Summary
In vitro studies suggest that the proapoptotic function of forkhead protein FKHR is probably inactivated by means of phosphorylation through the protein kinase B pathway. However, the clinical significance of FKHR in prostate cancer remains unclear. Six hundred forty radical prostatectomies were used for building tissue microarrays. Slides were stained with antibodies against FKHR and phosphorylated FKHR (p-FKHR). Correlations with clinicopathologic parameters were analyzed by Spearman rank test. Cox regression test and Kaplan-Meier test were used to determine the probability of disease recurrence, which is defined as a serum prostate-specific antigen (PSA) level greater than 0.4 ng/mL after radical prostatectomy. Nuclear FKHR level was higher in normal prostate than in benign prostatic hyperplasia and prostate cancer (P = .0000). Nuclear expression of FKHR was correlated with preoperative PSA level (ρ = 0.108, P = .029), extracapsular extension (ρ = 0.137, P = .005), and seminal vesicle invasion (ρ = 0.101, P = .039). FKHR expression was not a significant indicator of biochemical failure by either univariate or multivariate analysis. Nuclear p-FKHR expression correlated with patients’ age (ρ = 0.179, P = .0003), Gleason score (ρ = 0.130, P = .0083), extracapsular extension (ρ = 0.227, P = .0000), clinical stage (Union Internationale Contre le Cancer system) (ρ = 0.166, P = .0007), and lymph node status (ρ = 0.101, P = .0401). Cytoplasmic p-FKHR correlated with patients’ age (ρ = 0.146, P = .0030) and clinical stage (ρ = 0.117, P = .0180). Cytoplasmic p-FKHR was a significant indicator of biochemical recurrence (P = .0164; hazard ratio, 1.114–2.929). Nuclear p-FKHR strongly correlated with phosphorylated protein kinase B (ρ = 0.368, P = .0000), androgen receptor (ρ = 0.385, P = .0000), and Skp-2 (ρ = 0.170, P = .0036). Our data suggest that the proapoptotic role of FKHR is probably regulated by several signaling pathways in prostate cancer.
Keywords: Forkhead transcription factor, Apoptosis, Prostate cancer
1. Introduction
Prostate cancer (PCa) lesions are heterogeneous and contain a group of biologically distinct components, ie, androgen-sensitive and androgen-insensitive cells [1]. This could explain why PCa can respond to initial androgen withdrawal, but many patients with PCa end up with recurrence and androgen independence, leading to accelerated disease progression and death [2,3]. A number of regulatory pathways such as androgen receptor (AR) signaling pathway [4,5], mitogen-activated protein kinase signaling pathway [6], and Akt/PKB (protein kinase B) signaling pathway [7,8] may play critical roles in prosurvival/proliferation in PCa.
Malignant tumor is characterized by increased proliferation and/or decreased apoptosis. Increasing evidence indicates that the control of proliferation/apoptosis is tied to changes in activities of many regulatory pathways. Hence, understanding these regulatory pathways is of great importance because the discovery of the key regulators not only may yield precise prognostic information, but also may bring about a pharmaceutical breakthrough for PCa.
The Akt/PKB (protein kinase B) signaling pathway has been considered as one of the most important pathways in regulating cell survival. It has been demonstrated that cells with activated Akt/PKB pathway can withstand apoptotic stimuli [9]. The Akt/PKB can promote survival by regulating a number of transcription factors including forkhead transcription factors. The Forkhead (FoxO) family of transcription factors consists of 4 isoforms: FKHR/FoxO1, FoxO2, FKHRL1/FoxO3, and AFX/FoxO4 [10]. Forkhead proteins act as important downstream targets of Akt/PKB, and their phosphorylation by Akt/PKB can regulate cell survival by operating their target genes [10,11]. Besides, forkhead proteins may inhibit cell proliferation by down-regulating cyclin D and promote apoptosis by up-regulating Fas ligand [12,13].
Recently, forkhead transcription factors have drawn more and more attention because of their proapoptotic property and their involvement in many regulatory pathways. In PCa, FKHR might be an important target for both AKT-dependent and -independent survival signals [14]. Of note, androgens and AR seem to act synergistically to suppress FKHR-facilitated apoptosis in PCa. For instance, androgens negatively regulate FKHR via a proteolytic mechanism, whereas activated AR inhibits the ability of FKHR to bind DNA response elements and suppress FKHR-induced Fas ligand expression, thus impairing the ability of FKHR to induce apoptosis and cell cycle arrest in PCa cells [14,15]. More significantly, FKHR, one of the forkhead proteins and a potential biomarker, has been associated with disease progression in leukemia and alveolar rhabdomyosarcoma [16,17], but its clinical and pathologic significance in PCa has not been well documented. In this study, we examined expressions of both nonphosphorylated (FKHR) and phosphorylated forms (p-FKHR) of FKHR in a large cohort of radical prostatectomies using tissue microarray (TMA) technology. The levels of both forms of FKHR were then analyzed for their correlates with clinicopathologic markers as well as survival pathway–associated biomarkers in PCa.
2. Materials and methods
2.1. Clinical and pathologic characteristics
The initial cohort consisted of 1120 patients who underwent radical prostatectomy (RP) at Baylor College of Medicine (Houston, TX)–affiliated hospitals between 1983 and 1998. We qualified 640 cases for building TMAs based on the following criteria: (1) patients did not receive preoperative treatment, (2) patients were operated on between 1983 and 1998, and (3) sufficient PCa tissue is available for building TMA. The patients’ age ranged from 38 to 80 years (mean, 61.9; median, 59.0 years): 4% were younger than 50 years; 28% were aged 50 to 59 years; 52%, 60 to 69 years; and 16%, older than 70 years. Preoperative prostate-specific antigen (pre-PSA) level ranged from 0.3 to 97.5 ng/mL (mean, 10.36 ng/mL), with 30% of patients having a pre-PSA level greater than 10.36 ng/mL. Lower Gleason score (GS) (3–6) was present in 33% patients, whereas 67% had a higher GS (7–10). Biochemical recurrence occurred in 15.5% of patients, whereas 5.6% had lymph node (LN) metastases. Extracapsular extension (ECE) was found in 44.5% of patients. Seminal vesicle invasion (SVI) was present in 12.4% and surgical margins (SMs) in 15.3% of patients. Follow-up data included PSA recurrence (defined as PSA >0.4 ng/mL or 2 consecutive increases) and metastasis. Follow-up duration was up to 187 months (mean, 45 months; median, 84 months). Tissue recruitment was in accordance with institutional review board approval.
RP specimens from these patients were processed and prepared for whole-mount slides according to procedures described previously [18]. A single pathologist (T.M.W.) performed the pathologic analysis that included evaluation of staging, pathologic stage, SM, SVI, ECE, primary and secondary GS, LN status, and geographic location on RP specimens. Other clinical and pathologic data on patients who met the entry criteria were also available for analysis in the Baylor Prostate SPORE data bank.
2.2. Tissue microarray
The TMAs were constructed by using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD). Briefly, whole-mount slides were reviewed and mapped. The index tumor, defined as the largest and/or highest GS was identified on the slide, and areas representative of the highest GS were circled. Areas of normal peripheral zone away from the tumor and benign prostatic hyperplasia (BPH) were also circled. The chosen areas of normal prostate tissue were predominantly free of inflammation, atrophy, or other pathologic changes. Triplicate 0.6-mm cores were obtained from these areas and transferred to a recipient paraffin block. Sausage internal controls, which included up to 10 different types of tissues within each 0.6-mm control core, were also placed with the standard controls. The final tissue array set consisted of 15 blocks with 9 cores for every one of the 640 patients and controls for a total of approximately 6000 cores.
2.3. Immunohistochemistry
Immunohistochemical staining of FKHR and p-FKHR on TMA slides was conducted by using an automated immunostainer (DAKO, Carpinteria, CA). Briefly, sections were deparaffinized in xylene, rehydrated through decreasing concentrations of alcohol ending in phosphate-buffered saline, subjected to steam heat in 10 mmol/L citrate buffer (pH 6.0) for 40 minutes in a vegetable steamer, then allowed to cool off at room temperature for an additional 10 minutes. After endogenous peroxidase activity was quenched in 3% hydrogen peroxide solution in distilled water, sections were incubated with rabbit polyclonal antibody against FKHR and Afx at 65 to 70 kilodaltons (1:40, overnight at 4°C; cat no. 9462, which detects endogenous levels of FKHR/FOXO1 and FKHR/FOXO4) and rabbit antibody against p-FKHR at serine 256 (1:25, 30 minutes at room temperature; cat no. 9461, which detects endogenous levels of p-FKHR/FOXO1 and p-FKHR/FOXO4) (both from Cell Signaling Technology, Millville, NJ). Sections were washed and the bound antibody was detected by using a DAKO Envision Plus kit (DAKO) with diaminobenzidine as chromogen. Finally, sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were sections immunostained as above, but normal rabbit serum was used instead of primary antibody.
2.4. Image procurement and interpretation
An automated slide scanner (Bacus Laboratories, Lombard, IL) was used to digitize all the stained TMA slides to produce an image of every dot and also inform the dot coordinates on the slide. Each image was interpreted for immunoreactivity by using a 0 to 3+ semiquantitation scoring system for both the intensity of stain and percentage of positive cells (percent labeling frequency). For the intensity, the grading scale ranged from no detectable signal (0) to strong signal seen at low power (3); 2 corresponds to moderate signal seen at low to intermediate power, and 1 corresponds to weak signal seen only at intermediate to high power. Labeling frequency was scored as 0 (0%), 1 (1%–33%), 2 (34%–66%), or 3 (67%–100%). Because of the triplicate nature of the arrays, 3 values were obtained for every measurement. To represent the intensity of hot spots, the highest intensity value was used. The average of the 3 percentage values was used for analysis. The staining index was obtained by multiplying the score of intensity with that of percentage. In the case of nuclear and cytoplasmic expression, both nuclear and cytoplasmic staining signals were interpreted and recorded separately.
2.5. Statistical analysis
The differences of FKHR and p-FKHR were compared between normal prostate and cancer specimens by using matched pair analysis (Wilcoxon rank tests). The biological, clinical, and pathologic correlates of FKHR and p-FKHR were analyzed with the Spearman correlation test. The predictive value of FKHR and p-FKHR for biochemical recurrence–free survival was determined by Kaplan-Meier actuarial analysis and the log-rank test. In addition, the Cox proportional hazard regression model was used to assess the prognostic value of FKHR and p-FKHR in PCa. Risk ratios and 95% confidence intervals were recorded for each marker. All analyses were performed with statistical software (SPSS 11.0, SPSS, Chicago, IL).
3. Results
3.1. FKHR and p-FKHR in nonneoplastic prostate and PCa
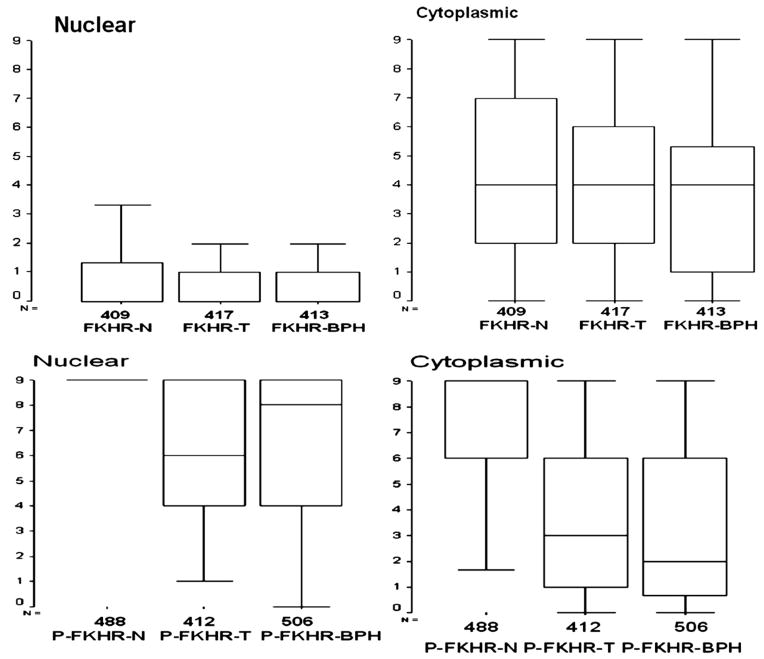
Because of repeated cutting, small foci of cancer can be lost due to 3-dimensional changes in tissue cores. Antigen-retrieval process also contributed to tissue damage and/or core loss. These cores were thus disqualified and excluded from the analysis. Therefore, variable numbers of cases were presented based on interpretability of data in normal prostate, PCa, and BPH (Fig. 2).
Fig. 2.
Comparison of FKHR and p-FKHR expression among normal prostate, BPH, and PCa. Top, Difference in nuclear and cytoplasmic expression of FKHR. Bottom, Difference in nuclear and cytoplasmic expression of p-FKHR among these 3 histologic types.
3.1.1. Forkhead protein FKHR
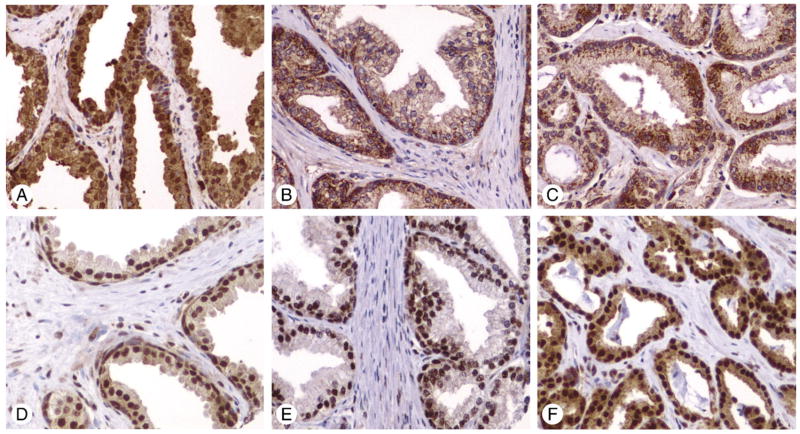
FKHR expression was located in either the nucleus or cytoplasm or both, but predominately in cytoplasm (Fig. 1A). Cytoplasmic expression of FKHR (mean ± SD) was higher in normal prostate (4.43 ± 2.96) and PCa (4.45 ± 2.74) than in BPH (3.61 ± 2.96) (P = .87, normal versus PCa; P < .0001, normal versus BPH and BPH versus PCa) (Fig. 2). Nuclear FKHR was higher in normal prostate (1.33 ± 2.16) than in BPH (1.00 ± 1.90) and PCa (0.82 ± 1.51) (P < .0001, normal versus PCa; P < .0001, normal versus BPH) (Figs. 1A–C and 2).
Fig. 1.
A–C, Immunostaining of FKHR. A, Normal prostate showing strong nuclear and cytoplasmic staining; B, BPH showing weak cytoplasmic expression; C, PCa showing moderate cytoplasmic staining. D–F, Immunostaining of p-FKHR. D, Normal prostate showing strong nuclear and weak cytoplasmic staining; E, BPH showing strong nuclear but no cytoplasmic expression; F, PCa showing both strong nuclear and cytoplasmic staining. (Immunohistochemistry, ABC method. Original magnification ×200).
3.1.2. p-FKHR
p-FKHR expression was strongly present in either nucleus or cytoplasm or both. Nuclear p-FKHR was consistently higher in normal prostate (mean ± SD, 8.57 ± 1.22) than in BPH (6.69 ± 2.67) and PCa (6.39 ± 2.51) (P < .0001, normal versus PCa; P < .0001, normal versus BPH). The cytoplasmic p-FKHR was also higher in normal prostate (6.86 ± 2.75) than in BPH (3.48 ± 3.28) and PCa (3.71 ± 3.17). (P < .0001, normal versus PCa; P < .0001, normal versus BPH) (Figs. 1D–F and 2).
3.2. Clinicopathologic correlates of FKHR and p-FKHR in PCa
Because forkhead proteins are DNA-binding proteins, nuclear localization of forkhead proteins seems to be essential for operating their biological functions. Indeed, it has been suggested that within the nucleus, FKHRL1 (one of the 4 isoforms of forkhead proteins) triggers apoptosis most likely by inducing the expression of genes that promote cell growth arrest and apoptosis [19]. Thus, cytoplasmic sequestration and/or phosphorylation of FKHR would disrupt FKHR’s proapoptotic function, which means an increased level of p-FKHR (either nuclear or cytoplasmic) might denote an enhanced survival. This antiapoptotic strategy by phosphorylation of FKHR is probably critical in PCa as our data showed that in cancer cells, the mean level of nuclear p-FKHR (6.39 ± 2.51) was significantly higher than that of nuclear FKHR (0.82 ± 1.51) (P < .001).
Nuclear but not cytoplasmic expression of FKHR correlated weakly with pre-PSA (ρ = 0.108, P = .029), ECE (ρ = 0.137, P = .005), and SVI (ρ = 0.101, P = .039). FKHR expression did not correlate with LN status, GS, and SM. In contrast, nuclear p-FKHR expression was more strongly correlated with patients’ age (ρ = 0.179, P = .0003), GS (ρ = 0.130, P = .0083), ECE (ρ = 0.227, P = .0000), and Union Internationale Contre le Cancer (UICC) clinical stage (ρ = 0.166, P = .0007) and weakly correlated with LN metastasis (ρ = 0.101, P = .0401), but was not correlated with pre-PSA, SVI, and SM. Cytoplasmic p-FKHR correlated weakly with patients’ age (ρ = 0.146, P = .0030) and UICC stage (ρ = 0.117, P = .0180) (Table 1).
Table 1.
Clinicopathologic correlations of FKHR and p-FKHR in prostate cancer
| Variables | FKHR |
p-FKHR |
||
|---|---|---|---|---|
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | |
| Pre-PSA | ||||
| ρ | 0.108 | 0.025 | −0.064 | 0.082 |
| P (2-tailed) | .029 | .613 | .194 | .097 |
| n | 407 | 406 | 413 | 413 |
| Age | ||||
| ρ | 0.011 | −0.073 | 0.179 | 0.146 |
| P (2-tailed) | .821 | .138 | .0003 | .003 |
| n | 417 | 415 | 412 | 412 |
| GS | ||||
| ρ | 0.021 | 0.070 | 0.1300 | 0.096 |
| P (2-tailed) | .675 | .155 | .008 | .510 |
| n | 417 | 416 | 413 | 413 |
| UICC stage | ||||
| ρ | −0.008 | −0.02 | 0.166 | 0.117 |
| P (2-tailed) | .875 | .678 | .0007 | .018 |
| n | 416 | 415 | 412 | 412 |
| LN | ||||
| ρ | 0.037 | 0.016 | 0.101 | 0.073 |
| P (2-tailed) | .453 | .749 | .0401 | .140 |
| n | 417 | 416 | 413 | 413 |
| ECE | ||||
| ρ | 0.137 | 0.076 | 0.227 | 0.094 |
| P (2-tailed) | .005 | .121 | .0000 | .057 |
| n | 417 | 416 | 413 | 413 |
| SVI | ||||
| ρ | 0.101 | −0.022 | 0.063 | 0.019 |
| P (2-tailed) | .0399 | .650 | .2016 | .704 |
| n | 417 | 416 | 413 | 413 |
| SM | ||||
| ρ | 0.005 | 0.002 | −0.041 | 0.096 |
| P (2-tailed) | .920 | .155 | .409 | .051 |
| n | 417 | 416 | 413 | 413 |
NOTE. Correlations between FKHR, p-FKHR, and prognostic factors in PCa by Spearman correlation test. Numbers in bold indicate significant correlations.
Abbreviations: ρ, correlation coefficient; n, number of patients.
3.3. Prognostic significance of FKHR and p-FKHR in PCa
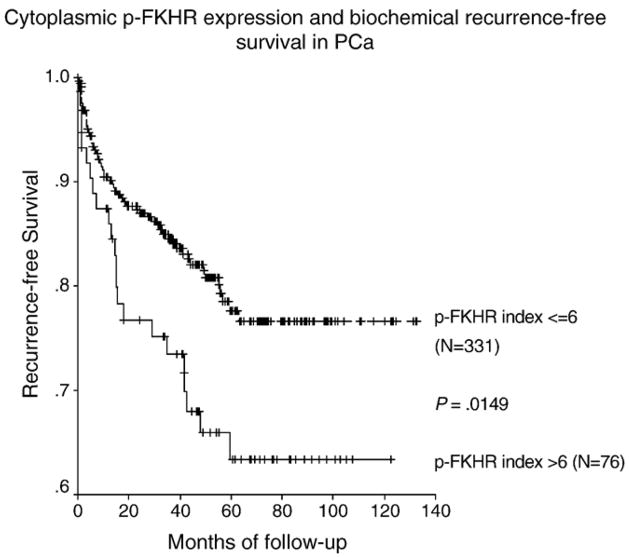
We tested the prognostic significance of FKHR and p-FKHR in terms of nuclear or cytoplasmic expression by Kaplan-Meier survival analysis and Cox regression model. We did not observe any significant predictive value of FKHR (either nuclear or cytoplasmic) and nuclear p-FKHR expression except for cytoplasmic p-FKHR. Cytoplasmic p-FKHR was inversely associated with biochemical recurrence–free survival. We did an extensive search for the cutoff value of cytoplasmic p-FKHR and found that when cytoplasmic expression of p-FKHR was grouped into high level (expression index >6) and low level (expression index ≤6) (Fig. 3), patients with high levels of cytoplasmic p-FKHR had a shorter recurrence-free survival than did patients with low levels of cytoplasmic p-FKHR (mean survival, 85.58 months in 76 patients with high levels versus 107.55 months in 331 patients with low levels, P = .0149). Cytoplasmic expression was a significant indicator of biochemical recurrence (P = .0164; hazard ratio [HR], 1.114–2.929) as determined by univariate analysis; however, it was not significant by multivariate analysis.
Fig. 3.
Kaplan-Meier survival analysis. Patients with high levels of cytoplasmic p-FKHR had a shorter biochemical recurrence–free survival than did patients with low levels of cytoplasmic p-FKHR (mean survival, 85.58 months in 76 patients with high levels versus 107.55 months in 331 patients with low levels, P = .0149).
3.4. Biological correlates of FKHR and p-FKHR in the prostate
Because forkhead proteins are the key molecules associated with several prosurvival/proliferation pathways, it would make sense to look at the correlations among FKHR and p-FKHR and relevant regulatory proteins such as p-Akt and AR. The p-Akt and AR data were available in the TMA master database and were published previously [4,8,20]. Significantly, there was a strong correlation between p-FKHR and p-Akt (ρ = 0.327, P < .0001, nuclear p-FKHR versus p-Akt; ρ = 0.368, P < .0001, cytoplasmic p-FKHR versus p-Akt), AR (ρ = 0.385, P <.0001, nuclear p-FKHR versus AR; ρ = 0.228, P <.0001, cytoplasmic p-FKHR versus AR), and Skp-2 (ρ = 0.170, P = .0036, nuclear p-FKHR versus Skp-2). Both nuclear and cytoplasmic FKHR were strongly correlated with p-Akt (ρ = 0.226, P < .0001; ρ = 0.370, P < .0001, respectively). Cytoplasmic but not nuclear FKHR was correlated with AR (ρ = 0.193, P = .0002, cytoplasmic FKHR versus AR). FKHR (either cytoplasmic or nuclear) and cytoplasmic p-FKHR did not correlate with Skp-2. These results indicated that forkhead proteins were probably associated with survival via Akt/PKB and/or AR signaling pathways or Skp-2 overexpression in PCa. Because p-FKHR was strongly expressed in normal prostate, we also explored the correlations among p-FKHR and p-Akt, AR, and Skp-2. Surprisingly, no correlations were found in normal prostate.
4. Discussion
We examined the biological and clinicopathologic correlates of FKHR and p-FKHR in PCa. Nuclear FKHR expression in PCa was significantly decreased compared with normal prostate, whereas cytoplasmic levels of FKHR were not significantly different among normal prostate, BPH, and PCa. p-FKHR was more strongly expressed in normal prostate compared with BPH and PCa. It seems that the distinct expression of FKHR and p-FKHR might be tied to their roles in the biology of PCa. Indeed, many established prognostic markers were correlated with p-FKHR and FKHR in this study. Taken together, our data suggest that a lower level of FKHR and/or high level of p-FKHR are the indicators of more aggressive biological features of PCa. Therefore, forkhead proteins might be important regulatory proteins associated with tumor growth and progression in PCa.
FKHR levels can be regulated by a variety of mechanisms such as FKHR inactivation by Akt/PKB [10] and/or AR signaling pathways [14,15]. It is known that activated Akt can phosphorylate many downstream proapoptotic proteins including forkhead proteins [10]. p-FKHR is then translocated from nucleus to cytoplasm where FKHR is not functionally active [19]. Our data demonstrated that both FKHR and p-FKHR were closely correlated with p-Akt. This is significant because it is evident that the Akt/PKB pathway is probably one of the major prosurvival mechanisms in regulating forkhead proteins. Moreover, our data also demonstrated a close correlation between AR levels and p-FKHR expression. As demonstrated in our previous study and elsewhere, AR signaling is critical in PCa growth and progression [4,14]. We believe that AR signaling might be another mechanism in blocking the proapoptotic effects of FKHR. Recently, overexpression of skp-2 has been suggested to inactivate FKHR via ubiquitination and proteasome degradation, thereby playing a key role in cell transformation and tumorigenesis [21]. This is plausible because our data demonstrated that PCa with high levels of Skp-2 also expressed high levels of p-FKHR.
Forkhead proteins function in tumor suppression by inducing cell growth arrest and apoptosis. Activation of these forkhead transcription proteins induces apoptosis through regulation of proapoptotic proteins including Fas ligand, TRAIL, and Bim in cancer cell lines [19,22,23]. Therefore, it seems possible that the higher the FKHR levels, the higher the tumor-suppressing effect FKHR would produce. This seems to be true as our data showed that normal prostate tissue expressed significantly higher levels of nuclear FKHR compared with FHKR levels in PCa, suggesting that FKHR might be an important regulatory protein for homeostasis in normal tissue, whereas the apoptosis-inducing role of FKHR is most likely inhibited in PCa. However, it is not clear why normal prostate also expressed a much higher level of p-FKHR. Interestingly, in normal prostate, p-FKHR was not correlated with p-Akt, AR, or Skp-2. Considering that both FKHR and p-FKHR were strongly expressed in normal prostate, the balance of apoptosis/proliferation in normal prostate might be controlled by some proapoptotic mechanisms other than prosurvival Akt/PKB and AR signaling pathways.
Based on our data, p-FKHR expression seems to be the key element associated with survival strategies in PCa, which may have significant clinical and pathologic implications. Our data revealed that p-FKHR was associated with more aggressive clinical and pathologic features. High levels of p-FKHR correlated with older age, higher GS, higher frequency of ECE, higher incidence of LN metastasis, and more advanced clinical stages. More significantly, higher levels of cytoplasmic expression of p-FKHR were associated with higher probability of biochemical failure after RP. This is significant because our data further confirm the notion that when FKHR is phosphorylated and/or cytoplasmically translocated, FKHR becomes obsolete and functionally inactive, leading to a more aggressive phenotype of PCa via an enhanced prosurvival mechanism.
In summary, our data suggest that in PCa, forkhead transcription proteins are probably associated with survival by inactivation of FKHR via phosphorylation and/or cytoplasmic sequestration, which might be accomplished via a plethora of regulatory pathways such as Akt/PKB and AR signaling pathways.
Footnotes
This study was funded in part by the Specialized Programs of Research Excellence in Prostate Cancer grant P50 CA58204 (Baylor College of Medicine SPORE Center, Houston, TX).
References
- 1.Isaacs JT, Coffey DS. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981;41:5070–5. [PubMed] [Google Scholar]
- 2.De Marzo AM, DeWeese TL, Platz EA, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–77. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 3.Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM. Molecular biology of prostate cancer progression. Cancer Surv. 1995;23:19–32. [PubMed] [Google Scholar]
- 4.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence–free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Nicosia SV, Bai W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem. 2001;276:20444–50. doi: 10.1074/jbc.M010226200. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, Steele R, Ray RB. c-myc Promoter–binding protein 1 (MBP-1) regulates prostate cancer cell growth by inhibiting MAPK pathway. J Biol Chem. 2005;280:14325–30. doi: 10.1074/jbc.M413313200. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Ittmann MM, Ayala G, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005:108–18. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 8.Ayala G, Thompson T, Yang G, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–8. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 9.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–6. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 10.Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 11.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Inhibition of Akt kinase signalling and activation of Forkhead are indispensable for upregulation of FasL expression in apoptosis of glioma cells. Oncogene. 2003;22:7617–27. doi: 10.1038/sj.onc.1207137. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Fernandez de Mattos S, van der Horst A, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves down-regulation of cyclin D. Mol Cell Biol. 2002;22:7842–52. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–18. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Muddiman DC, Tindall DJ. Androgens negatively regulate forkhead transcription factor FKHR (FOXO1) through a proteolytic mechanism in prostate cancer cells. J Biol Chem. 2004;279:13866–77. doi: 10.1074/jbc.M314143200. [DOI] [PubMed] [Google Scholar]
- 16.Cheong JW, Eom JI, Maeng HY, et al. Constitutive phosphorylation of FKHR transcription factor as a prognostic variable in acute myeloid leukemia. Leuk Res. 2003;27:1159–62. doi: 10.1016/s0145-2126(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2002;20:2672–9. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler TM, Lebovitz RM. Fresh tissue harvest for research from prostatectomy specimens. Prostate. 1994;25:274–9. doi: 10.1002/pros.2990250507. [DOI] [PubMed] [Google Scholar]
- 19.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Ayala G, De Marzo A, et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–26. [PubMed] [Google Scholar]
- 21.Huang H, Regan KM, Wang F, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–54. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor–related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–37. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]