Abstract
Obesity and insulin resistance (IR) increase the risk for coronary heart disease; however, much of this risk is not attributable to traditional risk factors. We sought to determine whether weight loss associated with supervised aerobic exercise beneficially alters biomarkers of oxidative stress and whether these alterations are associated with improvements in measures of insulin resistance. Twenty-five sedentary and overweight to obese [body mass index (BMI) = 33.0 ± 0.8 kg/m2] individuals, with characteristics of the metabolic syndrome, participated in a 4- to 7-mo weight loss program that consisted of energy restriction (reduced by ~500 kcal/day) and supervised aerobic exercise (5 days/wk, 45 min/day at 60% V̇o2max; ~375 kcal/day). IR and insulin sensitivity were assessed by the calculation of the homeostasis model assessment (HOMA) and quantitative insulin sensitivity check index (QUICKI), respectively. Oxidative stress was assessed by oxidized LDL (oxLDL), myeloperoxidase (MPO), and low- and high-density lipoprotein (LDL and HDL) lipid hydroperoxide concentrations in serum. Indexes for antioxidative status included apolipoprotein A1 (apoA1) concentrations and paraoxonase-1 (PON1) activity and protein concentrations. Exercise- and diet-induced weight loss (~10%) significantly (P < 0.05) increased insulin sensitivity and reduced IR, oxLDL, and LDL lipid hydroperoxides but did not alter HDL lipid hydroperoxides or MPO concentrations. Lifestyle modification impacted systemic antioxidative status by increasing apoA1 concentrations and reducing serum PON1 protein and activity. Changes in oxidative stress were not associated with alterations in HOMA or QUICKI. Diet- and exercise-induced weight loss (~10%) improves measures of insulin sensitivity and beneficially alters biomarkers of oxidative status.
Keywords: insulin resistance, oxidized low-density lipoprotein, paraoxonase-1, energy restriction
Americans are experiencing a weight gain epidemic, and recent epidemiological studies suggest an increased risk of coronary heart disease (CHD) and type 2 diabetes in overweight and obese individuals. In addition, patients with type 2 diabetes, impaired glucose tolerance, and insulin resistance (IR) are at elevated risk for CHD (24). Despite elevated levels of hypertension, obesity, and dyslipidemia in these individuals, epidemiological studies suggest that traditional risk factors do not explain all of the elevated risk of CHD (24, 29). This suggests that other underlying abnormalities are present and contributing to the etiology of the disease.
One possible link between IR and CHD is reduced levels of high-density lipoproteins (HDL) and increased oxidative stress. Low HDL cholesterol (HDL-C) predicts future CHD risk and is associated with type 2 diabetes and IR (20). Atherosclerosis is characterized by the accumulation of oxidatively modified low-density lipoprotein (LDL) (37), and circulating oxidized LDL (oxLDL) is elevated in patients with CHD (17) and type 2 diabetes (7). Furthermore, oxidative stress accompanies IR, and oxLDL may contribute directly to impaired insulin action (27).
In addition to facilitating reverse cholesterol transport (RCT), HDL and HDL-associated proteins apolipoprotein A1 (apoA1), lecithin-cholesterol acyltransferase, and paraoxonase-1 (PON1) possess antioxidant properties that prevent the oxidative modification of LDL (10). However, HDL is known to be oxidized by the leukocyte- and phagocyte-derived enzyme myeloperoxidase (MPO), reducing RCT capacity and the antioxidative functions (28).
It is well established that lack of physical activity is an independent predictor of CHD and type 2 diabetes and contributes to the progression of each disease. Lifestyle modifications targeted at increasing physical activity and reducing energy intake are recommended for optimal health (2, 23). Despite this recommendation, few studies have examined the impact of exercise training- and diet-induced weight loss on measures of oxidative stress and associated changes in IR. In the current investigation, we sought to determine whether weight loss via aerobic training and energy restriction was effective in beneficially altering biomarkers of oxidative stress (oxLDL, MPO, and HDL and LDL lipid hydroperoxides) and proteins with antioxidative activity (apoA1 and PON1) and improving measures of IR in sedentary, overweight, and obese adults with characteristics of the metabolic syndrome. We hypothesized that the intervention would beneficially alter oxidative status and that improvements in oxidative stress would be associated with reductions in measures of IR.
METHODS
Subjects
Twenty-one females and nine males, aged 18–50 yr, were recruited to participate in this study. Subjects were nonsmokers and weight stable (±3% current body weight for the previous 3 mo). The primary criteria for participation were an overweight to obese body mass index (BMI; 26–43 kg/m2) and a sedentary physical activity designation (<30 min/wk or 500 kcal/wk over the previous 4 mo). Subjects with diagnosed cardiovascular disease, diabetes, or disease symptoms that according to the American College of Sports Medicine (2) would limit exercise, were excluded. Additionally, subjects were excluded if they were taking weight-altering (including over-the-counter) medications. Subjects had at least two of five characteristics of the metabolic syndrome as defined by the American Heart Association/National Heart, Lung, and Blood Institute update of the National Cholesterol Education Program Adult Treatment Panel III report (15): waist circumference of ≥102 cm in men and ≥88 cm in women; serum TG concentration ≥150 mg/dl; HDL-C concentration of <40 mg/dl in men and <50 mg/dl in women; blood pressure ≥130/85; fasting glucose ≥100 mg/dl. Before participation in the study, participants gave written informed consent, as approved by the Health Sciences Institutional Review Board of the University of Missouri-Columbia, in accordance with the ethical standards of the University of Missouri Institutional Review Board and with the Helsinki Declaration of 1975, as revised in 1983.
Study Design
The duration of the study was 4–7 mo (mean = 6 mo), during which subjects adhered to a moderate energy restriction diet, reduced by ~500 kcal/day, and participated in supervised moderate-intensity aerobic exercise training, which progressed to 45 min/day, 5 day/wk, 1,500–2,000 kcal/wk, to induce 10% weight loss. Subjects weighed in once per week, and nutritional counseling was provided as needed to assist subjects with adherence to the modest energy restriction diet. Metabolic fitness and outcome measures were assessed at baseline and after weight loss.
Training Period
Exercise training consisted of brisk walking and/or slow jogging 5 days out of 7 days each week with occasional stationary cycling and elliptical exercise. The exercise training progressed to where at week 4, subjects were exercising 45 min/day, 5 days/wk, 60% V̇o2max. The exercise intensity for each session was monitored using Polar heart rate monitors (Polar CIC, Port Washington, NY). At least three exercise sessions per week were supervised within the Exercise Physiology Laboratory, and unsupervised sessions were monitored with heart rate monitors as with the supervised sessions.
Maximal Aerobic Capacity
Each subject completed a V̇o2max test on a treadmill at the beginning of the study and after weight loss to determine initial fitness and changes in fitness status, as well as to determine the appropriate exercise intensity for the submaximal training prescription. The baseline test was completed at University of Missouri Hospitals and Clinics in the Cardiology Diagnostic Laboratory and the post-weight loss test was completed in the Exercise Physiology Laboratory with the presence of a physician. The Bruce protocol was used for the V̇o2max test, as previously described (2). The speed and incline were increased in 3-min increments until volitional exhaustion. The highest V̇o2max value obtained was considered the subject’s V̇o2max.
Dietary Assessment
Baseline energy intake was assessed by 7-day food records and used for the energy intake prescription during the study. Before beginning participation, subjects were provided dietary education using references such as the U.S. Department of Agriculture Interactive Healthy Eating Index (4) to reduce energy intake by ~500 kcal/day. Subjects were encouraged to avoid excessive consumption of high-fat foods, reduce portion sizes, and to increase daily intake of complex carbohydrates, lean meats, dairy products, fruits, and vegetables. Subjects recorded a 7-day food record once per month throughout the duration of the study. The food records were examined by the study nutritionist and discussed throughout the entire study to assess dietary compliance, suggest modifications, and review progress toward goal weight. Subjects were asked to keep multivitamin usage constant during the intervention. Seven-day dietary food records, not including multivitamin supplements, were analyzed for total energy and macro/micronutrient content, including dietary antioxidants (vitamins A, C, and E) (Food Processor SQL, ESHA Research, Salem, OR) at baseline and after weight loss.
Blood Pressure and Body Composition
Systolic and diastolic pressure was measured using a standard aneroid sphygmomanometer. Body weight was measured to the nearest 0.05 kg and height to 0.1 cm, and used to calculate BMI (kg/m2). Body density was calculated from three-site skinfold measurements (men: chest, abdomen, and thigh; women: triceps, thigh, and suprailiac) using equations of Jackson and Pollack and the Siri equation to convert body density to percent body fat (2). Waist-to-hip ratio was determined by measuring waist circumference at the narrowest region between the costal margin and iliac crest and dividing by the hip circumference measured at its greatest gluteal protuberance.
Blood Collection and Preparation
Blood samples were collected following a 12-h fast and a 48-h no-exercise and dietary control period via a butterfly needle inserted into an antecubital forearm vein. All blood samples were collected into 10 ml EDTA, sodium heparin, or serum separator vacutainer tubes. All serum samples were allowed to clot, and then serum and plasma were separated by centrifugation at 4°C for 15 min at 2,000 g in a Marathon 21000R centrifuge (Fisher Scientific, Pittsburgh, PA). The separated serum and plasma were transferred to cryogenic vials and stored at −80°C until analyzed.
All subject samples were analyzed in duplicate and then averaged. In addition, each subject’s samples from the two testing sessions were analyzed in the same run to eliminate interassay variation. If taking birth control, women were asked to continue constant usage during the entire investigation. To help control for variation associated with cyclic hormones, each female subject had blood collected during the follicular phase of the menstrual cycle.
Biochemical Measurements
Cholesterol, glucose, triglyceride, insulin, and HDL-C analyses
Fasting cholesterol and triglycerides were analyzed in plasma using colorimetric diagnostic kits (Thermo, Arlington, TX). Glucose concentrations were determined by an automated analyzer (pHOx Plus L, Nova Biomedical, Waltham, MA) using the glucose oxidase method. The intra-assay coefficients of variation (CV) for cholesterol, glucose, and triglycerides in our laboratory are 1.9%, 1.8%, and 1.7%, respectively. Fasting serum insulin was determined by a chemiluminscence technique (Immulite 1000, Diagnostic Products, Los Angeles, CA). The intra-assay CV for insulin was 4.2%. LDL-C was calculated by the Friedewald equation (13).
Plasma HDL-C and subfractions were determined using a modified heparin-MnCl2-dextran sulfate method, as previously described by our group (42). HDL2-C was calculated by subtracting the value for HDL3-C from the HDL-C value. The intra-assay CVs are 1.0% and 2.0% for HDL-C and HDL3-C, respectively.
Insulin sensitivity
Surrogate markers of insulin sensitivity and IR were calculated using fasting insulin and glucose concentrations. Insulin resistance was assessed using the homeostasis model assessment (HOMA) on modeling of fasting insulin and glucose concentrations using the formula derived by Matthews et al. (26). Insulin sensitivity also was calculated by the quantitative insulin sensitivity check index (QUICKI) (21), as the authors suggest that QUICKI is a better correlate of the hyperinsulinemic-euglycemic clamp technique than other methods.
Insulin sensitivity also was assessed with a 3-h intravenous glucose tolerance test (IVGTT) as modified by Houmard et al. (18) from Bergman et al. (5). Insulin sensitivity was calculated using the minimal model and computer program developed by Bergman et al. (5). Because of technical difficulties, only 10 (7 females and 3 males) of the 25 participants had usable data in the IVGTT analyses.
HDL and LDL separation
HDL and LDL were isolated from plasma by single-spin density gradient ultracentrifugation, as previously described (9). Briefly, samples were adjusted to a density of 1.30 g/ml by the addition of solid KBr (0.4946 g/ml plasma) and overlaid with normal saline (0.15 M NaCl-0.01% EDTA, pH 7.4). HDL was separated in the density range ρ = 1.063–1.256 kg/l, and LDL was separated in the density range ρ = 1.022–1.063 kg/l by ultracentrifugation for 150 min at 65,000 rpm (Sorvall Discovery 100SE ultracentrifuge and a T-890 rotor). HDL and LDL fractions were removed by disposable Pasteur glass pipettes, and aliquots were dialyzed at 4°C for 24 h against 10 mM PBS at pH 7.4 (11). Lipoproteins were used within 24 h after isolation. The protein concentrations of LDL and HDL were determined by the method of Bradford (6).
PON1 and ApoA1 concentrations
PON1 protein concentrations were determined in serum by a commercially available ELISA (WAK-Chemie Medical GmbH, Steinbach, Germany). Serum concentrations of apoA1 also were determined by a commercially available ELISA (Alerchek, Portland, ME). The average intra-assay CV for PON-1 and apoA1 was 7.0% and 6.0%, respectively.
PON1 activity
PON1 paraoxonase activity in serum and associated with HDL (100 μg protein/ml) was determined by a highly sensitive fluorometric assay (excitation/emission maxima 360/450 nm) for the organophosphate activity of paraoxonase-1, based on the hydrolysis of a fluorogenic organophosphate analog (Molecular Probes, Eugene, OR). The structure of this analog is proprietary; thus, we are not able to discern its exact identity. However, Molecular Probes has custom synthesized novel fluorescent substrates with similar kinetic values to the current method with distinct specificity and sensitivity advantages over other substrates, such as phenylacetate (36). The average intra-assay CV for PON1 activity was 1.9%.
Systemic oxidative stress
Systemic levels of MPO and oxLDL were determined by commercially available ELISA (ALPCO Diagnostics, Salem, NH and Mercodia, Uppsala, Sweden, respectively). The Mercodia oxLDL ELISA uses the murine monoclonal antibody 4E6 for capture on the microtiter plate when apoB moieties have at least 60 lysine residues oxidatively modified. The average intra-assay CV for MPO and oxLDL were 4.9% and 4.7%, respectively.
In vitro HDL and LDL oxidation
The extent of lipid peroxidation of HDL and LDL was assessed as described previously (11, 19). HDL and LDL were isolated from each subject at each testing session (as described in Training Period), and the levels of lipid hydroperoxides from before and after treatment were assessed by the ferrous oxidation xylenol orange assay (19). Briefly, aliquots of HDL (200 μg) or LDL (100 μg) were resuspended in 10 mM PBS and incubated at 37°C for 30 min with ferrous oxidation xylenol orange reagent (Sigma; 100 μM xylenol orange, 250 μM Fe2+, 25 mM H2SO4, and 4 mM butylated hydroxytoluene in 90% methanol). After incubation, samples were centrifuged at 3,500 rpm for 15 min, and the supernatants, containing the lipid hydroperoxides, were used for the determination of the absorbance at 560 nm with a Beckman DU 530 spectrophotometer (Beckman Instruments, Fullerton, CA). Results were calculated as nanomoles of lipid hydroperoxides for 100 μg protein for LDL (nmol/100 μg) and 200 μg of protein for HDL (nmol/200 μg). t-Butyl-hydroperoxide solution was used as the standard. The average intra-assay CV was 7.0%.
Statistical analysis
The SPSS statistical package (SPSS/11.0, SPSS, Chicago, IL) was used for the calculations. Means were analyzed using one-way ANOVA with repeated measures. To assess associations among oxidative stress markers and measures of IR and sensitivity, Pearson correlations were performed. The threshold for significance was set at P <0.05, and values are reported as means ± SE.
RESULTS
Thirty subjects were recruited to participate in the current investigation; twenty-five (17 women and 8 men) completed both baseline and after weight loss testing and were included in the analyses. All of the subjects had at least two components of the metabolic syndrome, with ~40% of the participants having at least three components. Among the 25 subjects, compliance to the exercise intervention was more than 98% during the investigation.
Diet
All subjects decreased total energy, carbohydrate, protein, fat, and saturated fat intake from baseline to post-weight loss (Table 1). Percentage of total dietary energy from carbohydrate and protein was significantly increased, while percentage of energy derived from fat was significantly decreased from baseline to post-weight loss (data not shown). Intake of vitamin A and vitamin E remained unchanged during treatment; however, intake of vitamin C was significantly increased (Table 1).
Table 1.
Dietary intake
| Variables | Baseline | Post-Weight Loss |
|---|---|---|
| Total energy, kJ/day | 9,456±431 | 6,770±297* |
| Carbohydrate, g/day | 267±14 | 202±11* |
| Protein, g/day | 86±3 | 70±3* |
| Fat, g/day | 93±6 | 59±3* |
| Saturated fat, g/day | 32±2 | 20±1* |
| Vitamin A, IU/day | 5,359±688 | 6,284±642 |
| Vitamin E, mg/day | 4.2±0.5 | 4.3±0.7 |
| Vitamin C, mg/day | 58.6±6.4 | 76.8±8.2† |
Values are means ±SE.
Significantly different from baseline P <0.001
P <0.05.
Anthropometric and Physical Characteristics
Body weight (102.0 ± 5.1 vs. 94.1 ± 3.8 kg), body fat percent (28.1 ± 1.4 vs. 39.2 ± 0.9%), waist to hip ratio (1.00 ± 0.01 vs. 0.91 ± 0.01), and V̇o2max (3.2 ± 0.1 vs. 2.2 ± 0.4 l/min) differed significantly (P < 0.001) between men and women. Body weight, BMI, body fat percentage, waist circumference, hip circumference, and waist-to-hip ratio all were significantly reduced following 6 mo of exercise training- and diet-induced weight loss (Table 2). The intervention also significantly lowered resting heart rate and blood pressure.
Table 2.
Anthropometric and metabolic parameters
| Variables | Baseline | Post-Weight Loss | %Change |
|---|---|---|---|
| Body weight, kg | 96.6±3.1 | 87.6±2.7* | −9.3 |
| Body mass index, kg/m2 | 33.0±0.8 | 29.9±0.7* | −9.4 |
| Body fat, % | 35.6±1.3 | 32.1±1.4* | −9.8 |
| Waist circumference, cm | 110.6±1.8 | 101.8±1.6* | −8.0 |
| Hip circumference, cm | 118.0±1.8 | 112.2±1.8* | −4.9 |
| Waist to hip ratio | 0.94±0.01 | 0.91±0.01* | −3.2 |
| Resting heart rate, bpm | 79±2 | 69±2* | −12.7 |
| Systolic blood pressure, mmHg | 124±2 | 114±1* | −8.1 |
| Diastolic blood pressure, mmHg | 80±2 | 72±2* | −10.0 |
| V̇o2max, l/min | 2.5±0.1 | 2.8±0.2* | +12.0 |
| V̇o2max, ml · kg−1 · min−1 | 26.2±1.0 | 31.4±1.3* | +19.8 |
| EE, kcal/45 min | 356±18 | 398±22* | +11.8 |
Values are means ±SE.
Significantly different from baseline P <0.001.
EE, energy expenditure during a 45-min exercise session.
Absolute and relative V̇o2max and energy expenditure during a 45-min submaximal exercise session for women were significantly less than values found in the men; however, the percent improvement with the exercise intervention did not differ from men (data not shown). The relatively low V̇o2max values in the current investigation speak to the sedentary nature of the study participants; nevertheless, baseline values and training responses (+12%) were similar to previous reports in subjects with similar metabolic characteristics (22).
Blood Lipoproteins
Six months of exercise training- and diet-induced weight loss resulted in significant reductions in TG and TC (Table 3). HDL-C was not significantly altered by the intervention; however, HDL2-C was significantly increased and HDL3-C was significantly decreased following a 10% reduction in body weight (Table 3). LDL-C was significantly reduced with exercise training- and diet-induced weight loss, whereas the exercise training and dietary intervention resulted in a significant increase in apoA1 concentrations (Table 3).
Table 3.
Blood parameters
| Variables | Baseline | Post-Weight Loss | %Change |
|---|---|---|---|
| Triglycerides, mg/dl | 117.4±9.7 | 102.4±7.5† | −12.8% |
| Total cholesterol, mg/dl | 189.5±4.3 | 174.4±3.5* | −8.0% |
| HDL-C, mg/dl | 49.1±2.2 | 49.0±1.7 | NC |
| HDL2-C, mg/dl | 19.5±1.6 | 21.2±1.5† | +8.7% |
| HDL3-C, mg/dl | 29.7±0.9 | 27.7±0.5† | −6.7% |
| LDL-C, mg/dl | 116.8±4.1 | 104.9±3.1† | −10.2% |
| ApoA1, mg/dl | 108.3±3.1 | 116.4±3.1† | +7.5% |
| HOMA | 2.9±0.3 | 2.1±0.3* | −27.6% |
| QUICKI | 0.33±0.01 | 0.35±0.01* | +6.0% |
| Myeloperoxidase, ng/ml | 46.8±5.6 | 43.5±4.3 | −7.1% |
| HDL lipid hydroperoxides, nmol/200 μg proteins | 1.66±0.16 | 1.46±0.20 | −12.0% |
Values are means ±SE.
Significantly different from baseline P <0.001;
P < 0.05.
NC, no change.
Glucose Homeostasis
Sixty percent of subjects had baseline fasting insulin concentrations above 10.0 μIU/ml (range 4.3–32.1 μIU/ml) and HOMA values above 2.5 (range 1.0 –7.5). Insulin (12.3 ± 1.3 vs. 10.3 ± 1.2 μIU/ml), glucose (93.7 ± 2.1 vs. 85.5 ± 2.5 mg/dl), and HOMA were significantly reduced (P < 0.001) from baseline to after weight loss (Table 3). In addition, insulin sensitivity assessed by QUICKI (Table 3) and IVGTT (insulin sensitivity) also was significantly improved (baseline = 4.73 ± 1.26 mU · l−1 · min−1; post-weight loss = 7.72 ± 1.29 mU · l−1 · min−1; P < 0.05, n = 10).
Measures of Oxidative Stress
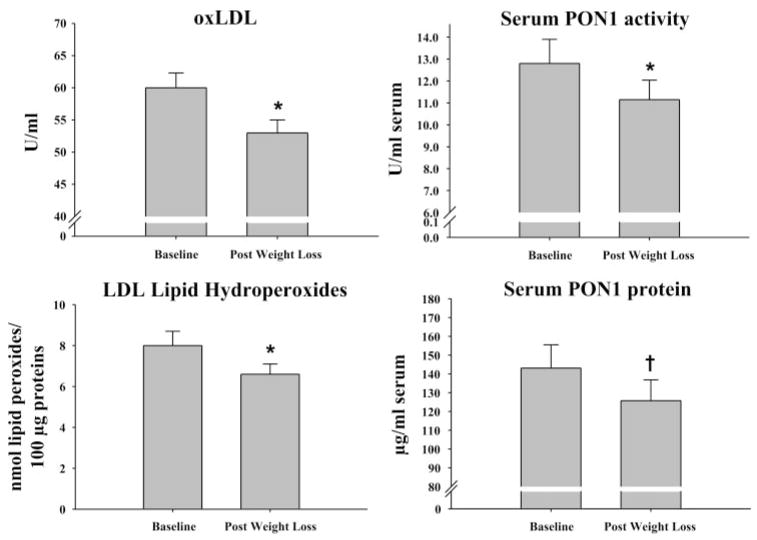
Exercise training- and diet-induced weight loss resulted in significant reductions in circulating concentrations of oxLDL and LDL lipid hydroperoxides (Fig. 1) but did not statistically reduce MPO or HDL lipid hydroperoxide concentrations (Table 3). Contrary to our hypothesis, PON1 activity per volume of serum was significantly reduced following the exercise training and weight loss intervention (Fig. 1). In addition, the current treatment also significantly reduced circulating levels of serum PON1 protein levels (Fig. 1). However, when controlling for the reductions in serum PON1 protein levels, serum PON1 activity (specific activity) was not altered with the current intervention (baseline = 114.0 ± 16.8; post-weight loss = 120.1 ± 19.3). Exercise training- and diet-induced weight loss also did not significantly (P > 0.05) alter the amount of PON1 activity (23.8 ± 4.8 vs. 20.0 ± 3.7 U/mg HDL) or PON1 protein concentrations associated with isolated HDL from baseline to post-weight loss (0.14 ± 0.03 vs. 0.11 ± 0.01 μg/mg HDL). Changes in dietary antioxidants (vitamins A, C, and E) were not associated with observed changes in our measures of oxidative stress (data not shown).
Fig. 1.
Effects of exercise training and weight loss on serum-oxidized LDL (oxLDL) concentrations, LDL lipid hydroperoxides, and serum paraoxonase-1 (PON1) protein concentrations and activity (means ± SE). Significantly different from baseline (*P < 0.01; †P = 0.02).
Correlations
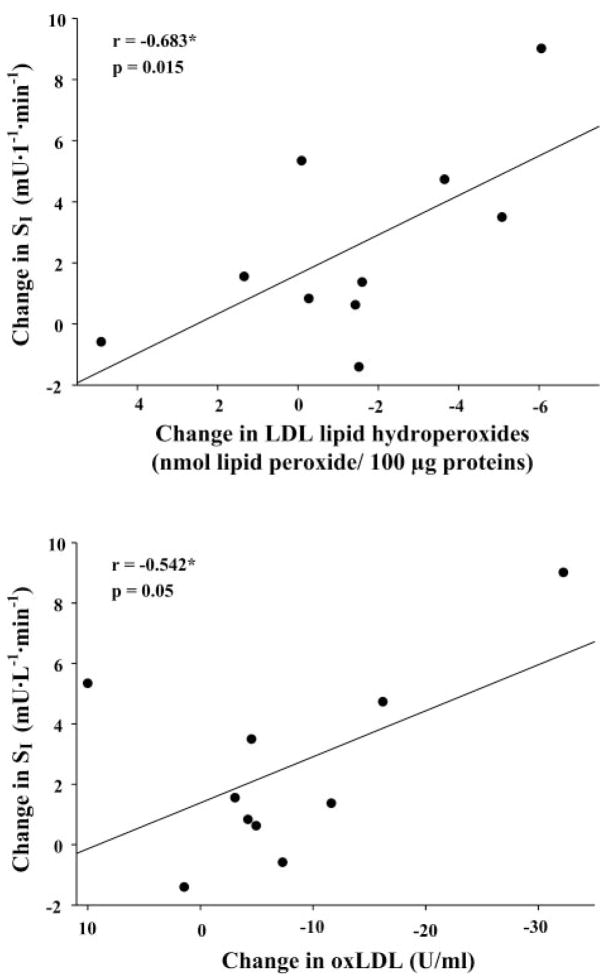
We report significant associations between baseline concentrations of oxLDL with baseline levels of HOMA (r = 0.521, P = 0.004), glucose (r = 0.695, P < 0.001), insulin (r = 0.421, P = 0.018), and inversely with QUICKI (P = −0.499, P = 0.006), suggesting that circulating oxLDL may be contributing to disruption in glucose homeostasis. However, we found no significant correlations among baseline levels of PON1, apoA1, or LDL lipid hydroperoxides and our assessments of insulin sensitivity and resistance (data not shown). Changes in oxidative stress markers were not associated with changes in QUICKI or HOMA (data not shown). However, we do report a significant negative association between reductions in oxLDL and LDL lipid peroxides with improvements in SI by IVGTT (r = −0.542, P = 0.05 and r = −0.683, P = 0.015, respectively; Fig. 2).
Fig. 2.
Associations between changes in oxLDL and LDL lipid hydroperoxide concentrations with insulin sensitivity (SI). *Changes in oxLDL and LDL lipid hydroperoxide concentrations were negatively associated with changes in SI by IVGTT (n = 10; P ≤0.05).
DISCUSSION
Patients with type 2 diabetes, impaired glucose tolerance, and IR are at elevated risk for CHD (24). Elevations in oxidative stress associated with obesity may represent a link between IR and CHD. Oxidative modification of LDL is an initiating event in the atherosclerotic process (37), and during the initial stages of LDL oxidation, endogenous antioxidants are consumed and lipid hydroperoxides accumulate. These lipid hydroperoxides then are converted to reactive aldehydes, which interact with lysine residues of apoB100 and continue oxidative modification of LDL (37). To our knowledge, we are the first group to demonstrate significant reductions in both early-stage (lipid hydroperoxides, −18%) and more complete oxidation (circulating oxLDL, −11%) of LDL in individuals with at least two components of the metabolic syndrome. Previous studies examining circulating oxLDL concentrations following exercise or weight loss interventions are mixed (39, 43). The current findings suggest that the oxidative modification of LDL can be targeted at multiple stages and that interventions aimed at reducing physical inactivity and body weight are an effective means to reduce oxidative stress.
There is evidence to suggest that oxidative stress contributes to the insulin-resistant condition. Oxidative stress is known to attenuate glucose uptake in cultured myocytes (25), and ox-LDL inhibits multiple steps in insulin signaling (27). In addition, glucose and insulin responses to an oral glucose tolerance test correlate with conjugated diene levels (8), and oxLDL is associated with SI in overweight and obese, nondiabetic men (16). The current findings are in agreement with previous reports, as significant associations were found between baseline levels of oxLDL with HOMA and inversely with SI and QUICKI. In addition, our findings of improvements in measures of insulin sensitivity are consistent with previous exercise training interventions (18). Although not a complete data set (n = 10), observed improvements in SI (but not HOMA or QUICKI) were negatively associated with reductions in ox-LDL and LDL lipid hydroperoxides. SI by IVGTT mimics glucose handling during postprandial conditions when IR typically is manifest and may be a more sensitive indicator of the effects of oxidative stress on IR. Further, our results are consistent with the postulate that circulating levels of oxLDL may contribute to IR.
We also report significant increases in apoA1 concentrations following exercise and weight loss in individuals with components of the metabolic syndrome, confirming some aerobic training studies (38, 41), but not all (33). Although it has been demonstrated that apoA1 contributes to the antioxidative capacity of HDL (10), apoA1 did not correlate with markers of IR or oxidative stress in the current investigation. Low HDL-C concentrations predict CHD risk and are associated with IR (20). Total HDL-C was not altered by our intervention, but we do report increases in HDL2-C and decreases in HDL3-C levels, suggesting cardioprotective effects, as the larger HDL2b and HDL2c offer the greater protection against CHD risk (12). It has been suggested that oxidized HDL might prove useful as a clinical tool in humans (35), and although not assessed, if apoA1 is more oxidized to prevent LDL oxidation, this may help explain the lack of reduction in HDL lipid hydroperoxides during the intervention. It is probable that elevations in apoA1 serve to facilitate prevention of oxidation of LDL and may help prevent the disruption of normal insulin signaling and atherosclerosis development. These possibilities deserve further investigation.
A potential source for disruption of HDL and apoA1 function is MPO. MPO generates hypochlorous acid, which oxidatively damages HDL and apoA1, impairing the antioxidative and RCT ability of each, and also increasing the conversion of LDL into oxLDL (28). Results from short-term lifestyle modifications aimed at increasing physical activity and decreasing MPO concentrations are mixed (14, 30, 32). A novel finding in the current investigation was that a longer-duration exercise program with more substantial weight loss did not alter MPO levels in sedentary, overweight, and obese adults with characteristics of the metabolic syndrome. The lack of diagnosed disease in the current study population may explain the discrepancy from previous reports, which included individuals with diabetes (32) and CHD (30) with MPO levels 3–7 times greater than the current report.
Another novel finding in the current investigation was that weight loss via exercise and energy restriction significantly reduced serum PON1 activity and protein concentrations. These findings were contrary to our original hypotheses, as it previously has been shown that PON1 hydrolyzes lipid hydroperoxides in oxidized lipoproteins (3), and PON1 activity is reduced in obesity (11) and the metabolic syndrome (34). Weight reduction (−20%) by gastric banding significantly increased PON1 activity (+25%) in morbidly obese individuals (40); whereas, serum PON1 activity was not altered with exercise training interventions of 3 (31), 12 (30), or 16 wk (39). Furthermore, short-term lifestyle modification failed to alter serum PON1 protein concentrations determined by Western blot analysis (31), contrary to current findings of reduced serum PON1 protein concentrations quantified by ELISA.
Multiple substrates have been identified to describe the activity of PON1, with previous weight loss and exercise interventions using either paraoxon (31, 39, 40) or phenylacetate (30). We chose two substrates to assess PON1 activity in the current investigation. With the use of phenylacetate, we found a nonsignificant reduction in serum PON1 activity (baseline = 92.7 ± 3.4 vs. post-weight loss = 90.0 ± 4.6 U/ml serum; data not shown), whereas with the novel fluorogenic organophosphate analog (Molecular Probes), we report significant reductions in serum PON1 activity (per ml serum).
It previously has been demonstrated that PON1 protein levels are normal or higher than normal in disease conditions (1), yet these populations exhibit reduced PON1 activity. Glycosylation or endogenous inhibitors may inactivate the protein, stimulating increased hepatic production. Weight loss, changes in dietary intake, or increasing physical activity could reduce these inhibitors, resulting in less need for hepatic synthesis. Changes in dietary antioxidant intake were not associated with changes in PON1 status; however, this hypothesis is supported by a significant association between reduced PON1 and reductions in body fat (r = 0.460, P = 0.01) and a negative association with reductions in visceral adipose tissue determined by computed tomography and changes in PON1 activity (data not shown). Furthermore, we found significant positive correlations between reductions in serum PON1 activity and reductions in IR as assessed by HOMA. Regardless of the mechanism, it does not appear that reductions in PON1 were detrimental, as increases in PON1 were not necessary for improvements in the oxidative status of LDL. Moreover, reductions in PON1 may be indicative of the improvement in the overall oxidative environment and in insulin action.
Weight loss in the current investigation was achieved via the combination of exercise training and energy restriction. It is recognized that weight loss is not as simple as energy intake and energy expenditure, and on the basis of calculations and dietary data obtained from the 7-day diet records, it appears that subjects were likely either underreporting or selectively reporting their energy intake during the weight loss intervention. Regardless of the reason for the discrepancy, the subjects lost the prescribed amount of weight.
In summary, we report that exercise training- and diet-induced weight loss cause significant reduction in biomarkers of oxidative stress, which may beneficially impact parameters of IR. Weight reduction by lifestyle modification is a means of reducing coprecipators of CHD and type 2 diabetes.
Acknowledgments
We thank Dr. John Thyfault for manuscript critique and Dr. Chelif Junor and Dr. Guru Govindarajan for stress test supervision. We also thank Meghan Ruebel, Joseph Company, Scott Naples, Michael Scott, and Melissa Linden for exercise training supervision.
GRANTS
This work was supported by National Institutes of Health (NIH) T32 AR48523 and NIH ROI DK67036.
References
- 1.Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1812–1818. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 2.American College of Sport Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 3.Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, Billicke S, Draganov D, Rosenblat M. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation. 2000;101:2510 –2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- 4.Basiotis PP, Carlson A, Gerrior SA, Juan WY, Lino M. The Healthy Eating Index: 1999–2000. U.S. Department of Agriculture, Center for Nutrition Policy and Promotion, Washington, DC: Government Printing Office; 2002. [Google Scholar]
- 5.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6:45– 86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248 –254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA. 1993;90:6434 – 6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000;152:203–208. doi: 10.1016/s0021-9150(99)00460-8. [DOI] [PubMed] [Google Scholar]
- 9.Chung BH, Segrest JP, Ray MJ, Brunzell JD, Hokanson JE, Krauss RM, Beaudrie K, Cone JT. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- 10.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473– 480. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti G, Bacchetti T, Moroni C, Savino S, Liuzzi A, Balzola F, Bicchiega V. Paraoxonase activity in high-density lipoproteins: a comparison between healthy and obese females. J Clin Endocrinol Metab. 2005;90:1728 –1733. doi: 10.1210/jc.2004-0486. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046 –1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499 –502. [PubMed] [Google Scholar]
- 14.Galassetti PR, Nemet D, Pescatello A, Rose-Gottron C, Larson J, Cooper DM. Exercise, caloric restriction, and systemic oxidative stress. J Investig Med. 2006;54:67–75. doi: 10.2310/6650.2005.05024. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 16.Ho RC, Davy K, Davy B, Melby CL. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002;51:1478 –1483. doi: 10.1053/meta.2002.35577. [DOI] [PubMed] [Google Scholar]
- 17.Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, Collen D, Muls E, Van de Werf F. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:844 – 848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 18.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384 –389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 20.Karhapaa P, Malkki M, Laakso M. Isolated low HDL cholesterol. An insulin-resistant state. Diabetes. 1994;43:411– 417. doi: 10.2337/diab.43.3.411. [DOI] [PubMed] [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 22.Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 23.Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 24.Laakso M, Kuusisto J. Epidemiological evidence for the association of hyperglycaemia and atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28:415– 418. doi: 10.3109/07853899608999101. [DOI] [PubMed] [Google Scholar]
- 25.Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404 – 410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412– 419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Maziere C, Morliere P, Santus R, Marcheux V, Louandre C, Conte MA, Maziere JC. Inhibition of insulin signaling by oxidized low density lipoprotein. Protective effect of the antioxidant Vitamin E. Atherosclerosis. 2004;175:23–30. doi: 10.1016/j.atherosclerosis.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 29.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 30.Richter B, Niessner A, Penka M, Grdic M, Steiner S, Strasser B, Ziegler S, Zorn G, Maurer G, Simeon-Rudolf V, Wojta J, Huber K. Endurance training reduces circulating asymmetric dimethylarginine and myeloperoxidase levels in persons at risk of coronary events. Thromb Haemost. 2005;94:1306 –1311. doi: 10.1160/TH05-03-0158. [DOI] [PubMed] [Google Scholar]
- 31.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol. 2006;101:1727–1732. doi: 10.1152/japplphysiol.00345.2006. [DOI] [PubMed] [Google Scholar]
- 32.Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol. 2006;100:1657–1665. doi: 10.1152/japplphysiol.01292.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ruano G, Seip RL, Windemuth A, Zollner S, Tsongalis GJ, Ordovas J, Otvos J, Bilbie C, Miles M, Zoeller R, Visich P, Gordon P, Angelopoulos TJ, Pescatello L, Moyna N, Thompson PD. Apolipoprotein A1 genotype affects the change in high-density lipoprotein cholesterol subfractions with exercise training. Atherosclerosis. 2006;185:65– 69. doi: 10.1016/j.atherosclerosis.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Senti M, Tomas M, Fito M, Weinbrenner T, Covas MI, Sala J, Masia R, Marrugat J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab. 2003;88:5422–5426. doi: 10.1210/jc.2003-030648. [DOI] [PubMed] [Google Scholar]
- 35.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high-density lipoprotein. Curr Opin Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 36.Soukharev S, Hammond DJ. A fluorogenic substrate for detection of organophosphatase activity. Anal Biochem. 2004;327:140 –148. doi: 10.1016/j.ab.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 38.Thompson PD, Yurgalevitch SM, Flynn MM, Zmuda JM, Spannaus-Martin D, Saritelli A, Bausserman L, Herbert PN. Effect of prolonged exercise training without weight loss on high-density lipoprotein metabolism in overweight men. Metabolism. 1997;46:217–223. doi: 10.1016/s0026-0495(97)90305-x. [DOI] [PubMed] [Google Scholar]
- 39.Tomas M, Elosua R, Senti M, Molina L, Vila J, Anglada R, Fito M, Covas MI, Marrugat J. Paraoxonase1-192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J Lipid Res. 2002;43:713–720. [PubMed] [Google Scholar]
- 40.Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes Surg. 2004;14:659 – 665. doi: 10.1381/096089204323093453. [DOI] [PubMed] [Google Scholar]
- 41.Williams PT, Krauss RM, Vranizan KM, Albers JJ, Wood PD. Effects of weight-loss by exercise and by diet on apolipoproteins A-I and A-II and the particle-size distribution of high-density lipoproteins in men. Metabolism. 1992;41:441– 449. doi: 10.1016/0026-0495(92)90082-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang JQ, Thomas TR, Ball SD. Effect of exercise timing on postpran-dial lipemia and HDL cholesterol subfractions. J Appl Physiol. 1998;85:1516 –1522. doi: 10.1152/jappl.1998.85.4.1516. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler S, Schaller G, Mittermayer F, Pleiner J, Mihaly J, Niessner A, Richter B, Steiner-Boeker S, Penak M, Strasser B, Wolzt M. Exercise training improves low-density lipoprotein oxidability in untrained subjects with coronary artery disease. Arch Phys Med Rehabil. 2006;87:265–269. doi: 10.1016/j.apmr.2005.09.025. [DOI] [PubMed] [Google Scholar]