Abstract
Background
White matter fiber tracts, especially those interconnecting the frontal and temporal lobes, are likely implicated in pathophysiology of schizophrenia. Very few studies, however, have focused on the fornix, a compact bundle of white matter fibers, projecting from the hippocampus to the septum, anterior nucleus of the thalamus and the mamillary bodies. Diffusion Tensor Imaging (DTI), and a new post-processing method, fiber tractography, provides a unique opportunity to visualize and to quantify entire trajectories of fiber bundles, such as the fornix, in vivo. We applied these techniques to quantify fornix diffusion anisotropy in schizophrenia.
Methods
DTI images were used to evaluate the left and the right fornix in 36 male patients diagnosed with chronic schizophrenia and 35 male healthy individuals, group matched on age, parental socioeconomic status, and handedness. Regions of interest were drawn manually, blind to group membership, to guide tractography, and Fractional Anisotropy (FA), a measure of fiber integrity, was calculated and averaged over the entire tract for each subject. The Doors and People test (DPT) was used to evaluate visual and verbal memory, combined recall and combined recognition.
Results
Analysis of variance was performed and findings demonstrated a difference between patients with schizophrenia and controls for fornix FA (P=0.006). Protected post-hoc independent sample t-tests demonstrated a bilateral FA decrease in schizophrenia, compared with control subjects (left side: P=0.048; right side P=0.006). Higher fornix FA was statistically significantly correlated with DPT and measures of combined visual memory (r=.554, p=.026), combined verbal memory (r=.647, p=.007), combined recall (r=.516, p=.041), and combined recognition (r=.710, p=.002) for the control group. No such statistically significant correlations were found in the patient group.
Conclusions
Our findings show the utility of applying DTI and tractography to study white matter fiber tracts in vivo in schizophrenia. Specifically, we observed a bilateral disruption in fornix integrity in schizophrenia, thus broadening our understanding of the pathophysiology of this disease.
INTRODUCTION
Prior to the advent of Diffusion Tensor Imaging (DTI), most magnetic resonance imaging studies of schizophrenia focused on gray matter (see review in Shenton et al., 2001). With the advent of DTI, however, we are now able to focus more closely on white matter integrity in schizophrenia. This technique is relatively recent and it depends upon the motion of water molecules to provide structural information in vivo (Basser, 1994; Pierpaoli, 1996). More specifically, DTI is dependent upon the structural environment of the brain, which modifies water according to the type of tissue involved. For example, myelin sheaths and nerve fibers restrict the motion of water in directions that are perpendicular to the fiber tracts. Other tissue properties such as the density of axons and dendrites, axon diameter, thickness of myelin tissue, as well as the organization and orientation of fibers, all affect the diffusion of water in the brain and thus provide important information about tissue integrity. Moreover, DTI is particularly useful for evaluating brain tissue where water diffusion is anisotropic, or unevenly restricted in direction (e.g., white matter), as opposed to isotropic or non-restricted, or evenly restricted diffusion (e.g., cerebral spinal fluid, gray matter).
DTI studies of white matter in schizophrenia have focused on large white matter regions (e.g., Buchsbaum et al., 1998; Lim et al., 1999; Kumra et al., 2004), as well as on smaller brain regions of interest (e.g., Burns et al., 2003; Kubicki et al., 2003; Wang et al., 2004) (See also reviews in Kubicki et al., 2005; Kanaan et al., 2005). Most of these studies have used rotationally invariant indices of diffusion anisotropy, most commonly Fractional Anisotropy (FA), a measure of the fraction of the magnitude of the tensor that constitutes the anisotropic diffusion (Basser, 1995). Most have also focused on region of interest (ROI) or voxel based morphology (VBM) analyses, and it is only more recently that tractography measures have been used to evaluate white matter fiber tracts in the brain (e.g., Jones et al., 2006; Kanaan et al., 2006).
Of further note, white matter fiber tracts, especially those interconnecting the frontal and temporal lobes, have long been thought to be involved in schizophrenia (e.g., Wernicke 1906, and more recently Weinberger et al., 1992; McGuire et al., 1996). Recently, DTI studies in schizophrenia have focused on individual association fiber bundles and have reported abnormalities in the cingulum bundle (Kubicki et al., 2003; Sun et al., 2003; Wang et al., 2004; Mori et al., 2007; Fujiwara et al., 2007), uncinate fasciculus (Kubicki et al 2002; Burns et al., 2003), and arcuate fasciculus (Burns et al., 2003; Hubl et al., 2004). The corpus callosum has also been evaluated where abnormalities have also been reported in schizophrenia (Fong et al., 2000; Agartz et al., 2001; Ardekani et al., 2003; Kannan et al., 2006; Mori et al., 2007).
The current study focuses on the fornix, a compact bundle of white matter fibers projecting from the hippocampus to the septum, anterior nucleus of the thalamus and the mamillary bodies. This structure is involved in important brain functions such as spatial memory (e.g., Gaffan 1994; Parker and Gaffan 1997), memory retrieval (e.g., Calabrese et al., 1995), and verbal memory (e.g., Calabrese et al., 1995; Mc Mackin et al., 1995). These are also all functions disturbed in schizophrenia, including spatial memory (e.g., Park and Holzman,1992; Carter et al., 1996 and Park et al., 1999) memory retrieval (e.g., Anderson and Spellman, 1995) and verbal memory (e.g., Park and Holzman,1992; Carter et al., 1998; Cohen et al., 1997; Callicott et al., 2000; Perlstein et al., 2001). Thus characterizing disruptions in fornix integrity might further our understanding of this disorder. It is nonetheless important to emphasize that the fornix is an integral part of both verbal and spatial memory networks, which also involves various other interconnected brain structures that we did not investigate here, including prefrontal cortex, temporal and limbic structures, as well as the parieto-occipital association cortex. Of further note, the hippocampus is one of the most frequently implicated brain structures that has been consistently reported to be abnormal in schizophrenia (e.g., Bogerts et al., 1985; Jeste and Lohr, 1989; Nelson et al., 1998; McCarley et al., 1999; Wright et al., 2000; Shenton et al., 2001; Heckers, 2001; Weiss et al., 2004), with volume reductions more prominent on the left side (see review in Shenton et al., 2001).
One of the main reasons for studying the fornix is because it is the main hippocampal output. Few studies have investigated the fornix in schizophrenia. Of those that have, Zahajszky and coworkers (2001), from our laboratory, found no MRI volumetric differences between healthy controls and patients with chronic schizophrenia. In contrast, Davies and coworkers (2001) showed an increase of cross-sectional area of the fornix in early onset schizophrenia. In the only post-mortem study evaluating the fornix, Chance and coworkers (1999) found increased fiber density in the left fornix in male subjects with schizophrenia.
To our knowledge there is only one DTI study of the fornix in schizophrenia (Kuroki et al., 2006). In this study, from our laboratory, the authors investigated a small, cross-sectional portion of the fiber tract, and found a decrease in fiber integrity in chronic schizophrenia subjects compared with controls. Recent advances in DTI post-processing-DT tractography, however, as proposed here, enable us to follow, and to quantify the entire tract, thus making it more reliable and more powerful, compared with region of interest or voxel-based analyses (Kanaan et al., 2005; Jones et al., 2005).
The main objective of the current study is to identify, separate and evaluate left and right fornix integrity in patients with chronic schizophrenia compared with healthy controls. Because previous work has demonstrated the relationship between verbal and spatial memory and fornix integrity (Nestor et al., 2007), we will also evaluate this relationship in schizophrenia.
METHODS
Subjects
DTI-MRI data was acquired to evaluate the right and the left fornix in 36 male patients diagnosed with chronic schizophrenia and 35 male healthy individuals. The population consisted of schizophrenic patients from the Brockton Veterans Administration Medical Center. Patients were diagnosed with schizophrenia based on the (DSM-IV) criteria. Normal controls were recruited through newspaper advertisement. The study was approved by the local IRB at both the VA and Brigham and Women’s Hospital. Following a description of the study, written informed consent was obtained from all subjects participating in the study. The clinical research interviews were conducted by a trained neuropsychologist. Groups were matched on age, gender (all males), handedness (Oldfield 1971), parental socio-economic status (PSES, Hollingshead 1965) and WRAT score for IQ (For details see Table 1).
Table 1.
Demographic data
| Mean (standard deviation) | p value | ||
|---|---|---|---|
| Schizophrenics n=36 | Controls n=36 | ||
| Sex (%male) | 100% | 100% | - |
| Age | 39.89 (9.06) | 39.59 (9.32) | 0.897 |
| PSES | 3.06 (.983) | 2.75 (1.34) | 0.278 |
| Handedness (%right) | 100% | 100% | - |
| WRAT | 97.29 (13.03) | 109.38(11.11) | 0.133 |
All patients were receiving antipsychotic medication (typical antipsychotics 9 of the 36; atypical antipsychotics 24 of the 36) and both (3 of the 36). All medication dosages were converted to chlorpromazine equivalents (Bezch-libnyk-Butler et al., 1996; Stoll 2001). Patients’ mean duration of illness was 17.5+/− 10.4 years, with a mean age of onset of 21.1+/− 4.1. Patients had no history of neurological problems, drug or alcohol abuse (within the last year). Clinical symptoms were measured using the Scale for the Assessment of Positive Symptoms (SAPS- Andreasen 1984) and the Scale for Assessment of Negative Symptoms (SANS- Andreasen 1981). In addition to the inclusion/exclusion criteria for patients, normal controls and their first-degree relatives had no history of mental disorder, drug or alcohol addiction. Additionally, we administered, the Doors and People test (DPT) to evaluate visual memory, verbal memory, combined recall and combined recognition (Baddeley et al., 1994).
MRI processing and acquisition
Images of all subjects were acquired with a 1.5 Tesla System GE Echospeed (General Electric Medical Systems, Milwaukee) using a Line Scan Diffusion MR imaging technique (Gudbjartsson et al 1996). This protocol is described in detail in our previous studies (Kubicki et al., 2003; Nakamura et al., 2005; Kuroki et al., 2006) and in contrast to single-shot EPI, which is reviewed in Kubicki et al. (2004). Briefly, we acquired coronal –oblique slices, aligned to the AC-PC line covering the entire brain. Six images with high diffusion-weighting (1000 sec/mm2) along six independent directions were collected. For low (5 sec/mm2) diffusion weighting, only two images were collected, because diffusion-related signal changes are minimal at low B values. The following scan criteria were used: field of view 220×165 mm, 128×128 scan matrix, slice thickness 4mm, interslice distance 1mm, NEX=1, TE (echo time) 64 msec, effective repetition time 2592 msec, scan time 60 sec/slice. The diffusion-weighted images were transferred to a workstation after reconstruction, where eigenvalue, eigenvector, and fractional anisotropy (FA) maps of the diffusion tensor were calculated. Motion-related artifact maps were also calculated, and later used for data correction.
Fiber Tracking
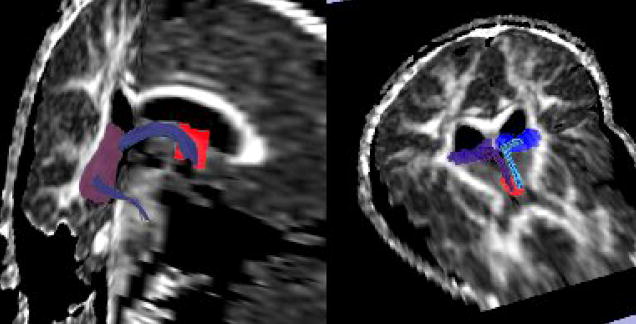
Fornix extraction and measurements were obtained using 3Dslicer software (www.slicer.org) and diffusion tensor tractography. Because the fornix is a small white matter structure and its fibers run close to other structures such as the corpus callosum and the anterior commissure, to segment the fornix is not a trivial issue. In order to ensure precise segmentation of the structure, we used multiple ROI method, which has been used before in several clinical tractography studies (e.g., Catani et al., 2002; Conturo et al.,1999; Jones et al., 2005). Five separate Regions of Interest (ROIs) were defined in order to tract the desired fibers, which are explained below. Only those pathways passing through all 5 ROIs were retained for further analysis. ROIs were manually drawn on the FA map, blind to diagnosis. The first ROI was placed on the most anterior coronal plane where the body of fornix is visible on the FA map, and then two more ROIs were placed on the next two consecutive slices using the corpus callosum, the contours of the lateral ventricles and the third ventricle, as landmarks. Finally, two additional ROIs, one on each side, were drawn on the two more posterior slices, including the hippocampus (tail), parahippocampal gyrus, and the crus fornicis (These ROIs can be seen in Figure 1). ROIs were drawn manually, with mean volumes being in the order of 8.30 cm2 on the left, 8.10 cm2 on the right and 2.91cm2 for the anterior ROI. After fornix tracts were extracted, the average values of FA and mean diffusivity were calculated for the entire tract, separately for each side, and subjected to statistical analysis.
Figure 1.
ROIs used to extract the fornix, along with the tracts (purple- right fornix, blue- left fornix). Middle ROIs were placed bilaterally.
Interrater Reliability
Interrater Reliability was calculated using an intraclass correlation coefficient for 7 cases, selected from the 71 cases at random, and redrawn using the same criteria, by a second rater blinded to diagnoses (KS). Intraclass correlation coefficients (Cronbach’s Alpha) achieved 0.985 for the right and 0.971 for the left fornix.
Sensitivity tool
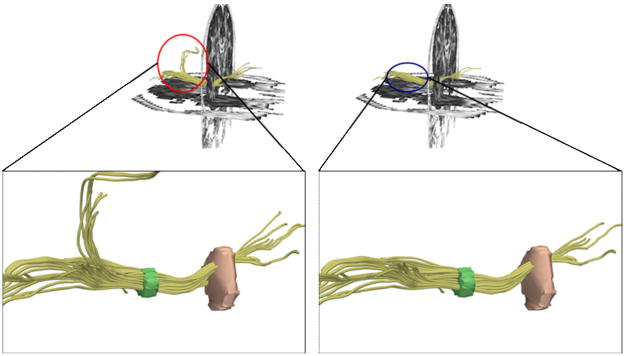
Because DTI scans are noisier and of lower resolution than the anatomical (structural) scans, fiber tractography usually produces a small percentage of extraneous “unwanted” fiber traces, that either need to be ignored, or removed. We developed a tool (currently part of the “slicer” package www.slicer.org) that can perform unbiased (automated) elimination of these fibers. This tool (described in detail in San Jose Estepar et al., 2006). For each point along the fiber, the sensitivity is computed by placing a Gaussian Kernel at that location and convolving the ROI image with the kernel. The output of the convolution is a value between 0 and 1, with higher values achieved by fibers running closer to the middle of the ROI. In addition, in order to accommodate fibers running in different orientations within single ROI, we use here an anisotropic kernel, with its width being proportional to the geodesic distant between the Diffusion Tensor at the fiber location and the neighbor tensors. In effect, if the principal diffusion directions (PDD) of the neighboring tensors are parallel to the PDD of the tensors at the fiber location, the kernel is an isotropic Gaussian with a std= 1.5 pixels. If the PDD of the neighboring tensors is not parallel, then the kernel width is reduced in a proportional way given by the geodesic distance between the tensors. Figure 2 demonstrates how the sensitivity tool can automatically eliminate erroneous fiber tracts (Figure 2).
Figure 2.
Figure demonstrating application of “sensitivity” tool to extract fibers of interest. Tool was able to exclude anatomically incorrect fibers (left panel before, right after tool application).
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS v.12.0). To test for group differences in FA within the fornix, analysis of variance (ANOVA) was performed, with side as the within- subject factor, and group as the between- subject factor. Protected post-hoc independent sample t-tests were then used to evaluate differences between groups on the left and on the right side.
RESULTS
There were no group differences in gender (all males) or handedness (all right), and mean age did not differ either between normal subjects and schizophrenics (39.5, SD= +/−9.3, versus 39.8, SD=+/−9.06). Parental socioeconomic status also showed no significant difference (2.75, SD=+/−1.34 versus 3.06, SD=+/−0.983) and there was no difference between groups on IQ (.133, SD=+/−0.129). (Table1).
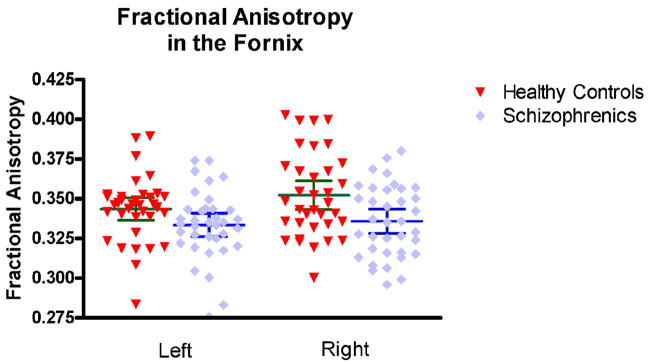
There was a group effect for Fornix FA in both left (F=4.049; df=1,69; p=0.048) and right FA (F=7.902; df=1,69; p=0.006). Further post-hoc protected T-tests showed decreased FA in the left (t [69] =2.012, p=0.048) and the right (t [69] =2.811, p=0.006) fornix in schizophrenia, compared with control subjects.
We also observed a side effect (F=3.846; df=1; p=0.054), but no side x group interaction (F=1.242; df=1; p=0.269). Both groups were characterized by mean FA values greater on the right side than on the left (Figure 3), however only control subjects showed this difference to be statistically significant (t [34]= −1.986, p=0.055 for NC, and (t[35]= −.665, p=0.510 for SZ).
Figure 3.
Results of Fractional Anisotropy comparison.
No correlation between FA measures and medication dosage were found. Pearson’s correlation showed FA Left + CPZ equivalent=.230 (p=.212) and FA Right + CPZ equivalent= .0459 (p=.806).
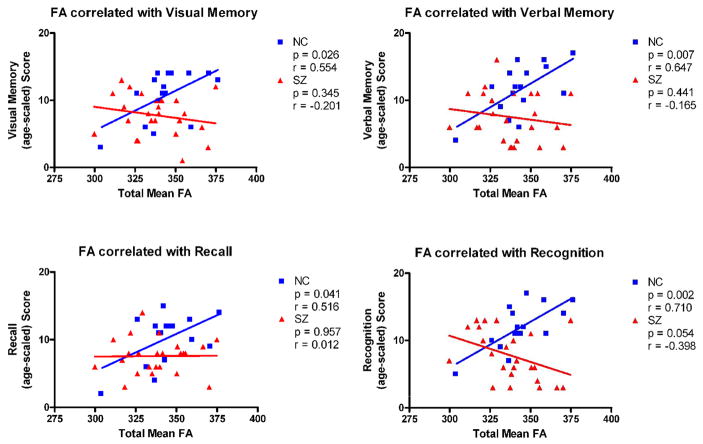
Associations were found for the control group, with higher FA of the fornix correlating statistically significantly with DPT (Doors and people Test described in detail in Nestor et al., 2007) measures of combined visual memory (r=.554, p=.026), combined verbal memory (r=.647, p=.007), combined recall (r=.516, p=.041) and combined recognition (r=.710, p=.002). No such statistically significant correlations were found in the patient group. (Table 2) (Figure 4).
Table 2.
Doors and People Test (DPT) correlation between neuropsychological and brain measures for patients with schizophrenia (SZ) and normal control s(NC).
| NC | SZ | |||
|---|---|---|---|---|
| r | p | r | p | |
| Combined Visual Memory | 0.554 | 0.026* | −.201 | .354 |
| Combined Verbal Memory | 0.647 | 0.007* | −.165 | .441 |
| Combined Recall | 0.516 | 0.041* | 0.012 | .957 |
| Combined Recognition | 0.71 | 0.002* | −.398 | .054 |
P < .05.
Figure 4.
Scattergrams for fornix FA and Doors and People Test (DPT) measures of visual, verbal, recall and recognition memory for normal controls and patients with schizophrenia.
Discussion
Fornix Integrity and Schizophrenia
In this study, fiber integrity of the fornix was measured in patients with chronic schizophrenia and in normal controls. Our results showed bilateral reduction of FA (indicative of white matter integrity) in the fornix of patients with schizophrenia compared to normal control subjects. Although we did not find neuropsychological correlations with measures of FA for the fornix in schizophrenic patients, we did find statistically significant correlations between fornix integrity and combined visual and verbal memory and combined recall and recognition in the control group . Previous studies also failed to demonstrate a significant fornix-declarative memory relationship in patients with schizophrenia (Nestor et al., 2007). Reduced FA in the fornix thus may be more associated with executive failures but not to memory anomalies in schizophrenia. The significant correlation in this study that we reported in controls, however, between episodic memory and FA in the fornix, is consistent with considerable evidence demonstrating that the fornix, a subcortical white matter tract carrying axons to and from the hippocampus, plays a vital role in normal episodic memory in the human brain (Graffan, 2005; Squire Zola-Morgan, 1991).
Few Studies of Fornix in Schizophrenia
Despite the important role of the fornix as the largest connection of the hippocampus with other brain structures, there are only a few studies that have evaluated this structure and its relationship to hippocampal volumes in schizophrenia. Our investigation includes only white matter measures. Because of the anatomical relationship between fornix and hippocampus, where the fornix serves as an important connective pathway carrying axons to and from the hippocampus, it is important to examine both gray and white matter measures in future studies in order to understand further the relationship between hippocampus and fornix.
The only postmortem study that investigated the fornix in schizophrenia was the study by Chance et al. (1999), who found a higher fiber density on the left side in men with schizophrenia than in comparison subjects, with no significant differences in the total number of fibers. The subjects in his study were older and only descending columns of the fornix were analyzed. An imaging study was later conducted by Zahajsky and coworkers (2001), who used structural MRI to measure and compare fornix volumes between patients with schizophrenia and controls, and where no significant differences in total volume between groups was observed, although these investigators were able to identify correlations between fornix and hippocampus volumes. Finally, the only study investigating fornix integrity in schizophrenia using Diffusion Tensor Imaging was a previous study conducted in our laboratory, which found reduced FA in the fornix in the schizophrenia patients compared with normal control subjects. This study, however, was limited to a small part of the fornix, the body of the fornix, and thus the entire structure was not included in vivo in schizophrenia.
Implications of Bilateral Fornix Findings in Schizophrenia
We reported bilateral findings for reduced FA in patients with schizophrenia compared with healthy controls. Of further note, anisotropic asymmetry differences were observed for the fornix for right-higher-than left anisotropy in healthy controls but not in patients with schizophrenia. The absence of anisotropy asymmetry differences in the fornix in patients is interesting although it is unclear what it means (i.e., lack of lateralization for fornix structure and function in schizophrenia?) and thus further investigation is needed. In fact, the relevance of brain asymmetry and schizophrenia is still poorly understood, although a number of studies have characterized differences in brain asymmetries between patients with schizophrenia and controls (e.g., Bartley et al., 1993; Crow et al., 1989, 1997, 2000; DeLisi et al., 1994; Hoff et al., 1992). The fornix, in fact, is one of several structures where asymmetry abnormalities have been reported. Future studies are thus needed to clarify further asymmetry, or the lack there of, in the fornix in schizophrenia.
Fiber Tractography to Quantify Fornix Anisotropic Diffusion
Fiber Tractography itself is a new approach to DTI data analysis (e.g., Mori et al., 1999; Basser et al., 2000; Catani et al., 2002). This new approach makes it possible to observe and to quantify white matter properties along the entire fiber bundles. This technique was introduced in 1999 (Mori et al.), and has been shown since to solve some of the problems associated with difficulties in the manual definition of long tracts (Kannan et al., 2005). Fiber tractography also has higher specificity and sensitivity compared to the conventional approach of using measurements from manually drawn ROIs (see Kannan et al., 2005). More specifically, after tracts are generated, they are used as labels to quantify fractional anisotropy, considered to be a measure of axonal integrity, density and myelination (Beaulieu 2002). This technique has also proven to be sensitive to white matter changes in different pathological conditions such as Multiple Sclerosis (Wilson et al., 2003) and Congenital Hemiparesis (Glenn et al., 2003).
Our findings using fiber tractography indicate abnormalities in the integrity of the fornix, which provides connectivity between the hippocampus and other brain regions. This kind of relationship has been demonstrated before in epilepsy. Kuzniecky et al. (1999), for example, found fornix atrophy in epilepsy patients with unilateral hippocampal atrophy and in a higher proportion in patients with bilateral hippocampal atrophy.
Advantages and Limitations of the Study
There are some limitations that require further studies. First, we don’t know the effect of antipsychotic medication on DTI measures, although we found no significant correlation between FA measures and medication dosage. There are, nevertheless, only a few other studies that have evaluated this relationship, although different results have been obtained, with some reporting an association (Minami et al., 2003) and others not (Buchsbaum et al., 1998; Foong et al., 2000). It is possible that long- term treatment with antipsychotics also affects schizophrenics and thus further longitudinal studies might clarify this issue. Second, FA is observed to change as a function of the normal aging process (Pfefferbaum et al., 2005; Salat et al., 2005). Our study, however, included subjects who were younger compared to the participants of normal aging studies, and thus further studies are needed to compare FA changes in patients with schizophrenia and normal controls. We note, however, that Rosenberger et al. (2008), in our group, showed a negative correlation between FA in a group of 20–55 year olds compared to a group of healthy controls in the same age range. Moreover, medication dosage was not correlated with this finding. These findings thus suggest that white matter integrity may show progressive changes even in a restricted age range in patients with schizophrenia. Third, while our sample size was large and it provided adequate power, the subject population included only men and thus studies are needed that include both men and women in order to evaluate gender effects. Finally, further studies should be conducted in first episode patients as well as longitudinal studies in order to help identify whether or not abnormalities in schizophrenia are static or progressive in nature.
Conclusion
We used DTI and a tractographic approach to make measurements of fractional anisotropy in a specific white matter tract and found this to be a useful tool. Our results point to bilateral disruption in the fornix integrity in schizophrenia. Considering the role of the fornix in connecting key brain structures involved in superior cognitive functions, this study can help broaden our understanding of the pathophysiology of schizophrenia.
Acknowledgments
We thank Nancy Maxwell and Jennifer Goodrich for administrative support; Marek Kubicki, M.D., Ph.D., and Martha Shenton, Ph.D. for personal support; and Georgia Bushell, B.A., Kate Smith, B.A. and Usman Khan, B.A. for their support as research assistants.
The authors would like to thank Nancy Maxwell for her administrative assistance. Additionally, we gratefully acknowledge the support of the National Institute of Health (K05 MH070047 and R01 MH 50740 to MES, R01 MH 40799 to RWM and R01 MH 074794 to CFW, P50 MH 080272 to RWM, MES), the Department of Veterans Affairs Merit Awards (MES, RWM), and the VA Schizophrenia Center Grant (RWM/MES). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (MK, RK, MES).
Role of the Funding Source
This study was supported, in part, by grants from the National Institute of Health (K05 MH070047 and R01 MH 50740 to MES, R01 MH 40799 to RWM and ROI MH 074794 to CFW, P50 MH 080272 to RWM, MES), the Department of Veterans Affairs Merit Awards (MES, RWM), and the VA Schizophrenia Center Grant (RWM/MES). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (MK, RK, MES). All of the study sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest that require disclosure.
Contributors
Jennifer Fitzsimmons, M.D., and Marek Kubicki, M.D., Ph.D. designed the study and wrote the protocol. Jennifer Fitzsimmons, M.D also wrote the first draft of the manuscript. Marek Kubicki, M.D, Ph.D. and Carl-Fredrik Westin, Ph.D. supervised the MRI data acquisition and processing, and provided guidance on technical aspects of diffusion tensor imaging. Paul Nestor, Ph.D., Margaret Niznikiewicz, Ph.D., and Robert W. McCarley, M.D managed the recruitment and collected clinical information of participants. Martha E. Shenton, Ph.D., Marek Kubicki, M.D., Ph.D., and Robert W. McCarley, M.D. supervised the statistical analyses and edited multiple iterations of the manuscript, they also provided guidance on the study design and implementation of the study. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001;12:2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Andreason NC. Scale for the Assessment of Negative Symptoms. University of Iowa College of Medicine; Iowa City, IA: 1981. [Google Scholar]
- Andreason NC. Scale for the Assessment of Negative Symptoms. University of Iowa College of Medicine; Iowa City, IA: 1984. [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The Doors and People Test. Thames Valley Test; Bury St. Edmunds, UK: 1994. [Google Scholar]
- Barch DM. The basis of anisotropic water diffusion in the nervous system - a technical review. Biol Psychiatry. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The Basis of Anisotropic Water Diffusion in the Nervous System- A Technical Review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk-Butler, Jeffries . Clinical Handbook of Psychotropic Drugs. 5. Hogrefe and Huber; Seattle: 1996. [Google Scholar]
- Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry. 1985;42:784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Calabrese P, Markowitsch HJ, Harders AG, Scholz M, Gehlen W. Fornix damage and memory. A case report. Cortex. 1995;31:555–564. doi: 10.1016/s0010-9452(13)80066-4. [DOI] [PubMed] [Google Scholar]
- Callicott J, Bertolino A, Mattay V, Langheim F, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter C, Robertson L, Nordahl T, Chaderjian M, Kraft L, O’Shora-Celaya L. Spatial working memory deficits and their relationship to negative symptoms in unmedicated schizophrenia patients. Biol Psychiatry. 1996;40:930–932. doi: 10.1016/S0006-3223(96)00350-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chance SA, Highley JR, Esiri MM, Crow TJ. Fiber content of the fornix in schizophrenia: lack of evidence for a primary limbic encephalopathy. Am J Psychiatry. 1999;156:1720–1724. doi: 10.1176/ajp.156.11.1720. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res. 2004;130:71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar KO, Barch DM, Braver TS. Issues concerning relative speed of processing hypotheses, schizophrenic performance deficits, and prefrontal function: comment on Schooler et al. (1997) J Exp Psychol Gen. 1997;126:37–41. doi: 10.1037//0096-3445.126.1.37. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D, Wardell A, Woolsey R, James A. Enlargement of the fornix in early-onset schizophrenia: a quantitative MRI study. Neurosci Lett. 2001;6:163–166. doi: 10.1016/s0304-3940(01)01637-8. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, Fukuyama H, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Widespread cortical networks underlie memory and attention. Science. 2005;309:2172–2173. doi: 10.1126/science.1119445. [DOI] [PubMed] [Google Scholar]
- Glenn OA, Henry RG, Berman JI, Chang PC, Miller SP, Vigneron DB, et al. DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. J Magn Reson Imaging. 2003;18:641–648. doi: 10.1002/jmri.10420. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson H, Maier S, Mulkern R, Morocz I, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996;36:509–519. doi: 10.1002/mrm.1910360403. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Lohr JB. Hippocampal pathologic findings in schizophrenia. A morphometric study. Arch Gen Psychiatry. 1989;46:1019–1024. doi: 10.1001/archpsyc.1989.01810110061009. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, et al. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005;13:1092–1099. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, et al. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, McMeniman M, Vogel J, Augustin R, Becker DE, et al. Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biol Psychiatry. 2004;55:1138–1145. doi: 10.1016/j.biopsych.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60:22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniecky R, Bilir E, Gilliam F, Faught E, Martin R, Hugg J. Quantitative MRI in temporal lobe epilepsy: evidence for fornix atrophy. Neurology. 1999;53:496–501. doi: 10.1212/wnl.53.3.496. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A. Cortical gray matter deficit in patients with bipolar disorder. Schizophr Res. 1999;40:219–227. doi: 10.1016/s0920-9964(99)00063-8. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- McMackin D, Cockburn J, Anslow P, Gaffan D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir (Wien) 1995;135:12–18. doi: 10.1007/BF02307408. [DOI] [PubMed] [Google Scholar]
- Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, et al. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mori S, Van Ziji P. Fiber tracking: Principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, et al. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Kuroki N, Gurrera RJ, Niznikiewicz M, Shenton ME, et al. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007;155:21–28. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Piech R, Allen C, Niznikiewicz M, Shenton M, McCarley RW. Retrieval-induced forgetting in schizophrenia. Schizophr Res. 2005;75:199–209. doi: 10.1016/j.schres.2005.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biol Psychiatry. 1999;46:392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Parker A, Eacott MJ, Gaffan D. The recognition memory deficit caused by mediodorsal thalamic lesion in non-human primates: a comparison with rhinal cortex lesion. Eur J Neurosci. 1997;9:2423–2431. doi: 10.1111/j.1460-9568.1997.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia. 1997;35:1093–1102. doi: 10.1016/s0028-3932(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, Niznikiewicz M, Westin CF, Kikinis R, Saykin JA, McCarley RW, Shenton ME. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Jose Estepar R, Kubicki M, Shenton M, Westin CF. A kernal-based approach for user-guided fiber bundling using diffusion tensor data. IEEE EMBS. 2006;28:2626–2629. doi: 10.1109/IEMBS.2006.259829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stoll . The Psychopharmacology References Card Antipsychotic Treatment Guide. McLean Hospital; Belmont, MA: 2001. [Google Scholar]
- Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14:1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, et al. Disrupted integrity of the fornix is associated with impaired memory organization in schizophrenia. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Wang DS, Bennett DA, Mufson EJ, Mattila P, Cochran E, Dickson DW. Contribution of changes in ubiquitin and myelin basic protein to age-related cognitive decline. Neurosci Res. 2004;48:93–100. doi: 10.1016/j.neures.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Grundrisse der Psychiatrie. Leipzig: Thieme; 1906. [Google Scholar]
- Wilson M, Tench CR, Morgan PS, Blumhardt LD. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry. 2003;74:203–207. doi: 10.1136/jnnp.74.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zahajszky J, Dickey CC, McCarley RW, Fischer IA, Nestor P, Kikinis R, et al. A quantitative MR measure of the fornix in schizophrenia. Schizophr Res. 2001;47:87–97. doi: 10.1016/s0920-9964(00)00051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]