Abstract
The classical concept of linear pathways is being increasingly challenged by network representations, which emphasize the importance of interactions between components of a biological system, and motivates for adopting a system‐level approach in biology. We have developed a dynamical system that integrates quantitative, dynamic and topological representation of network of ERK5 (Extracellular signal‐regulated kinases 5), JNK(c‐Jun N‐terminal kinases) and P38 kinase cascades. We have observered that, the transient activation of ERK5, JNK1 and P38β kinase, and the persistent activation of JNK2, JNK3 and P38 δ kinase does not get affected due to the cross‐talks between ERK5, JNK and P38 kinase cascades. But it is due to the cross ‐ talks, the transiently activated P38α kinase become inactivated, and the transiently activated P38γ kinase become persistently activated. The impacts of one‐way cross‐talks between the cascades are insignificant and differ from the impact of two‐way cross‐talks. We generate a hypothesis that, signaling pathways should be studied as a system by considering the cross‐talks between the two adjacent cascades.
Keywords: cross‐talks, ERK5, JNK, P38 kinase cascades, systems approach
Background
Cells need to adjust to changes in the external environment, to respond to signals from neighboring cells. Binding of extracellular signaling molecules to cell‐surface receptors such as G protein‐coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) activates limited number of signaling pathways. These pathways do not simply transmit, but they also process, encode and integrate internal and external signals. MAPK (mitogen‐activated protein kinase) signal transduction pathways regulate diverse cellular processes ranging from proliferation and differentiation to apoptosis [1]. Five families of MAPKs have been defined in mammalian cells: extra cellular signal‐regulated kinases (ERK1 and ERK2); c‐Jun N‐terminal kinases (JNK1, JNK2 and JNK3); P38 kinase isozymes (P38α, P38β, P38γ and P38δ); ERK3/ERK4; and ERK5. By and large, activation of ERKs has been linked to cell survival, whereas JNKs and P38 kinases are linked to induction of apoptosis. This dichotomy, however, is an oversimplification, and the actual roles of each MAPK cascade are highly cell‐type specific and context‐dependent [2].
In the processes of cellular signaling, protein‐protein interactions play a central role. Protein kinases are enzymes that covalently attach phosphate to the side chain of serine, threonine, or tyrosine of specific proteins inside cells and protein phosphatases remove the phosphates that were transferred to the protein substrate by the kinase. In this manner, the action of MAPKs and protein phosphatases reciprocally and rapidly alter the behavior of cells as they respond to changes in their environment [2]. A MAPKKK that is activated by extracellular stimuli phosphorylates a MAPKK on its serine and threonine residues. This MAPKK activates a MAPK through phosphorylation of its serine and tyrosine residues. The activated MAPKs, in turn, phosphorylate specific serines and threonines of target protein substrates and regulate cellular activities ranging from gene expression, mitosis, movement, metabolism, and programmed death [2]. Based on the information's given in JNK and P38 pathway diagrams deposited in the Science's STKE (Signal Transduction Knowledge Environment) database [3,4], we have drawn the diagram (Figure 1) to depict ERK5, JNK and P38 Kinase cascades with cross‐talks.
Figure 1.
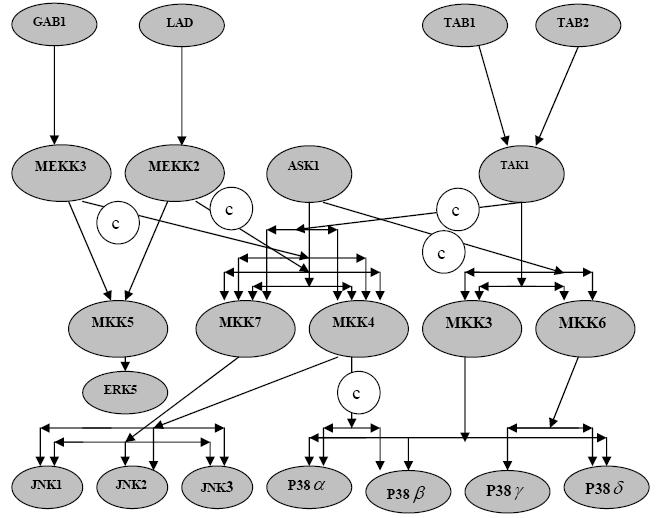
ERK5, JNK and P38 kinase cascades with cross‐talks. © = cross talk
ERK5 is a MAP Kinase regulated by a wide range of mitogens and cellular stresses. The potentially crucial role of ERK5 in cancers and heart diseases make this cascade highly attractive for the development of new therapeutic strategies to treat pathological conditions that are resistant to current therapies [5]. The JNKs consist of three isoforms: JNK1 and JNK2 are the products of alternative splicing of a single gene and are expressed in many tissues, but JNK3 is specifically expressed in neuronal tissue brain. Members of the JNK family play crucial roles in regulating responses to environmental stress, radiation, and growth factors, and in neural development, inflammation, and apoptosis [6]. Four isoforms of P38 MAP kinase: P38α, P38β, P38γ and P38δ have been identified. The P38 MAPKs play an important role in asthma and autoimmunity in humans and are activated by numerous physical and chemical stresses, including hormones, UV irradiation, ischemia, cytokines including interleukin‐1 and tumor necrosis factor, osmotic shock and heat shock [7].
Methodology
See supplementary material for methodology and mathematical formulation. References [8], [9] and [10] are used in supplementary material.
Discussion
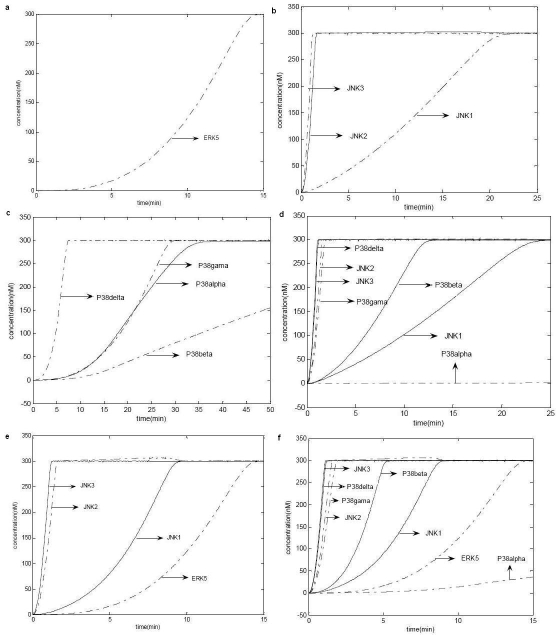
We have assist‐MATLAB to numerically integrate (simulation) the system of differential equations using Adams‐Bash forth algorithm and considered the plots, which represents the activation of ERK5, JNKs and P38 kinases only for analysis. We have observed that, the qualitative behavior of the stimulus / response curves does not change for a range of input signals (1‐5 nM) and for a range of reaction rate constants (0.001 ‐ 3 nM/s). See Figure 2 for simulation results. It might be due to the robust nature of the kinase cascades. Also, we have taken the molar concentrations for MAPKs, MAPKKs and MAPKKKs such that molar concentration of MAPKs are lesser than that of MAPKKs and MAPKKKs, and the molar concentration of MAPKKs and MAPKKKs are equal as it has been observed and used in practice. We have taken the values of Michaelis‐Menten's constants such that, the ratio of the molar concentration of enzymes and the Michaelis‐Menten's constants are in the range of 10 ‐ 20.
Figure 2.
Results of simulation is given for (a) ERK5 cascade with unit‐step input signals; (b) JNK cascade; (c) P38 kinase cascade with unit‐step input signal; (d) JNK and P38 kinase cascades with cross‐talks and unit‐step input signal; (e) ERK5 and JNK cascades with cross‐talks and unit‐step input signals; (f) ERK5, JNK and P38 kinase cascades with cross‐talks and unit‐step input signals.
We have observed that, the transient activation of ERK5, JNK1 and P38β kinase, and the persistent activation of JNK2, JNK3 and P38δ kinase does not get affected due to the cross ‐ talks between ERK5, JNK and P38 kinase cascades. But it is due to the cross ‐ talks, the transiently activated P38α kinase become inactivated, and the transiently activated P38γ kinase become persistently activated. Also, the influence of ERK5 cascade on JNK and P38 kinase cascades is insignificant, might be due to the one way nature of the cross ‐ talks between ERK5 and JNK cascades. Transient activation of ERK5 may trigger cell proliferation [11], persistent activation of JNK2 and JNK3 is associated with apoptosis and the transient activation of JNK1 is associated with cell survival [12,13]. The persistent activation of P38γkinases might mediate process like mitogenesis, cell fate induction [14], and may result in an anti‐angiogenic phenotype that contributes to endothelial dysfunction [15].
Conclusion
The impacts of one‐way cross‐talks between the cascades are insignificant and differ from the impact of two‐way cross‐talks. We generate a hypothesis that, signaling pathways should be studied as a system by considering the cross ‐ talks between the two adjacent cascades.
Supplementary material
Footnotes
Citation:Sundaramurthyet al., Bioinformation 3(6): 244-249 (2009)
References
- 1.Chang L, Karin M. Nature. 2001;410:37. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 2.Johnson GL, Lapadat R. Science. 2002;298:1911. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 3. http://stke.sciencemag.org/cgi/cm/stkecm;CMP_10827
- 4. http://stke.sciencemag.org/cgi/cm/stkecm;CMP_10958
- 5.Kato Y, et al. EMBO J. 1997;16:7054. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis RJ. Cell. 2000;103:239. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 7.Han J, et al. Nature. 1997;20:296. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 8.Wolkenhauer O, et al. Biochem SocTrans. 2005;33:507. [Google Scholar]
- 9.Heinrich R, et al. Mol Cel. 2002;9:957. doi: 10.1016/s1097-2765(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 10.Kholodenko BN, et al. Proc Natl Acad Sci U S A. 2002;99:12841. doi: 10.1073/pnas.192442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scapoli L, et al. Oncogene. 2004;23:805. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- 12.Shaulian E, et al. Cell. 2000;103:6. doi: 10.1016/s0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 13.Reinhard C, et al. EMBO J. 1997;16:5. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrell JE. Trends Biochem Sci. 1996;21:12. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 15.McMullen ME, et al. J Biol Chem. 2005;280:22. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.