Abstract
We report the self-assembly of a hydrophilic 8-(m-acetylphenyl)-2′-deoxyguanosine (mAG) derivative into a discrete and thermally stable hexadecameric supramolecule in aqueous media. We demonstrate that this hexadecamer is isostructural to the one formed by a related lipophilic derivative in organic media. This mAG moiety represents a rare example of a small-molecule recognition motif that is capable of assembling isostructurally and with high fidelity in both organic and aqueous media.
Water plays a central role in how weak noncovalent interactions dictate the outcome of vital processes such as protein folding as well as the formation of protein−protein and protein−nucleic acid complexes.(1) Recently, there has been an increased interest in the construction of synthetic self-assembled supramolecular nanostructures in water due to their potential use in biomedical applications.(2) Even though the field of supramolecular chemistry has made great strides in the construction of complex self-assembled structures in organic media, similar advances in aqueous environments have lagged behind.(3) Nonetheless, progress in this area has been achieved primarily in the construction of stacked and micellar assemblies of indefinite sizes.(4) The synthesis of discrete and well-defined structures in water has been mostly limited to systems that use coordinative covalent bonds for their formation, in particular, in the construction of capsular supramolecules.(5) In contrast, examples of discrete supramolecular assemblies made exclusively with noncovalent interactions have remained somewhat elusive.(5c)
Recently, Schrader and co-workers(6) reported an elegant system based on aminopyrazole peptides that self-assembled into discrete hexameric nanorosettes in water. Unfortunately, the prevalence of Shrader’s hexamer, even at 275 K, was just 40% at 45 mM and 15% at 23 mM in peptide monomers. This example underscores the challenges of developing self-assembling small molecules from scratch. Herein we report the self-assembly of a hydrophilic 8-(m-acetylphenyl)-2′-deoxyguanosine (mAG) derivative into a discrete and thermally stable hexadecameric supramolecule in aqueous media. Furthermore, we demonstrate that this hexadecamer is isostructural to the one formed by a related lipophilic mAG derivative in organic media.
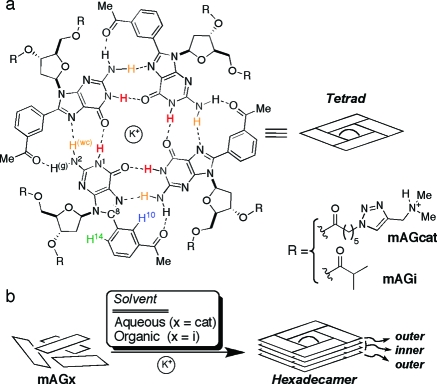
We have previously demonstrated(7) that the self-assembly of lipophilic 8-phenyl-2′-deoxyguanosine derivatives into G-quadruplexes(8) (GQs) could be modulated by replacement of the H8 in the guanine base with a functionalized phenyl group (Figure 1). The position of the functional group in the phenyl ring enables the modulation of the molecularity and thermal stability of the resulting supramolecules. In particular, the mAG scaffold forms hexadecamers in organic media very reliably, regardless of the group attached to the sugar. We recently reported taking advantage of this strategy to construct hexadecameric self-assembled dendrimers.(7b)
Figure 1.
(a) Chemical structure of the mAG tetrad. (b) Cartoon representation for the formation of a hexadecamer formed by mAGx derivatives in aqueous or organic media. A hexadecamer is composed of two sets of tetrads that occur in distinct chemical environments.
In this study, we aimed at elucidating if the mAG scaffold could also self-assemble in water, and if it did, what would it form. We hypothesized that, because the GQs were held together by a combination of noncovalent interactions,(9) the self-assembly in water was possible as long as we could synthesize soluble derivatives. We achieved this by making the dimethylamino derivatives mAGcat and the control compound dGcat (which has no 8-phenyl substitution) using a synthetic methodology similar to that previously described by our group (Scheme S1).(7) At neutral pH, the positively charged ammonium groups render both derivatives soluble in water.
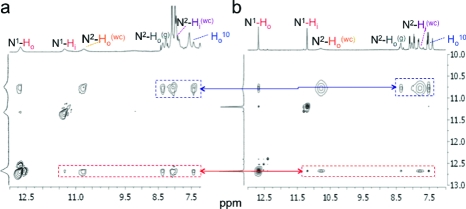
1H NMR experiments with mAGcat in H2O−D2O (9:1, with water suppression) reveal many of the signatures of a quadruplex assembly in a solution containing KCl (1 M).(10) The presence of two peaks between 11 and 13 ppm is characteristic of the N1H protons, which are in slow exchange with the solvent (Figure 2). A comparison with the spectrum of mAGi in CD3CN reveals striking similarities between the two species in key areas of their spectra. In contrast, control experiments with the parent compound dGcat show no evidence of self-assembly even at higher concentrations (Figure S18).(11) These findings underscore the importance of the 8-phenyl group in the self-assembly, which enhances the noncovalent interactions such as hydrophobic contacts, stacking, and H bonds.
Figure 2.
1H NMR and partial 2D NOESY spectra showing signature cross-peak patterns of (a) mAGcat (10 mM) in H2O−D2O (9:1) with KCl; (b) mAGi (50 mM) in CD3CN saturated in KI (o = outer, i = inner). (See Figure S24 for more details.)
The CD spectra of both mAGcat and mAGi exhibit very similar signatures with a negative band near 330 nm and a positive band near 290 nm (Figure S14). Despite their different environments, the close correlation of their CD spectra is a strong indication they share a similar structure at the core.(12)
The hexadecamers of both mAGcat and mAGi display parallel signature cross-peak patterns in their 2D NOESY spectra in H2O−D2O (9:1) and CD3CN, respectively (Figure 2). These experiments offer further evidence to support that mAGcat and mAGi form supramolecules of the same molecularity (hexadecamers). Furthermore, parallel to the CD results, the NOESY spectra indicate that the stereochemical arrangement of the mAG moieties within the core of the quadruplex is nearly identical (i.e., isostructural).
In order to get a sense of the size of the (mAGcat)16•3KCl, we performed 1H DOSY NMR experiments to determine its molecular translational coefficient (D). We found that D = (0.776 ± 0.020) × 10−10 m2 s−1 at 298 K, which corresponds to a hydrodynamic radius (r′H) of 25.5 Å.(13) This value is in close agreement with 24.8 Å, which was obtained from a molecular model of the mAGcat hexadecamer (Figures S25 and S26).(14) In contrast, (mAGi)16•3KI has a D = (6.430 ± 0.114) × 10−10 m2 s−1 at 298 K in CD3CN, which corresponds to a smaller r′′H of 10.0 Å. The differences in sizes are due to the shorter isopropyl side chains in mAGi and the fact that the side chains in mAGcat are more extended due to the repulsion between the cationic ammonium groups (Figure 3).
Figure 3.
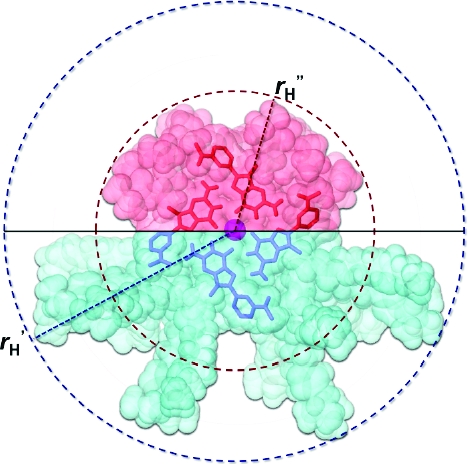
Comparison of the relative sizes for the hexadecamers of mAGcat (blue, r′H) and mAGi (red, r′′H) highlighting the hydrodynamic radii. Only the top tetrads are highlighted for clarity. The purple sphere represents a potassium cation.(14)
Analysis of the melting curve of mAGcat, obtained by variable temperature (VT) NMR, reveals a Tm of 60.7 °C, which is once again remarkably close to the value obtained for mAGi in CD3CN of 56.4 °C (Figure S27).(7) A detailed thermodynamic profile using differential scanning calorimetry (DSC) shows a monophasic transition at 55.1 °C with a favorable ΔG (293 K) value, −2.1 kcal/mol (ΔH = −33.7 kcal/mol, TΔS −31.6 kcal/mol) for the formation of the quadruplex and results from the compensation of a favorable enthalpy with an unfavorable entropy contribution (assembly of 16 subunits and 3 cations).(15) Compared to dGcat, mAGcat has a larger surface area and greater hydrophobicity, both of which have been shown to stabilize supramolecular structures in water due to enhanced van der Waals and π-stacking.(16) The mAGcat tetrads can also form up to four additional H bonds due to the expanded Hoogsteen edge (Figure 1a).7,17
In conclusion, the mAG moiety represents a remarkable example of a small-molecule recognition motif that is capable of assembling isostructurally in organic or aqueous media with high fidelity and stability. We expect that this and related derivatives will offer important insights into the rules that govern the self-assembly of small molecules in aqueous media. We are currently using this and related compounds to construct self-assembled nanostructures in aqueous media for biomedical applications.(2)
Acknowledgments
We thank Prof. Gian Piero Spada (Universitá di Bologna) and Prof. Beatriz Zayas (UMET, PR) for assistance with CD and MS studies, respectively. We also thank Ivonne Andújar and José E. Betancourt for their helpful technical assistance. G.H. thanks NIH-SCoRE (2506GM08102), and M.G-A. thanks NIH-RISE (2R25GM61151), NSF-EPSCoR (EPS0223152), PRLSAMP-HRD-0114586, and SLOAN for graduate fellowships.
Supporting Information Available
Detailed experimental protocols and spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ball P. Chem. Rev. 2008, 108, 74–108. [DOI] [PubMed] [Google Scholar]
- For examples of self-assembled nanostructures used for biomedical applications, see:; a Dankers P. Y. W.; Meijer E. W. Bull. Chem. Soc. Jpn. 2007, 80, 2047–2073. [Google Scholar]; b Jun H.; Paramonov S. E.; Hartgerink J. D. Soft Matter 2006, 177–181. [DOI] [PubMed] [Google Scholar]
- Oshovsky G. V.; Reinhoudt D. N.; Verboom W. Angew. Chem., Int. Ed. 2007, 46, 2366–2393. [DOI] [PubMed] [Google Scholar]
- a Fenniri H.; Deng B.-L.; Ribbe A. E.; Hallenga K.; Jacob J.; Thiyagarajan P. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fenniri H.; Mathivanan P.; Vidale K. L.; Sherman D. M.; Hallenga K.; Wood K. V.; Stowell J. G. J. Am. Chem. Soc. 2001, 123, 3854–3855. [DOI] [PubMed] [Google Scholar]; c Hartgerink J. D.; Beniash E.; Stupp S. I. Science 2001, 294, 1684–1688. [DOI] [PubMed] [Google Scholar]; d Hartgerink J. D.; Beniash E.; Stupp S. I. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ryu J. H.; Hong D. J.; Lee M. Chem. Commun. 2008, 1043–1054. [DOI] [PubMed] [Google Scholar]; f Kato T.; Mizoshita N.; Kishimoto K. Angew. Chem., Int. Ed. 2006, 45, 38–68. [DOI] [PubMed] [Google Scholar]
- For representative examples of capsular assemblies made with coordinative covalent bonds, see:; a Fujita M.; Tominaga M.; Hori A.; Therrien B. Acc. Chem. Res. 2005, 38, 369–378. [DOI] [PubMed] [Google Scholar]; b Fiedler D.; Leung D. H.; Bergman R. G.; Raymond K. N. Acc. Chem. Res. 2005, 38, 349–358. [DOI] [PubMed] [Google Scholar]; For an example of a hydrophobically driven self-assembled capsule, see:; c Gibb C. L. D.; Gibb B. C. J. Am. Chem. Soc. 2004, 126, 11408–11409. [DOI] [PubMed] [Google Scholar]
- Rzepecki P.; Hochdorffer K.; Schaller T.; Zienau J.; Harms K.; Ochsenfeld C.; Xie X.; Schrader T. J. Am. Chem. Soc. 2008, 130, 586–591. [DOI] [PubMed] [Google Scholar]
- a Gubala V.; Betancourt J. E.; Rivera J. M. Org. Lett. 2004, 6, 4735–4738. [DOI] [PubMed] [Google Scholar]; b Betancourt J. E.; Rivera J. M. Org. Lett. 2008, 10, 2287–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hobley G.; Gubala V.; Rivera-Sánchez M. C.; Rivera J. M. Synlett 2008, 1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Davis J. T.; Spada G. P. Chem. Soc. Rev. 2007, 36, 296–313. [DOI] [PubMed] [Google Scholar]; b Davis J. T. Angew. Chem., Int. Ed. 2004, 43, 668–698. [DOI] [PubMed] [Google Scholar]
- Rehm T.; Schmuck C. Chem. Commun. 2008, 801–813. [DOI] [PubMed] [Google Scholar]
- Although the studies reported here were performed using 1 M KCl, titration experiments of mAGcat with KCl show the presence of 31% hexadecamer even with 0.6 mM KCl (Figure S17)
- Studies with 5′-GMP have also shown that it self-assembles with low fidelity in aqueous KCl solutions but only at very high concentrations (0.15−1.0 M), giving a mixture of columnar aggregates of indefinite length (stacks of <24−87 tetrads):; a Fisk C. L.; Becker E. D.; Miles H. T.; Pinnavaia T. J. J. Am. Chem. Soc. 1982, 104, 3307–3314. [Google Scholar]; b Bouhoutsos-Brown E.; Marshall C. L.; Pinnavaia T. J. J. Am. Chem. Soc. 1982, 104, 6576–6584. [Google Scholar]; c Wong A.; Ida R.; Spindler L.; Wu G. J. Am. Chem. Soc. 2005, 127, 6990–6998. [DOI] [PubMed] [Google Scholar]
- From the CD results, it can be inferred that the helical twist between the planar tetrads is very similar for the hexadecamers of mAGcat and mAGi due to the relative stereochemical arrangements of the subunits. For further information, see:; Paramasivan S.; Rujan I.; Bolton P. H. Methods 2007, 43, 324–331. [DOI] [PubMed] [Google Scholar]
- a Cohen Y.; Avram L.; Frish L. Angew. Chem., Int. Ed. 2005, 44, 520–554. [DOI] [PubMed] [Google Scholar]; b Kaucher M. S.; Lam Y. F.; Pieraccini S.; Gottarelli G.; Davis J. T. Chem.—Eur. J. 2004, 11, 164–173. [DOI] [PubMed] [Google Scholar]
- Minimizations were performed using:; AMBER* (MacroModel), Version 9.5, Maestro 8.0.315; Schrödinger, LLC: New York, 2007, representing water as a continuum solvent.
- These thermodynamic parameters are similar to those determined from the calorimetric measurements obtained for DNA aptamers capable of forming G-quadruplexes. See:; a Olsen C. M.; Gmeiner W. H.; Marky L. A. J. Phys. Chem. B 2006, 110, 6962–6969. [DOI] [PubMed] [Google Scholar]; b Mergny J. L.; De Cian A.; Ghelab A.; Saccà B.; Lacroix L. Nucleic Acids Res. 2005, 33, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rachwal P. A.; Fox K. R. Methods 2007, 43, 291–301. [DOI] [PubMed] [Google Scholar]
- a Grimme S. Angew. Chem., Int. Ed. 2008, 47, 3430–3434. [DOI] [PubMed] [Google Scholar]; b Guckian K. M.; Schweitzer B. A.; Ren R. X. F.; Sheils C. J.; Tahmassebi D. C.; Kool E. T. J. Am. Chem. Soc. 2000, 122, 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Krueger A. T.; Lu H.; Lee A. H.; Kool E. T. Acc. Chem. Res. 2007, 40, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins L. J.; Reinhoudt D. N.; Timmerman P. Angew. Chem., Int. Ed. 2001, 40, 2382–2426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.