values deemed to reflect folate insufficiency were <317 nmol/L (<140 μg/L) for RBCF, >14 μmol/L for plasma tHcy, and <6.8 nmol/L (<3 μg/L) for SF (19). In light of these cutoff values, our data strongly suggest that the 1998 folate RDA, 400 μg DFE/d, is not sufficient for Mexican American men, particularly for those with the MTHFR 677TT genotype. Specifically, among those with the 677TT genotype, 23 of 29 (79%) had plasma tHcy concentrations >14 μmol/L and 27 of 29 (93%) had SF concentrations in the suboptimal (59%; 6.8–13.6 nmol/L) or deficient (34%) range. Among men with the MTHFR 677CC genotype, 2 of 31 (~7%) had plasma tHcy concentrations >14 μmol/L and 24 of 31 (77%) had SF concentrations in the suboptimal (61%) or deficient (16%) range. RBCF did not approach the cutoff value of 317 nmol/L in either genotype; however, RBCF continued to decline throughout the study period as did UF.
Our second objective was to examine the influence of differing choline intakes [300, 550 (choline AI level), 1100, or 2200 mg/d] on homocysteine and folate metabolism. In this regard, others have reported a homocysteine-lowering effect (range: 11–20%) after daily supplementation with phosphatidylcholine, 34 g containing 2.6 g choline for 2 wk (6), or betaine with intakes ranging from 1.5 to 6 g for 6 wk (5,35,36). However, the lack of effect of choline intake on plasma tHcy response in this study suggests that supplemental choline can not serve as an alternative to folate as a homocysteine lowering agent in this population. This finding is consistent with the observation that supplementation with betaine, but not choline, suppressed plasma homocysteine elevation induced by folate deficiency in rats (37). Why choline is not effective as a homocysteine lowering agent is unclear; however, measurements of choline and its derivatives in plasma are underway to further elucidate the relationship between choline, betaine, and homocysteine.
Unlike choline intake, the MTHFR C677T genotype was a strong modifier of plasma tHcy. Specifically, plasma tHcy increased 170% in men with the MTHFR 677TT genotype compared with 18% in men with the 677CC genotype. Although a robust interaction between folate and the MTHFR C677T genotype is widely recognized, the MTHFR C677T may also interact with choline/betaine (4,38), riboflavin (39,40), and vitamin B-12 (41) to affect homocysteine. In this study, the response of plasma tHcy to various choline intakes did not differ between the MTHFR 677TT or CC genotypes. Riboflavin and vitamin B-12 were provided in RDA amounts by the diet, although measurements of these analytes in blood were not performed. Thus, like folate, it is possible that men with the MTHFR 677TT genotype have higher requirements for riboflavin and vitamin B-12 compared with men with the 677CC genotype.
In addition to gene-nutrient interactions, the large variation in plasma tHcy concentrations (i.e., 8.6 to 62 μmol/L at wk 12) among men with the MTHFR 677TT genotype under controlled dietary conditions suggests that the MTHFR 677TT genotype may be interacting with other relevant genetic variants (i.e., gene-gene interactions). In this regard, Lucock and Yates (42) recently reported that as the number of folate-related variant alleles increased within the MTHFR 677TT genotype, plasma tHcy decreased. Additional findings by these authors suggested that homocysteine trans-sulfuration may be positively selected within the MTHFR 677TT genotype as the number of folate-related variant alleles increased (42). Work is underway in our laboratory to examine the possibility of gene-gene interactions on plasma tHcy within the MTHFR 677TT genotype.
To conclude, the findings of this controlled feeding strongly suggest that the 1998 folate RDA of 400 μg DFE/d is not adequate for Mexican American men, especially for those with the MTHFR 677TT genotype. Additional studies involving other ethnic groups and several dosing levels are needed to more fully ascertain folate requirements in men. Data from this study also suggest that choline intakes of up to 4 times the choline AI cannot compensate for the diminished availability of folate and potentially other B vitamins associated with remethylation of homocysteine.
Acknowledgments
The authors thank Christian Abratte for his creation of the figure and helpful discussions.
Footnotes
Supported by NIH Grant S06GM053933 and funds from the California Agricultural Research Initiative.
Author disclosures: C. Solis, K. Veenema, A. A. Ivanov, S. Tran, R. Li, W. Wang, D. J. Moriarty, C. V. Maletz, and M. A. Caudill, no conflicts of interest.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AI, adequate intake; DFE, dietary folate equivalents; MTHFR, methylenetetrahydrofolate reductase; RBCF, RBC folate; RDA, recommended dietary allowance; SF, serum folate; tHcy, plasma total homocysteine; THF, tetrahydrofolate; UF, urinary folate.
Literature Cited
- 1.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenete-trahydrofolate reductase. (Letter) Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 2.Guinotte CL, Burns MG, Axume JA, Hata H, Urrutia TF, Alamilla A, McCabe D, Singgih A, Cogger EA, et al. Methylenetetrahydrofolate reductase 677C→T variant modulates folate status response to controlled folate intakes in young women. J Nutr. 2003;133:1272–80. doi: 10.1093/jn/133.5.1272. [DOI] [PubMed] [Google Scholar]
- 3.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–7. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- 4.Abratte CM, Wang W, Li R, Moriarty DJ, Caudill MA. Folate intake and the MTHFR C677T genotype influence choline status in young Mexican American women. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2007.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133:4135–8. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- 6.Olthof MR, Brink EJ, Katan MB, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and post-methionine-loading plasma homocysteineconcentrations inhealthymen. AmJ ClinNutr. 2005;82:111–7. doi: 10.1093/ajcn.82.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Holm PI, Hustad S, Ueland PM, Vollset SE, Grotmol T, Schneede J. Modulation of the homocysteine-betaine relationship by methylenete-trahydrofolate reductase 677 C->t genotypes and B-vitamin status in a large-scale epidemiological study. J Clin Endocrinol Metab. 2007;92:1535–41. doi: 10.1210/jc.2006-1471. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J, Seyoum E, Pomforte EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- 9.Caudill M. The role of folate in reducing chronic and developmental disease risk: An overview. J Food Sci. 2004;69:SNQ55–8. [Google Scholar]
- 10.Miller JW. Folate, cognition, and depression in the era of folic acid fortification. J Food Sci. 2004;69:SNQ61–4. [Google Scholar]
- 11.Moyers S, Bailey LB. Fetal malformations and folate metabolism: review of recent evidence. Nutr Rev. 2001;59:215–35. doi: 10.1111/j.1753-4887.2001.tb07013.x. [DOI] [PubMed] [Google Scholar]
- 12.Rozen R. Folate and genetics. J Food Sci. 2004;69:SNQ65–7. [Google Scholar]
- 13.Zeisel SH, Da Costa K, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]
- 14.da Costa KA, Badea M, Fischer L, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–70. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 15.Collinsova M, Strakova J, Jiracek J, Garrow TA. Inhibition of betaine homocysteine S-methyltransferase causes hyperhomocysteinemia in mice. J Nutr. 2006;136:1493–7. doi: 10.1093/jn/136.6.1493. [DOI] [PubMed] [Google Scholar]
- 16.da Costa KA, Niculescu MD, Craciunescu CN, Fischer LM, Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer D. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine; National Academy of Sciences USA. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 20.Shelnutt KP, Kauwell GP, Chapman CM, Gregory JF, Maneval DR, Browdy AA, Theriaque DW, Bailey LB. Folate status response to controlled folate intake is affected by the methylenetetrahydrofolate reductase 677C→T polymorphism in young women. J Nutr. 2003;133:4107–11. doi: 10.1093/jn/133.12.4107. [DOI] [PubMed] [Google Scholar]
- 21.Yang TL, Hung J, Caudill MA, Urrutia TF, Alamilla A, Perry CA, Li R, Hata H, Cogger EA. A long-term controlled folate feeding study in young women supports the validity of the 1.7 multiplier in the dietary folate equivalency equation. J Nutr. 2005;135:1139–45. doi: 10.1093/jn/135.5.1139. [DOI] [PubMed] [Google Scholar]
- 22.Hung J, Yang TL, Urrutia TF, Li R, Perry CA, Hata H, Cogger EA, Moriarty DJ, Caudill MA. Additional food folate derived exclusively from natural sources improves folate status in young women with the MTHFR 677 CC or TT genotype. J Nutr Biochem. 2006;17:728–34. doi: 10.1016/j.jnutbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Esfahani ST, Cogger EA, Caudill MA. Heterogeneity in the prevalence of methylenetetrahydrofolate reductase gene polymorphisms in women of different ethnic groups. J Am Diet Assoc. 2003;103:200–7. doi: 10.1053/jada.2003.50030. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine; National Academy of Sciences USA. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 25.Institute of Medicine; National Academy of Sciences USA. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 26.Institute of Medicine; National Academy of Sciences USA. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, zinc. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 27.Food and Nutrition Board. Recommended Dietary Allowances. 10. Washington, DC: National Academy of Sciences; 1989. [Google Scholar]
- 28.Tamura T, Mizuno Y, Johnston KE, Jacob RA. Food folate assay with protease, alpha amylase, and folate conjugase treatments. J Agric Food Chem. 1997;45:135–9. [Google Scholar]
- 29.Gregory JF, Engelhardt R, Bhandari SD, Bustafson SK. Adequacy of extraction techniques for determination of folates in foods and other biological materials. J Food Compos Anal. 1990;3:134–44. [Google Scholar]
- 30.Tamura T. Microbiological assay of folates. In: Picciano MF, Stokstad ELR, Gregory JF, editors. Folic Acid Metabolism in Health and Disease. New York: John Wiley & Sons; 1990. pp. 121–37. [Google Scholar]
- 31.Koc H, Mar M-H, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–40. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- 32.Choudhary G, Hart B, Cho D, Caudill MA. App. Note 334: Determination of Choline and Its Metabolites using a Finnigan LTQ Linear Ion Trap Mass Spectrometer. San Jose (CA): Thermo Electron Corporation; [Google Scholar]
- 33.Vester B, Rasmussen K. High performance liquid chromatograph method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem. 1991;29:549–54. doi: 10.1515/cclm.1991.29.9.549. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 35.Schwab U, Torronen A, Toppinen L, Alfthan G, Saarinen M, Aro A, Uusitupa M. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76:961–7. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- 36.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–5. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 37.Yagisawa M, Doi Y, Uenohara T, Toda M, Shigematsu N, Nakata R. Betaine supplementation suppresses plasma homocysteine level elevation induced by folate deficiency in rats. Nutr Res. 2006;26:266–70. [Google Scholar]
- 38.Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J, Mar M-H, Zeisel SH, Castro C, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–4. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 39.Jacques PF, Kalmbach R, Bagley PJ, Russo GT, Rogers G, Wilson PWF, Rosenberg IH, Selhub J. The relationship between riboflavin and plasma total homocysteine in the Framingham Offspring cohort is influenced by folate status and the C677T transition in the methylenetetrahydrofolate reductase gene. J Nutr. 2002;132:283–8. doi: 10.1093/jn/132.2.283. [DOI] [PubMed] [Google Scholar]
- 40.McNulty H, Dowey LRC, Strain JJ, Dunne A, Ward M, Molloy AM, McAnena LB, Hughes JP, Hannon-Fletcher M, et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C→T polymorphism. Circulation. 2006;113:74–80. doi: 10.1161/CIRCULATIONAHA.105.580332. [DOI] [PubMed] [Google Scholar]
- 41.Bailey LB, Duhaney RL, Maneval DR, Kauwell GPA, Quinlivan EP, Davis SR, Cuadras A, Hutson AD, Gregory JF. Vitamin B-12 status is inversely associated with plasma homocysteine in young women with C677T and/or A1298C methylenetetrahydrofolate reductase polymorphisms. J Nutr. 2002;132:1872–8. doi: 10.1093/jn/132.7.1872. [DOI] [PubMed] [Google Scholar]
- 42.Lucock M, Yates Z. Synergy between 677 TT MTHFR genotype and related folate SNPs regulates homocysteine level. Nutr Res. 2006;26:180–5. [Google Scholar]