Abstract
Approximately 40% of patients with type 2 diabetes present with concurrent hypertension at the time of diabetes diagnosis. Increases in peripheral vascular resistance and correspondingly enhanced vasoconstrictor capacity could have profound implications for the development of hypertension and the progression of insulin resistance to overt diabetes. The purpose of this study was to determine whether skeletal muscle arteriolar vasoconstrictor dysfunction precedes or occurs concurrently with the onset of diabetes and hypertension. Male Zucker diabetic fatty (ZDF) rats were studied at 7, 13, and 20 wk of age to represent prediabetic and short-term and long-term diabetic states, respectively. Conscious mean arterial pressure (MAP), fasted plasma insulin and glucose, vasoconstrictor responses, and passive mechanical properties of isolated skeletal muscle arterioles were measured in prediabetic, diabetic, and age-matched control rats. Elevated MAP was manifest in short-term diabetes (control 117 ± 1, diabetic 135 ± 3 mmHg) and persisted with long-term diabetes (control 113 ± 2, diabetic 135 ± 3 mmHg). This higher MAP was preceded by augmented arteriolar vasoconstrictor responses to norepinephrine and endothelin-1 and followed by diminished β-adrenergic vasodilation and enhanced myogenic constriction in long-term diabetes. Furthermore, we demonstrate that diminished nitric oxide (NO) signaling underlies the increases in vasoconstrictor responsiveness in arterioles from prediabetic and diabetic rats. Arteriolar stiffness was not different between control and prediabetic or diabetic rats at any time point studied. Collectively, these results indicate that increases in vasoconstrictor responsiveness resulting from diminished NO signaling in skeletal muscle arterioles precede the development of diabetes and hypertension in ZDF rats.
Keywords: type 2 diabetes, vasoconstriction, nitric oxide, norepinephrine, endothelin-1
Diabetes Mellitus Affects approximately 16 million Americans (15, 29), and the cardiovascular complications associated with the disease are the leading cause of the morbidity and mortality among patients (1). The presence of diabetes increases cardiovascular disease risk two- to sixfold over that of normal healthy adults (2, 5, 27, 30), with 40% of type 2 diabetic patients presenting with concurrent hypertension at the time of diagnosis (54). This early onset of hypertension suggests that cardiovascular complications may precede the onset of overt diabetes and result from a long-term impaired glucose tolerance and hyperinsulinemia (28). Alterations in vasoconstrictor responses during this prediabetic state may have profound implications for both the development of cardiovascular complications and the progression of subclinical glucose intolerance to overt type 2 diabetes.
The skeletal muscle is the site of the largest percentage of glucose removal from the blood under insulin-stimulated conditions (55), with the rate of clearance primarily determined by the blood flow delivered to the tissue (3, 7). Capillary perfusion is determined by the pressure gradient across a capillary bed, which is itself a function of blood flow and precapillary resistance determined primarily at the level of first-, second-, and third-order arterioles (26, 36, 53). Therefore, tone of this resistance vasculature will determine the magnitude of capillary perfusion and consequently glucose utilization in skeletal muscle (2, 3). In addition, the skeletal muscle microvasculature is a primary determinant of peripheral vascular resistance (12), so alterations in the responsiveness of the skeletal muscle resistance vasculature to vasoconstrictor agonists may have profound implications for the development and progression of glucose intolerance and hypertension.
Despite the critical role of skeletal muscle blood flow in the regulation of glucose uptake, there is a paucity of data describing alterations in skeletal muscle vasoreactivity in type 2 diabetes. A number of studies have described altered vasomotor responses in the skeletal muscle vasculature of the obese Zucker fatty (OZR) rat (19–24, 40, 46, 52), a model of the metabolic syndrome. However, unlike the Zucker diabetic fatty (ZDF) rat, the OZR rat does not experience a clearly delineated decline in pancreatic function and thus remains hyperinsulinemic throughout most of its life span. The ZDF rat was chosen for the present study because it has been shown to initially develop peripheral insulin resistance followed by frank type 2 diabetes at predictable ages (17, 32). Moreover, these rats display many of the same conditions as prediabetes and type 2 diabetes in humans, such as obesity (6), hypertension (32), and abnormal blood lipid profiles (6, 8, 17, 49, 50), making them a clinically relevant model. Furthermore, although changes in function of large arterial rings (9, 48), intestinal arterioles (4), and coronary and mesenteric arteries and arterioles (41) have been described during the progression of type 2 diabetes, a similar examination of the skeletal muscle vasculature is lacking. Therefore, the purposes of the present study were to determine whether 1) there is an increased responsiveness of skeletal muscle arterioles to vasoconstrictor agonists during the natural progression of type 2 diabetes and whether they are associated with elevations in mean arterial pressure (MAP), 2) alterations in arterial stiffness contribute to these putative increases in tone, 3) impairment of endothelium-dependent and -independent vasodilation occurs during the progression of type 2 diabetes, and 4) the nitric oxide (NO) signaling pathway contributes to alterations in vasoconstrictor and endothelium-dependent vasodilator responses.
RESEARCH DESIGN AND METHODS
All animal procedures were approved by the Texas A&M University Laboratory Animal Care Committee and complied with the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (National Academy Press, revised 1996).
Male diabetic (ZDF:Gmi fa/fa) and age-matched control (ZDF:Gmi +/?) rats were obtained from Charles River Laboratories/Genetic Models and studied at 7, 13, and 20 wk of age. Animals were housed individually and allowed free access to Purina 5008 diet and water. Animals were housed in a temperature-controlled (23 ± 2°C) room with a 12:12-h light-dark cycle. Information obtained from Charles River (www.criver.com) and previous studies (8, 17, 18, 25, 42, 50, 51, 57) has demonstrated that male ZDF rats maintained on the Purina 5008 diet develop mild hyperglycemia and marked hyperinsulinemia by 7 wk of age, a condition referred to in the present study as prediabetes. This rat strain then develops marked hyperglycemia by 10–12 wk of age (8, 17, 18, 51), a condition referred to in the 13-wk-old ZDF rats of the present study as short-term diabetes. A progressive decline in beta cell function resulting in normo- to hypoinsulinemia (8, 17, 18, 42, 51) by 20 wk of age is referred to as long-term diabetes in the present study.
The day before the functional arteriolar studies, rats were anesthetized with isoflurane (5% balanced in 95% oxygen) via inhalation, and a polyurethane (Braintree Scientific, Micro-renathane; ID 0.36 mm, OD 0.84 mm) catheter filled with heparinized saline was inserted into the carotid artery. Rats were fasted overnight, and the following morning electronically averaged MAP was recorded during conscious standing in the cage as previously performed (11). After blood pressure measurement, blood was collected via the carotid catheter for assessment of plasma glucose and insulin content. The rat was then euthanized via exsanguination while under anesthesia, and the gastrocnemius-plantaris-soleus muscle complex was carefully excised from each hindlimb.
After excision, the muscle complex was placed in cold (4°C) physiological saline solution (PSS) that contained (mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 3.0 MOPS buffer, with 1 g/100 ml bovine serum albumin, pH 7.4. Gastrocnemius muscle first-order arterioles were isolated with the aid of a dissecting microscope (Olympus SVH10) as previously described (37, 38). After cannulation, the chambers were transferred to the stage of an inverted microscope (Olympus IX70) equipped with a video camera (Panasonic BP310), a video caliper (Microcirculation Research Institute), and a data acquisition system (MacLab/Macintosh) for recording of luminal diameter. Arterioles were initially pressurized to 44 mmHg with two independent hydrostatic pressure reservoirs. Leaks were detected by pressurizing the vessel and then closing the reservoirs to verify that diameter remained constant. Arterioles that exhibited leaks were discarded. Arterioles free of leaks were warmed to 37°C and allowed to develop initial spontaneous tone during a 30- to 60-min equilibration period.
Study 1: Vasoconstrictor responsiveness
To determine whether increases in adrenergic vasoconstriction accompany the progression of type 2 diabetes, changes in luminal diameter were measured in response to the cumulative addition of the α-receptor agonist norepinephrine (NE, 10−9–10−5 M). To determine whether enhanced vasoconstrictor responses were specific to adrenergic signaling, vasoconstriction was induced by endothelin-1 (ET-1, 10−11–10−8 M) and the non-receptor-mediated vasoconstrictor isotonic KCl (10–100 mM).
Study 2: Mechanisms of enhanced vasoconstrictor responses
Results from study 1 demonstrated enhanced vasoconstriction of gastrocnemius muscle arterioles to NE and ET-1 in the prediabetic condition. Therefore, we sought to determine whether altered mechanical properties of the vessel wall and subsequent alterations in stiffness contribute to these increases in vasoconstrictor responses. To do so, we determined both the active (myogenic vasoconstriction) and passive pressure-diameter relations (0–110 mmHg). Medial wall thickness, circumferential stretch and stress, and incremental stiffness were calculated from the passive relations as previously described (38).
Because both NE and ET-1 can stimulate production and release of the vasodilator substance NO from endothelial cells, we next sought to determine whether an impairment of the NO signaling pathway could account for the enhanced vasoconstrictor responses. To assess the role of NO signaling in modulating vasoconstrictor responses to NE and ET-1, dose responses were determined after a 30-min incubation with the nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10−5 M) (39).
Study 3: Endothelium-dependent and -independent vasodilation
To determine whether endothelium-dependent vasodilation is diminished during the progression of type 2 diabetes, dose responses to acetylcholine (ACh, 10−9–10−4 M) in the presence and absence of L-NAME were determined. To assess endothelium-independent vasodilator responses, KCl (10 mM)-mediated and sodium nitroprusside (SNP, 10−10–10−4 M)-induced vasodilation were also determined. Furthermore, because adrenergic stimulation can result in both α-receptor-mediated vasoconstriction and β-receptor-mediated vasodilation, the combination of these influences will contribute to the overall tone in the vessel. To determine whether decreases in adrenergic vasodilator responses accompany the progression of type 2 diabetes, dose-response relations for the β-receptor agonist isoproterenol (Iso, 10−9–10−5 M) were also assessed.
To determine whether the decreased NOS signaling is related to diminished expression of endothelial NOS (eNOS) or superoxide dismutase (SOD), relative differences in eNOS and SOD-1 protein content were assessed in gastrocnemius first-order arterioles by Western blot as described previously (31). eNOS protein content was evaluated with a monoclonal antibody (1:1,600; catalog no. N30020, BD Biosciences), and SOD-1 protein content was assessed with a polyclonal antibody (1:1,600; catalog no. SOD-100, Stressgen). Immunoblots were evaluated by enhanced chemiluminescence (ECL, Amersham) and densitometry with NIH Image Software (National Institutes of Health, Bethesda, MD). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard to control for small differences in protein loading. GAPDH protein content was assessed with a monoclonal antibody (1:10,000; catalog no. MAB374, Chemicon). The data were normalized to GAPDH expression to account for differences in protein loading.
Stock solutions of drugs were prepared in distilled water and frozen. Fresh dilutions of these stocks were prepared daily. All drugs were purchased from Sigma (St. Louis, MO).
Data presentation and analysis
The development of spontaneous tone was expressed as the percent vasoconstriction relative to maximal diameter and was calculated as previously described (14, 37–39). Vasoconstrictor responses to NE, KCl, and ET-1 were expressed as percent change from baseline diameter as previously described (14, 38). Vasodilator responses were recorded as actual diameters and subsequently expressed as percent maximal vasodilation (37–39).
Two-way repeated-measures ANOVA was used to detect differences between (control vs. prediabetic or diabetic) and within (drug concentration or pressure level) factors. The agonist concentration that produced 50% of the maximal vasoconstrictor or vasodilator response was designated EC50 or IC50, respectively. All EC50 and IC50 values were converted to log values for statistical comparison. A one-way ANOVA was used to determine the significance of differences between groups in animal and muscle mass and vessel characteristics. Duncan’s multiple-range test was used to determine the significance of difference between groups. All data are presented as means ± SE. Significance was set at P ≤ 0.05.
RESULTS
Animal and vessel characteristics
ZDF rats during prediabetes and short-term diabetes had a greater body mass than age-matched control animals (Control-7, Control-13, respectively), whereas the body mass of ZDF rats during long-term diabetes did not differ from that of age-matched controls (Control-20) (Table 1). The ZDF rats became hyperglycemic during short-term diabetes compared with age-matched controls, and this persisted with long-term diabetes (Table 1). Prediabetic and short-term diabetic rats displayed hyperinsulinemia that subsequently declined in long-term diabetes (Table 1). MAP was not different between prediabetic and Control-7 rats (Table 1). However, MAP was elevated 15% during short-term diabetes and 19% with long-term diabetes. The gastrocnemius muscle-to-body mass ratio was lower at all time points in the prediabetic and frankly diabetic rats compared with age-matched controls (Table 1).
Table 1. Animal, muscle, and arteriolar characteristics from in vitro studies of prediabetic, short-term and long-term diabetic, and age-matched control rats.
| Cnt-7 | Pre | Cnt-13 | ST | Cnt-20 | LT | |
|---|---|---|---|---|---|---|
| n | 21 | 24 | 24 | 24 | 23 | 20 |
| Body mass, g | 171±8 | 221±10* | 328±8 | 359±11* | 408±8 | 391±9 |
| Glucose, mg/dl | 80±4 | 104±8 | 83±4 | 230±23* | 91±7 | 258±15* |
| Insulin, mU/ml | 1.1±0.2 | 4.7±1.1* | 1.3±0.1 | 5.3±1.4* | 1.7±0.3 | 1.8±0.2 |
| Mean arterial pressure, mmHg | 117±1 | 122±3 | 117±1 | 135±3* | 113±2 | 135±3* |
| Gastrocnemius muscle | ||||||
| Muscle mass, g | 0.99±0.07 | 1.03±0.03 | 1.88±0.02 | 1.45±0.05* | 2.07±0.16 | 1.35±0.10* |
| Muscle-to-body mass ratio, g muscle/g body mass × 100 | 0.49±0.13 | 0.40±0.04* | 0.53±0.03 | 0.39±0.02* | 0.47±0.13 | 0.33±0.08* |
| Maximal diameter, μm | 96±7 | 101±3 | 126±10 | 116±4 | 130±4 | 110±7* |
| Spontaneous tone, % | 38±6 | 47±6 | 44±1 | 44±5 | 34±4 | 32±5 |
| Tone with L-NAME, % | 56±5† | 65±5† | 64±2† | 61±5† | 52±6† | 39±7 |
Values are means ± SE for n animals studied per group. ZDF, Zucker diabetic fatty; Pre, prediabetic; ST, short-term diabetic ZDF:( fa/fa); LT, long-term diabetic ZDF:(fa/fa); Cnt-7, Cnt-13, Cnt-20, lean ZDF:(+/?) age matched to Pre, ST, and LT, respectively; L-NAME, NG-nitro-L-arginine methyl ester.
Significant difference vs. age-matched control;
significant difference from spontaneous tone (P < 0.05).
Maximal arteriolar lumen diameter determined in vitro from the gastrocnemius muscle was 15% smaller in long-term diabetes but did not differ at any other time point between groups (Table 1). Furthermore, spontaneous tone did not differ between control and prediabetic or diabetic rats at any time point.
From histological examination of gastrocnemius muscle arterioles to determine medial wall thickness (study 2), no differences in maximal diameter between control and prediabetic or diabetic rats were evident at any time point (Table 2). There was, however, a trend for smaller maximal diameters in arterioles from the long-term diabetic rats (P < 0.1), which is consistent with results obtained from in vitro experiments. Furthermore, no differences in medial wall thickness were measured between groups at any time point. Both the wall cross-sectional area and wall-to-lumen ratios were higher in arterioles from prediabetic compared with age-matched control rats (Table 2).
Table 2. Morphological characteristics of gastrocnemius muscle arterioles from prediabetic, short-term and long-term diabetic, and age-matched control ZDF rats.
| Group | n | Maximal Diameter, μm | Wall Thickness, μm | Wall Cross-Sectional Area, μm2 | Wall-to-Lumen Ratio, % |
|---|---|---|---|---|---|
| Cnt-7 | 9 | 101±6 | 10.0±0.9 | 2,142±358 | 8.8±0.8 |
| Pre | 8 | 102±6 | 12.2±0.4 | 3,780±749* | 11.8±0.8* |
| Cnt-13 | 8 | 118±7 | 13.0±1.8 | 3,772±848 | 10.4±1.2 |
| ST | 8 | 119±10 | 11.4±1.1 | 3,013±453 | 9.6±1.2 |
| Cnt-20 | 9 | 131±9 | 10.6±0.9 | 2,528±313 | 8.1±0.8 |
| LT | 9 | 112±10 | 10.7±1.1 | 2,651±410 | 10.3±1.1 |
Values are means ± SE for n vessels studied histologically.
Significant difference from age-matched control (P ≤ 0.05).
Adrenergic vasoconstriction
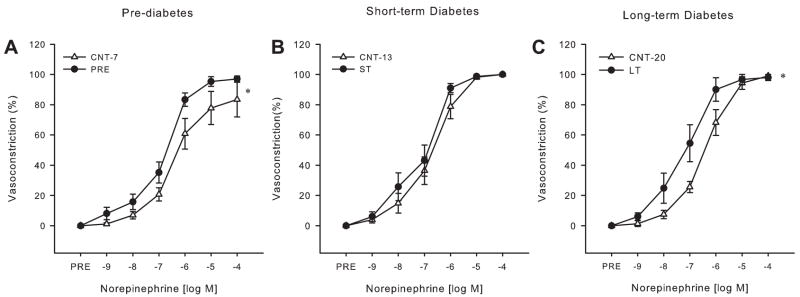
A greater vasoconstriction for a given dose of NE was found during prediabetes [Fig. 1A; NE (log M): −7: P ≤ 0.1, −6: P < 0.05, −5: P ≤ 0.1], as well as a trend toward an increase in sensitivity to NE during prediabetes (EC50: Control-7 4.3E −7 ± 1.5E−7 M vs. prediabetic 2.0E−7 ± 0.4 E−7 M; P ≤ 0.1). No differences were found in NE-mediated vasoconstriction in arterioles from short-term diabetic rats (Fig. 1B). Although there was no difference in maximal NE-induced vasoconstriction during long-term diabetes (Fig. 1C), there was a significant dose by group interaction resulting in increased vasoconstriction at given doses of NE [NE (log M): −8: P ≤ 0.1, −7: P < 0.05, −6: P ≤ 0.1] and an increase in arteriolar sensitivity (EC50: Control-20 2.4E−7 ± 0.2E−7 M vs. long term 1.1E−7 ± 0.4 E−7 M; P < 0.01).
Fig. 1.
Gastrocnemius muscle arteriole vasoconstrictor reactivity. Concentration-response relations for the cumulative addition of norepinephrine (NE) in gastrocnemius muscle arterioles from prediabetic (Pre, A) and short-term (ST, B) and long-term (LT, C) diabetic Zucker diabetic fatty (ZDF) rats compared with age-matched control rats (Cnt-7, Cnt-13, Cnt-20, respectively) (n = 9–11/group). Values are means ± SE. *Significant dose by group interaction in dose-response relations (P < 0.05).
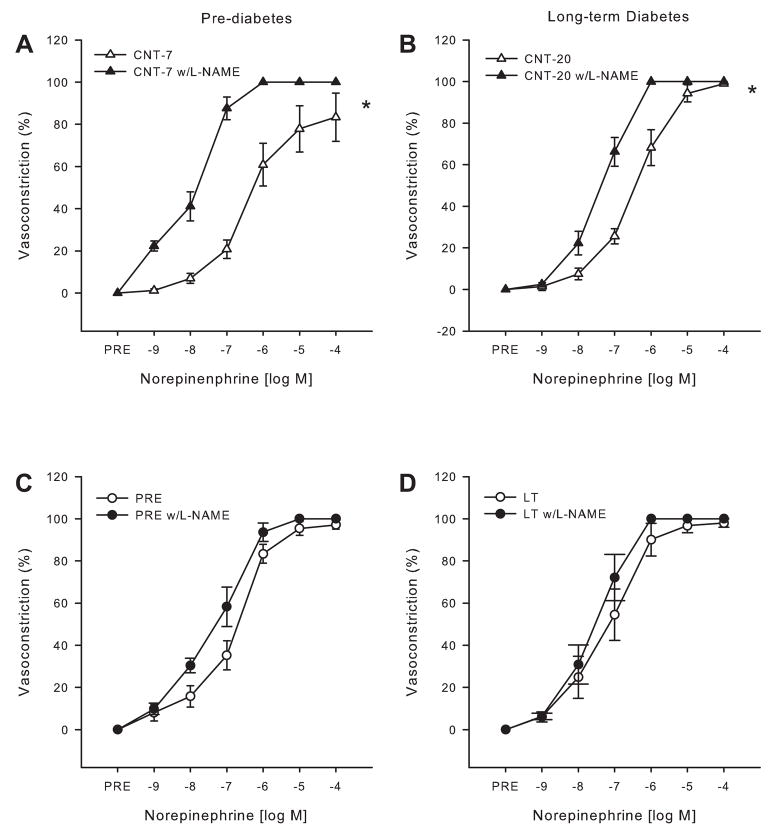
To assess the role of the NOS signaling pathway in mediating the enhanced NE-mediated vasoconstrictor responses in gastrocnemius muscle arterioles, NE dose responses were repeated after NOS inhibition. These experiments were performed at time points that had previously demonstrated enhanced vasoconstrictor reactivity. Specifically, we examined the effects of NOS inhibition on NE-mediated vasoconstriction during prediabetes and long-term diabetes. NOS inhibition increased tone in the gastrocnemius muscle arterioles from the prediabetic and short-term diabetic rats as well as their age-matched controls (Table 1), indicating that tonic NO bioavailability is similar between the groups. However, L-NAME increased tone in the gastrocnemius muscle arterioles from Control-20 rats but did not affect tone in their diabetic counterparts, indicating that with long-term diabetes there is a decrease in tonic NO bioavailability. Tone after NOS inhibition did not differ between the diabetic and fatty rats at any time point studied (Table 1).
Enhanced vasoconstriction found in the prediabetic arteriolar responses to NE was reversed after NOS inhibition, such that arterioles from control rats demonstrated increased vasoconstriction to NE relative to those from prediabetic rats when preincubated in L-NAME (group difference: P < 0.01). Likewise, the augmented vasoconstriction found in the long-term diabetic rat arterioles compared with the age-matched controls was abolished by NOS inhibition (group difference: P = 0.4). Neither the vasoconstrictor responsiveness (Fig. 2C) nor the sensitivity to NE (EC50: PSS only 1.8E−7 ± 4.2E−8 M vs. L-NAME 1.0E−7 ± 2.8E−8 M; P = 0.18) was altered by L-NAME in arterioles from prediabetic rats, whereas NOS inhibition in the arterioles from Control-7 rats resulted in a significant dose by treatment interaction resulting in increased vasoconstriction at given doses of NE [Fig. 2A; NE (log M): −9, −8, −7, −6: P < 0.01, −5: P ≤ 0.1] and an increased sensitivity to NE (EC50: PSS only 0.4E−8 ± 1.5E−7 M vs. L-NAME 2.2E−8 ± 4.6E−9 M; P < 0.05). Similarly, NOS inhibition led to a significant dose by treatment interaction resulting in increased vasoconstriction in the arterioles of Control-20 rats at given NE doses [Fig. 2B; NE (log M): −8: P < 0.05, −7 and −6: P < 0.01] but did not alter the responses to NE in the long-term diabetic rats (Fig. 2D). These data indicate that the modulator effect that NO has on NE-induced vasoconstriction under control conditions is ameliorated in arterioles from prediabetic and diabetic rats.
Fig. 2.
Vasoconstrictor responses of gastrocnemius muscle arterioles to NE (n = 8–10/ group) during nitric oxide synthase (NOS) inhibition with NG-nitro-L-arginine methyl ester (L-NAME). A and B: 7 (A)- and 20 (B)-wk-old control gastrocnemius muscle arteriolar responses to NE with and without L-NAME. C and D: prediabetic (C) and long-term diabetic (D) rat gastrocnemius muscle arteriolar responses to NE with and without L-NAME. Values are means ± SE. *Significant group by dose interaction for dose-response relations (P < 0.05).
ET-1 vasoconstriction
Similar to the NE response, there was a significant dose by group interaction resulting in increased vasoconstriction to given doses of ET-1 and an increased maximal vasoconstriction to ET-1 in the prediabetic gastrocnemius muscle arterioles [Fig. 3A; ET-1 (log M): −8.5 and −10.5: P ≤ 0.1; −8, −9, −9.5, and −10: P ≤ 0.05]. In the short-term diabetic rats, there was a greater sensitivity to ET-1 (EC50: Control-13 6.2E−9 ± 2.0E−9 M vs. short-term 10.0E−10 ± 4.4E−10 M; P < 0.05), although no dose by group interaction was found at this time point (Fig. 3B). Furthermore, no dose by group interaction, difference in maximal vasoconstriction, or change in sensitivity to ET-1 was found during long-term diabetes (Fig. 3C). Sensitivity to ET-1 did not differ among age groups (7 wk vs. 13 wk vs. 20 wk) either within the prediabetic and diabetic groups or within the control groups.
Fig. 3.
Vasoreactivity to endothelin-1 (ET-1) in gastrocnemius muscle arterioles. Concentration-response relations for ET-1 in gastrocnemius muscle arterioles from prediabetic (A) and short-term (B) and long-term (C) diabetic ZDF rats compared with age-matched control rats are shown (n = 8/group). Values are means ± SE. *Significant group by dose interaction for dose-response relations; †significant differences in maximal responses (P < 0.05).
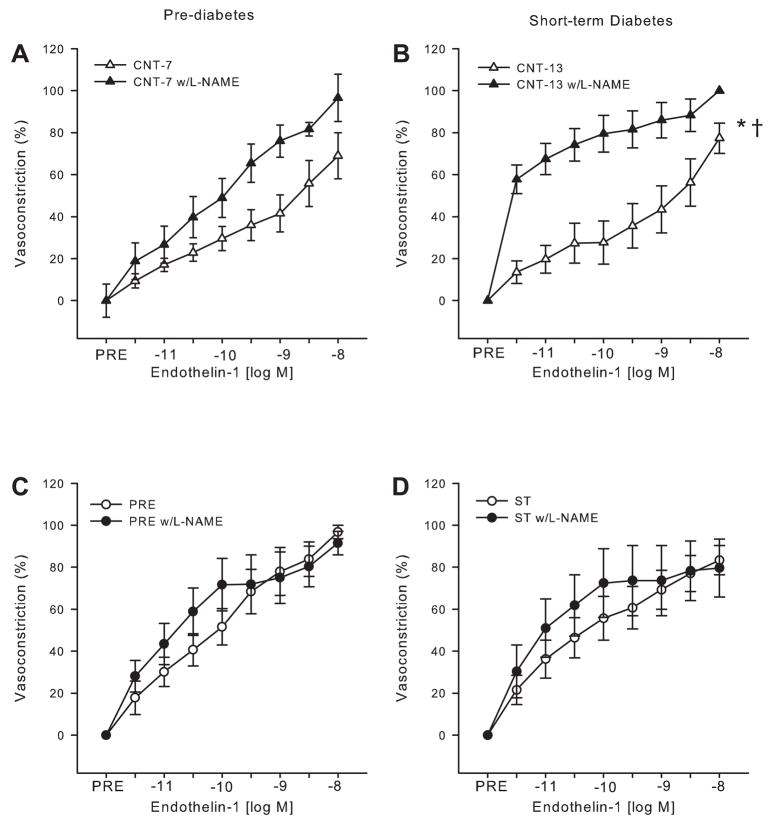
To determine the influence of the NOS signaling pathway on ET-1-elicited vasoconstriction, ET-1 dose responses were repeated after NOS inhibition. These experiments were performed at time points that had previously demonstrated enhanced ET-1 vasoconstrictor reactivity, i.e., the prediabetic and short-term diabetic conditions. NOS inhibition increased tone in the gastrocnemius muscle arterioles from prediabetic and short-term diabetic rats as well as their age-matched controls (Table 1).
Group differences occurring in the original dose-responses to ET-1 were abolished after incubation with L-NAME. Inhibition of NOS resulted in a trend (P ≤ 0.1) for greater maximal vasoconstriction of arterioles from Control-7 rats (Fig. 4A), whereas inhibition of NOS resulted in greater vasoconstriction of arterioles from Control-13 rats at all doses of ET-1 [Fig. 4B; ET-1 (log M): −11.5, −11, −10.5, −10, −9.5, −9, and −8: P ≤ 0.01; −8.5: P < 0.05]. Maximal vasoconstriction induced by ET-1 in arterioles from prediabetic (Fig. 4C) and short-term diabetic (Fig. 4D) rats was unchanged by L-NAME. These results suggest that the increases in sensitivity to NE and ET-1 that were originally found in the absence of L-NAME inhibition are an indirect effect of a decrease in NO bioavailability rather than a specific change in receptor sensitivity to the agonists.
Fig. 4.
Vasoconstrictor responses of gastrocnemius muscle arterioles to ET-1 (n = 6–8/ group) during NOS inhibition with L-NAME. A and B: 7 (A)- and 13 (B)-wk-old control gastrocnemius muscle arteriolar responses to ET-1 with and without L-NAME. C and D: prediabetic (C) and short-term diabetic (D) rat gastrocnemius muscle arteriolar responses to ET-1 with and without L-NAME. Values are means ± SE. *Significant group by dose interaction for dose-response relations (P < 0.05); †significant differences in maximal responses (P < 0.05).
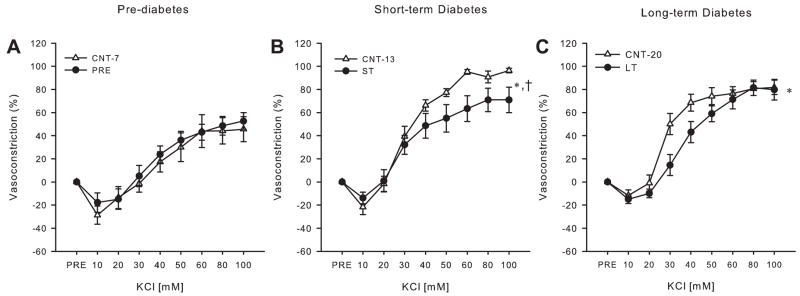
KCl vasoconstriction
In contrast to the NE and ET-1 responses, KCl-induced vasoconstriction was not different between Control-7 and prediabetic rats (Fig. 5A). During short-term diabetes maximal KCl-mediated vasoconstriction, but not sensitivity, was diminished in gastrocnemius muscle arterioles [Fig. 5B; KCl (mM): 50 and 80: P ≤ 0.1; 60 and 100: P < 0.05], a time point at which no differences in NE-mediated and increased ET-1-mediated vasoconstriction were observed. Moreover, a significant dose by group interaction and resultant decreases in vasoconstriction to given doses of KCl were found during long-term diabetes [Fig. 5C; KCl (mM): 30 and 40: P < 0.05]. In addition, there was a decrease in sensitivity to KCl in arterioles from the long-term diabetic rats (EC50: Control-20 29.6 ± 2.4 mM vs. long-term 40.4 ± 3.3 mM; P ≤ 0.01) (Fig. 5C). There were no differences in EC50 in the gastrocnemius muscle arterioles from diabetic rats across age groups, although sensitivity was higher in Control-13 and Control-20 rats relative to Control-7 rats.
Fig. 5.
Vasoreactivity to isotonic KCl in gastrocnemius muscle arterioles. Concentration-response relations for isotonic KCl in gastrocnemius muscle arterioles from prediabetic (A) and short-term (B) and long-term (C) diabetic ZDF rats compared with age-matched control rats are shown (n = 9–11/group). Values are means ± SE. *Significant group by dose interaction for dose-response relations; †significant differences in maximal responses (P < 0.05).
Arteriolar mechanical properties
Myogenic vasoconstriction was not altered in the prediabetic condition, although the passive distension of gastrocnemius muscle arterioles was greater at low intraluminal pressures [Fig. 6A (mmHg): 0: P < 0.05, 11: P < 0.1]. In short-term diabetic rats, no differences in the active myogenic response or passive distension occurred between groups (Fig. 6B). However, myogenic vasoconstrictor responsiveness was greater in long-term diabetic rats (Fig. 6C; group difference: P ≤ 0.01). Concurrent with this increase in active vasoconstriction to changes in intraluminal pressure, vessel passive distension was not different between long-term diabetic rats and age-matched controls (Fig. 6C). Furthermore, examination of the passive mechanical properties of the arterial wall revealed no differences in incremental stiffness in the gastrocnemius muscle arterioles between prediabetic or diabetic rats and their age-matched controls (data not shown).
Fig. 6.
Active (n = 8–10/group) and passive (n = 11–13/group) pressure-diameter relations of gastrocnemius muscle arterioles in prediabetic (A) and short-term (B) and long-term (C) diabetic ZDF rats. Values are means ± SE. *Significant differences in pressure-diameter relations (P < 0.05).
Adrenergic vasodilation
No differences in sensitivity (IC50: Control-7 4.6E −8 ± 1.7E−8 M vs. prediabetic 4.4E−8 ± 2.1E−8 M; P= 0.92) or dose by group interaction to Iso were found between prediabetic and age-matched control rats. However, maximal Iso-induced vasodilation (10−6 M) was lower in prediabetes compared with age-matched control rats (Fig. 7A, P < 0.05). With short-term diabetes, these differences became more pronounced such that significant dose by group interactions resulting in reductions in vasodilation to given doses of Iso and a lower maximal vasodilation were found during both short-term [Fig. 7B; Iso (log M): −7, −6, −5: P < 0.05] and long-term [Fig. 7C; Iso (log M): −7, −6, −5: P < 0.05] diabetes. No differences in sensitivity to Iso were found during either short-term or long-term diabetes compared with respective age-matched controls.
Fig. 7.
Concentration-response relations to isoproterenol in gastrocnemius muscle arterioles from prediabetic (A) and short-term (B) and long-term (C) diabetic ZDF rats compared with age-matched control rats (n = 8–10/group). Values are means ± SE. *Significant group by dose interaction for dose-response relations; †significant differences in maximal responses (P < 0.05).
Endothelium-dependent vasodilation
Unlike that which was observed for Iso, there was no impairment of ACh-mediated vasodilation of arterioles from prediabetic and diabetic rats relative to age-matched controls (Table 3). However, NOS inhibition with L-NAME indicated that although the dilator responses were preserved in prediabetic and diabetic arterioles, there was a diminished NO component to this vasodilation (Table 3). In the control rats, L-NAME significantly blunted ACh-mediated vasodilation at all time points examined. Although still significant, by 20 wk of age the contribution of NO to ACh-induced vasodilation diminished from ~50% in Control-7 and Control-13 animals to ~30% in Control-20 rats; however, this reduction in the NO contribution across age failed to reach significance (P = 0.11). In ZDF rats, although there appears to be a reduction in the vasodilation to ACh with L-NAME blockade across the age groups, this difference failed to reach significance (P = 0.13). Despite the preserved maximal ACh-mediated dilation in ZDF rats compared with their age-matched controls, NO did not significantly contribute to ACh-induced vasodilation in ZDF rats. This is in contrast to the control rats, in which NO accounted for ~30–50% of the dilation in response to ACh.
Table 3. Maximal responses and concentrations eliciting half-maximal response to acetylcholine in absence and presence of nitric oxide synthase inhibitor L-NAME, sodium nitroprusside, and KCl in gastrocnemius muscle arterioles from prediabetic, short-term and long-term diabetic, and age-matched control rats.
| ACh (n = 8–12) |
ACh + L-NAME (n = 5–10) |
SNP (n = 7–9) |
|||||
|---|---|---|---|---|---|---|---|
| Group | Max, % | IC50 (× 10−7 M) | Max, % | IC50 (× 10−7 M) | Max, % | IC50 (× 10−7 M) | 10 mM KCl Max, % (n = 8–12) |
| Cnt-7 | 80±5 | 1.6±0.9 | 33±24*† | 7.1±6.3 | 59±11 | 26±13 | 30±9 |
| Pre | 76±7 | 1.7±0.7 | 83±7 | 1.1±0.7 | 73±6 | 0.2±0.09* | 22±12 |
| Cnt-13 | 83±6 | 1.3±0.7 | 29±15*† | 11.3±6.9 | 79±4 | 1.3±0.5 | 25±8 |
| ST | 66±11 | 1.2±0.7 | 68±9 | 2.8±0.7 | 73±7 | 3.2±1.6 | 17±7 |
| Cnt-20 | 87±4 | 0.6±0.2 | 61±10† | 1.2±0.4 | 56±9 | 0.6±0.2 | 18±7 |
| LT | 68±11 | 0.5±0.2 | 57±11 | 0.5±0.3 | 66±10 | 0.4±0.1 | 17±4 |
Values are means ± SE for n animals studied per group. ACh, acetylcholine; Max, maximal response; IC50, concentration eliciting half-maximal response; SNP, sodium nitroprusside;
Significant difference vs. age-matched responses;
significant difference compared with ACh Max (P < 0.05).
Endothelium-independent vasodilation
Maximal vasodilator responses to SNP did not differ between Control-7 and prediabetic arterioles or between arterioles from short-term or long-term diabetic rats and their age-matched controls. Sensitivity to SNP was enhanced in the prediabetic arterioles, whereas no differences in arteriolar sensitivity to SNP were evident in short-term or long-term diabetes. Isotonic KCl, which elicits membrane hyperpolarization at 10 mM, did not result in any impairment of potential-mediated smooth muscle vasodilator responses in prediabetic and diabetic rats compared with age-matched controls (Table 3).
eNOS and SOD-1 protein expression
The eNOS-to-GAPDH protein ratio (Control-7 7.5 ± 3.6 vs. prediabetic 6.4 ± 2.7; Control-13 wk 3.5 ± 1.2 vs. short-term 2.0 ± 1.4; Control-20 1.0 ± 0.5 vs. long-term 0.6 ± 0.1) and the SOD-1-to-GAPDH protein ratio (Control-7 7.5 ± 3.4 vs. prediabetic 4.5 ± 1.2; Control-13 2.7 ± 0.7 vs. short-term 1.5 ± 0.7; Control-20 1.8 ± 1.1 vs. long-term 1.1 ± 0.2) were not different between groups at any age studied (n = 8–10/group).
DISCUSSION
Microvascular reactivity has previously been shown to be related to both peripheral insulin sensitivity and blood pressure in normal healthy subjects (47), so the emergence of a proconstrictor phenotype in the microcirculation could be a significant contributor to the development of both overt diabetes and hypertension. Although a number of studies have examined vascular function in the metabolic syndrome (19–24, 40, 46, 52), to our knowledge only one has examined changes in vascular function during a time course of type 2 diabetes (41) and none has done so in the skeletal muscle microcirculation or included a time point prior to the emergence of hyperglycemia.
Vascular research conducted with the OZR rat, a model of the metabolic syndrome, may not be as specific to type 2 diabetes as a result of the extreme obesity of these animals. By 15 wk of age the OZR rat has been reported to have almost double the body mass of its lean age-matched counterpart (~600 vs. ~350 g) (20, 23). Because obesity is known to impair endothelial function independent of diabetes, it is impossible to separate the effects of obesity from those of diabetes in the OZR model. In contrast, the body mass of the ZDF rat is slightly greater than that of its lean counterpart during prediabetes (~220 vs. ~170 g), a time at which the rat is hyperinsulinemic but not yet hyperglycemic. With the onset of overt diabetes, body mass remains greater than that of control animals (~360 vs. ~330 g) but never reaches the same magnitude as that observed in the OZR animals, and at this time the ZDF rats are both hyperglycemic and hyperinsulinemic (short-term diabetes). Finally, as diabetes is sustained (long-term diabetes) there is no longer a difference in body mass between ZDF and control rats (~410 vs. ~390 g). In fact, the absolute body mass of the ZDF rat is lower than that of the lean age-matched control animals, and the animals remain hyperglycemic but are no longer hyperinsulinemic. Thus the present study was designed to take advantage of these known changes in glucose and insulin to examine vascular function at distinct time points in type 2 diabetes independent of the dramatic increases in body and fat mass observed in the OZR model.
In the present study, the data demonstrate that enhanced skeletal muscle arteriolar reactivity to NE and ET-1 occur before the onset of hypertension and these alterations are largely due to reductions in NO signaling. Although a reduction in NO bioavailability was shown previously in type 2 diabetes, a novel finding of the present study is that this reduction in NO signaling in the skeletal muscle vasculature precedes the development of overt diabetes and contributes to the enhanced vasoconstrictor responses in this vascular bed. With short-term diabetes, the onset of hypertension occurs concurrently with greater arteriolar sensitivity to ET-1 and KCl, whereas long-term diabetes and the concurrent hypertension are associated with enhanced NE and myogenic vasoconstriction in skeletal muscle arterioles. Furthermore, during prediabetes and each stage in the progression of diabetes there is diminished β-adrenergic vasodilation.
The ZDF rat exhibits a period of insulin resistance at 7 wk of age (8, 17, 18, 25, 42, 50, 51, 57), a condition referred to as prediabetes, such that there is a significant decrease in glucose uptake in isolated skeletal muscles (17). During this prediabetic condition, normal fasted blood glucose and elevated plasma insulin are being manifested (Table 1). At ~10–12 wk of age a transition to overt diabetes occurs, with the development of marked hyperglycemia (Table 1). In the present study, we demonstrate that the development of overt diabetes is also associated with the emergence of hypertension. As a result, the vasculature of this rat model is exposed to a changing chemical milieu and mechanical environment that have the potential to alter vasomotor responsiveness. Thus, with the onset of overt short-term diabetes, the chemical environment of the arterioles has progressed from one of transient postprandial hyperglycemia in the face of chronic hyperinsulinemia to one of marked chronic hyperglycemia and hyperinsulinemia. With the further progression of long-term diabetes, the ZDF rat can be characterized as being hyperglycemic and hypoinsulinemic (Table 1). Because changes in both plasma glucose (33, 34) and insulin (43) concentration have been shown to affect vascular function, these divergent factors may contribute to differential alterations in vascular responsiveness. This progression is in contrast to the OZR rat, which remains hyperinsulinemic throughout at least 32 wk of age and is a model representative of metabolic syndrome and not type 2 diabetes per se (41).
Increased responsiveness to the vasoconstrictor effects of both NE (Fig. 1A) and ET-1 (Fig. 3A), as well as reduced β-adrenergic-mediated vasodilation (Fig. 7A), occur before the onset of diabetes in arterioles from the gastrocnemius, a skeletal muscle composed predominantly of fast-twitch fibers (10). The resultant effect of this proconstrictor phenotype in arterioles from fast-twitch skeletal muscle of prediabetic rats is potentially significant, since fast-twitch low-oxidative muscle accounts for ~70% of the total skeletal muscle mass in the rat (10) and skeletal muscle as a whole receives ~18% of the total cardiac output at rest (12). Therefore, an increase in vasoconstrictor tone in this muscle type could contribute significantly to the development of hypertension and insulin resistance. Furthermore, previous investigations have reported that both sympathetic nerve activity (16) and plasma ET-1 concentration (13) are increased in obesity. If these observations are applicable to ZDF rats, enhanced vasoconstrictor responsiveness in the face of increased sympathetic drive and elevated ET-1 concentrations would act to further exacerbate vasoconstrictor tone, increases in blood pressure, and insulin resistance.
In the present study there were no alterations in maximal vasodilator responses to ACh, 10 mM KCl, and SNP, whereas adrenergic-mediated vasodilation was diminished. This suggests that there may be β2-receptor-specific defects in vasodilator function rather than a generalized defect in vasodilator capacity that occurs in conjunction with increases in vasoconstriction. Interestingly, the increases in vasoconstriction observed in the present study appear to result from decrements in NO bioavailability (Figs. 2 and 4), which is consistent with the ACh responses, where the contribution of NO to the vasodilator responses was diminished in prediabetic and diabetic rats. In response to ACh administration, however, it appears that other vasodilator substances, such as endothelium-derived hyperpolarizing factors or prostanoid dilators, are able to compensate for the reduction in NO bioavailability. Thus our data demonstrate that reduced NO-mediated vasodilation opposing the adrenergic- and ET-1-mediated vasoconstriction leads to a net increase in vasoconstriction that may be further exacerbated by reductions in adrenergic vasodilator responses. The resultant effect of these two influences would be a proconstrictor phenotype in the skeletal muscle arterioles of the prediabetic and diabetic rats.
Although an NO-mediated mechanism for Iso-induced vasodilation has been described with venous occlusion plethys-mography in humans (44), studies from our laboratory (14) and others (35) have demonstrated that Iso induces vasodilation in an endothelium- and NO-independent manner in both rat skeletal muscle and coronary arterioles, respectively. The diminished adrenergic vasodilation could result from a generalized smooth muscle vasodilator dysfunction, but this does not appear to be the case since ACh-, SNP-, and KCl-mediated vasodilation were preserved in these arterioles. Given that β-adrenergic receptor-mediated vasodilation to Iso occurs through the activation of the voltage-gated potassium channels and the activation of adenylate cyclase (35), whereas NO-induced vasodilation occurs predominantly through cGMP-mediated reductions in intracellular calcium (56), these data suggest that there may be a specific defect in adrenergic signaling through cAMP.
The diminished NO counterinfluence to the NE and ET-1 vasoconstrictor responses is consistent with the notion of diminished NO bioavailability in this model and is further supported by the reduction in NO-mediated dilation in response to stimulation by ACh (Table 3). Reduced substrate or cofactors (e.g., L-arginine, tetrahydrobiopterin) and increases in reactive oxygen species could contribute to a loss of NO bioavailability. Oltman et al. (41) demonstrated increased lipid peroxidation in the serum of ZDF rats as young as 8 wk of age, with the level of oxidation increasing through 40 wk. This suggested that reactive oxygen species may indeed play a role in reducing the activity of NO in these rats. To determine whether a reduction in the enzyme responsible for NO production, eNOS, or a decrease in the free radical scavenger SOD-1 is associated with the altered NOS signaling mechanism, we measured gastrocnemius muscle arteriolar protein content of these enzymes. However, we found no differences in protein expression for either enzyme, suggesting that the quantity of these proteins is not affecting NO signaling. However, the possibility remains that enzyme activity and not protein content per se is being altered in the prediabetic and diabetic conditions, a possibility that requires further elucidation.
In conclusion, the present study examined changes in vascular function resulting from type 2 diabetes at time points that are distinct in the progression of this disease. The skeletal muscle microvasculature was chosen because of its large contribution to both glucose uptake and peripheral resistance, and first-order arterioles from the gastrocnemius muscle were selected to represent the resistance vasculature in fast-twitch skeletal muscle tissue. The present study expands current knowledge by including an examination of the time course of both the changes in vascular function and the emergence of hypertension in the ZDF model. In this skeletal muscle arteriole, increased vasoconstrictor responses to NE and ET-1 in prediabetes are mediated through a decrement in the counter-influence of the NOS signaling mechanism. This enhanced vasoconstriction, combined with diminished β-adrenergic vasodilation, could contribute to the later increases in MAP and further deterioration of insulin sensitivity. Our present working model suggests that an early augmentation in adrenergic- and ET-1-mediated vasoconstriction contributes to the coordinated emergence of hypertension and diabetes. The enhancement in vasoconstrictor responsiveness in this prediabetic state may result from increases in sympathetic nervous system activity (16), circulating ET-1 (13), and increased plasma insulin (45). However, in long-term diabetes prolonged exposure to these agonists augments myogenic vasoconstriction, which may act to maintain the increases in blood pressure even in the face of adrenergic and ET-1 receptor desensitization (Figs. 1 and 3). Furthermore, these results suggest that interventions aimed at increasing NO bioavailability during the prediabetic state may serve to combat the hypertension associated with the onset of type 2 diabetes.
Acknowledgments
This study was supported by National Aeronautics and Space Administration Grants NAG2-1340 and NCC2-1166 and National Institutes of Health Grants AG-00988, AG-525622, DC-006459, HL-58503, HL-36088, and P01-HL-077670.
References
- 1.Anonymous Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation. 1999;100:1132–1133. doi: 10.1161/01.cir.100.10.1132. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD, Clark MG. Role of blood flow in the regulation of muscle glucose uptake. Annu Rev Nutr. 1997;17:487–499. doi: 10.1146/annurev.nutr.17.1.487. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG, Lash JM. Endothelial-dependent vasodilation is preserved in non-insulin-dependent Zucker fatty diabetic rats. Am J Physiol Heart Circ Physiol. 1995;268:H2366–H2374. doi: 10.1152/ajpheart.1995.268.6.H2366. [DOI] [PubMed] [Google Scholar]
- 5.Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–2114. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA. The Zucker-fatty rat: a review. Fed Proc. 1977;36:148–153. [PubMed] [Google Scholar]
- 7.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 8.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis. 2000;148:231–241. doi: 10.1016/s0021-9150(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 9.Cox RH, Kikta DC. Age-related changes in thoracic aorta of obese Zucker rats. Am J Physiol Heart Circ Physiol. 1992;262:H1548–H1556. doi: 10.1152/ajpheart.1992.262.5.H1548. [DOI] [PubMed] [Google Scholar]
- 10.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 11.Delp MD, Manning RO, Bruckner JV, Armstrong RB. Distribution of cardiac output during diurnal changes of activity in rats. Am J Physiol Heart Circ Physiol. 1991;261:H1487–H1493. doi: 10.1152/ajpheart.1991.261.5.H1487. [DOI] [PubMed] [Google Scholar]
- 12.Delp MD, O’Leary DS. Integrative control of the skeletal muscle micro-circulation in the maintenance of arterial pressure during exercise. J Appl Physiol. 2004;97:1112–1118. doi: 10.1152/japplphysiol.00147.2003. [DOI] [PubMed] [Google Scholar]
- 13.Donatelli M, Colletti I, Bucalo ML, Russo V, Verga S. Plasma endothelin levels in NIDDM patients with macroangiopathy. Diabetes Res. 1994;25:159–164. [PubMed] [Google Scholar]
- 14.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 16.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 17.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000;49:684–688. doi: 10.1016/s0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 18.Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 19.Frisbee JC. Enhanced arteriolar α-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–772. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 20.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1124–R1134. doi: 10.1152/ajpregu.00239.2003. [DOI] [PubMed] [Google Scholar]
- 21.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289:R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 22.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2003;285:H104–H111. doi: 10.1152/ajpheart.00118.2003. [DOI] [PubMed] [Google Scholar]
- 23.Frisbee JC. Vascular adrenergic tone and structural narrowing constrain reactive hyperemia in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290:H2066–H2074. doi: 10.1152/ajpheart.01251.2005. [DOI] [PubMed] [Google Scholar]
- 24.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283:H2160–H2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- 25.Garnett KE, Chapman P, Chambers JA, Waddell ID, Boam DS. Differential gene expression between Zucker Fatty rats and Zucker Diabetic Fatty rats: a potential role for the immediate-early gene Egr-1 in regulation of beta cell proliferation. J Mol Endocrinol. 2005;35:13–25. doi: 10.1677/jme.1.01792. [DOI] [PubMed] [Google Scholar]
- 26.Granger HJ, Goodman AH, Granger DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res. 1976;38:379–385. doi: 10.1161/01.res.38.5.379. [DOI] [PubMed] [Google Scholar]
- 27.Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol. 1999;84:11J–14J. doi: 10.1016/s0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- 28.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 29.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 30.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44:1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 31.Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol. 1999;87:1476–1482. doi: 10.1152/jappl.1999.87.4.1476. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Koshimura K, Murakami Y, Tsumori M, Gonda T, Kato Y. Antihypertensive effect of insulin via nitric oxide production in the Zucker diabetic fatty rat, an animal model for non-insulin-dependent diabetes mellitus. Eur J Endocrinol. 1999;140:341–349. doi: 10.1530/eje.0.1400341. [DOI] [PubMed] [Google Scholar]
- 33.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 34.Lash JM, Sherman WM, Hamlin RL. Capillary basement membrane thickness and capillary density in sedentary and trained obese Zucker rats. Diabetes. 1989;38:854–860. doi: 10.2337/diab.38.7.854. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP- mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 36.Lindbom L, Arfors KE. Mechanisms and site of control for variation in the number of perfused capillaries in skeletal muscle. Int J Microcirc Clin Exp. 1985;4:19–30. [PubMed] [Google Scholar]
- 37.McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol. 2000;89:398–405. doi: 10.1152/jappl.2000.89.1.398. [DOI] [PubMed] [Google Scholar]
- 38.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- 39.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 40.Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R154–R161. doi: 10.1152/ajpregu.00245.2005. [DOI] [PubMed] [Google Scholar]
- 41.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780–H1787. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 42.Peterson R, Shaw W, Neel M, Little L, Eichberg J. Zucker diabetic fatty rat as a model for non-insulin dependent diabetes mellitus. ILAR J. 1990;32 doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pieper GM, Meier DA, Hager SR. Endothelial dysfunction in a model of hyperglycemia and hyperinsulinemia. Am J Physiol Heart Circ Physiol. 1995;269:H845–H850. doi: 10.1152/ajpheart.1995.269.3.H845. [DOI] [PubMed] [Google Scholar]
- 44.Schlaich MP, Ahlers BA, Parnell MM, Kaye DM. β-Adrenoceptor-mediated, nitric-oxide-dependent vasodilatation is abnormal in early hypertension: restoration by L-arginine. J Hypertens. 2004;22:1917–1925. doi: 10.1097/00004872-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the Zucker Diabetic Fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol. 2003;163:21–28. doi: 10.1016/S0002-9440(10)63626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder CA, Jr, Chen YL, Messina EJ. Inhibition of NO synthesis or endothelium removal reveals a vasoconstrictor effect of insulin on isolated arterioles. Am J Physiol Heart Circ Physiol. 1999;276:H815–H820. doi: 10.1152/ajpheart.1999.276.3.H815. [DOI] [PubMed] [Google Scholar]
- 47.Serne EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJ, Gans RO. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- 48.Sexl V, Mancusi G, Raberger G, Schutz W. Age-related changes in vascular reactivity in genetically diabetic rats. Pharmacology. 1995;50:238–246. doi: 10.1159/000139288. [DOI] [PubMed] [Google Scholar]
- 49.Sparks JD, Phung TL, Bolognino M, Cianci J, Khurana R, Peterson RG, Sowden MP, Corsetti JP, Sparks CE. Lipoprotein alterations in 10-and 20-week-old Zucker diabetic fatty rats: hyperinsulinemic versus insulinopenic hyperglycemia. Metabolism. 1998;47:1315–1324. doi: 10.1016/s0026-0495(98)90298-0. [DOI] [PubMed] [Google Scholar]
- 50.Sparks JD, Shaw WN, Corsetti JP, Bolognino M, Pesek JF, Sparks CE. Insulin-treated Zucker diabetic fatty rats retain the hypertriglyceridemia associated with obesity. Metabolism. 2000;49:1424–1430. doi: 10.1053/meta.2000.17736. [DOI] [PubMed] [Google Scholar]
- 51.Sreenan S, Keck S, Fuller T, Cockburn B, Burant CF. Effects of troglitazone on substrate storage and utilization in insulin-resistant rats. Am J Physiol Endocrinol Metab. 1999;276:E1119–E1129. doi: 10.1152/ajpendo.1999.276.6.E1119. [DOI] [PubMed] [Google Scholar]
- 52.Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282:H816–H820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- 53.Sweeney TE, Sarelius IH. Arteriolar control of capillary cell flow in striated muscle. Circ Res. 1989;64:112–120. doi: 10.1161/01.res.64.1.112. [DOI] [PubMed] [Google Scholar]
- 54.Tooke JE. Microvasculature in diabetes. Cardiovasc Res. 1996;32:764–771. [PubMed] [Google Scholar]
- 55.Wiernsperger N. Vascular defects in the aetiology of peripheral insulin resistance in diabetes. A critical review of hypotheses and facts. Diabetes Metab Rev. 1994;10:287–307. doi: 10.1002/dmr.5610100305. [DOI] [PubMed] [Google Scholar]
- 56.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 57.Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, Ohlstein EH, Jucker BM. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554 –562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]