Abstract
Objectives
To characterise the natural history of metastatic prostate cancer after radical prostatectomy (RP) in patients followed expectantly for rising prostate-specific antigen (PSA) (noncastrate metastases).
Methods
Cox proportional hazards analyses were used to assess predictors of survival among 95 patients who developed clinically detectable noncastrate metastases after RP. The initial metastatic phenotype was characterised as minimal (nodal or axial skeletal involvement) or extensive (appendicular skeletal involvement or visceral metastases). Estimates of survival after diagnosis of metastases were generated with the Kaplan-Meier method.
Results
Median disease-specific survival from diagnosis of noncastrate metastases was 6.6 yr (95% confidence interval [CI], 5.2, 7.9). The initial site of metastatic disease was bone, lymph node, and viscera in 63%, 36%, and 6% of patients, respectively. Thirteen patients (14%) had extensive disease at their first metastatic manifestation. Longer PSA doubling time in the rising PSA state (hazard ratio [HR] 0.8 for each month increase in doubling time; 95%CI, 0.67–0.94) and the initial metastatic phenotype (HR 0.3 for minimal vs. extensive disease; 95%CI, 0.1–0.6) were associated with improved survival. The prostatectomy Gleason score, lymph node status at RP, PSA level at diagnosis of metastases, and interval from surgery to diagnosis of metastases did not correlate with outcome.
Conclusion
Men who develop noncastrate metastases after RP may have a durable survival. Favourable prognostic indicators include longer PSA doubling time preceding diagnosis of metastases and initial involvement of axial skeleton or lymph nodes.
Keywords: Hormonal therapy, Metastasis, Natural history, Prostate cancer, Prostatectomy
1. Introduction
Of men experiencing prostate-specific antigen (PSA) failure after radical prostatectomy (RP), in the absence of additional treatment an estimated 65% will develop metastatic disease within 10 yr [1]. Because there are no uniform criteria dictating management in a state of rising PSA, patients may be followed expectantly with no further treatment until they develop clinical noncastrate metastases or may be treated with salvage therapies or androgen-deprivation therapy (ADT) early in the course of their relapse [2]. Although early hormonal therapy has been advocated in the setting of locally advanced cancer treated by radiation [3] and for patients with minimal nodal involvement undergoing RP [4], the merits of androgen deprivation for a rising PSA after definitive local therapy remain unclear [2,5]. Given the protracted interval from biochemical failure to development of metastases in the postprostatectomy setting [1], the adverse effects of long-term castration [6], and the lack of clinical trials showing a survival benefit, physicians may recommend deferring hormonal therapy until an end point of radiographic progression has been reached [1,2].
Metastatic prostate cancer is essentially incurable, rendering patients at substantial risk of dying from their cancer. ADT is considered the standard of care for patients with documented metastatic disease because it provides palliation by reducing bone pain and decreasing the risk of pathologic fracture, spinal cord compression, and ureteral obstruction [6]. Recognising that docetaxel may prolong survival in patients with progressive castrate metastatic disease [7], further assessing the benefits of cytotoxic agents in earlier clinical states is important. Patients in the noncastrate metastatic state are proper candidates for enrollment in clinical trials because, unlike those in the state of rising PSA after local therapy, they often experience rapid disease progression and are all at significant risk of dying from cancer.
Although the prognosis of patients who present with metastatic prostate cancer is generally poor, with a median survival of approximately 30 mo [8–10], the outcome of those who develop noncastrate metastases after RP is not well defined. Some studies suggest that, for those who were first diagnosed with apparently localised cancer, the course of metastatic disease may be altered by treatment of the primary tumour [11–13]. In the present study we sought to characterise the natural history of noncastrate metastatic prostate cancer after RP.
2. Patients and methods
Between 1987 and 2003, 4054 patients with clinically localised or locally advanced prostate cancer underwent RP with curative intent at Memorial Sloan-Kettering Cancer Center. Follow-up data were available for 3995 patients (98.5%). After obtaining institutional review board approval, we reviewed our multidisciplinary prostate cancer registry to identify 95 patients (2.3%) who developed metastatic disease without previously being treated with ADT for PSA relapse. Because the study end point was cancer-specific survival, and evidence from randomised trials has shown no differences in cancer control (judged by PSA outcomes) for patients treated with hormonal therapy prior to RP [14], we allowed for inclusion of 34 patients (36%) who received a short course of ADT before surgery and in whom serum testosterone was above castrate levels (>50 ng/ml) at initial metastatic manifestation. Sixteen of the 95 patients (17%) received salvage radiation therapy for a presumptive isolated local recurrence; all had experienced persistent PSA elevation during or immediately after radiation, and hence were considered to harbour micrometastases at the time of treatment and were included in the present analysis. Clinical, laboratory, and imaging data were retrieved from the charts and reviewed to characterise the course of disease after entry into the noncastrate metastatic disease state.
Median follow-up from surgery was 7.1 yr. Patients were typically followed postoperatively with rectal examinations and serum PSA determinations every 3 mo for the first 3 yr, semiannually during years 4 and 5, and annually thereafter. Patients with a rising PSA who deferred ADT were generally monitored carefully with diagnostic imaging studies and serial PSA measurements at intervals of 3–6 mo. Bone metastases were diagnosed based on new uptake by radionuclide scans and confirmed by additional imaging modalities including plain radiographs, computed tomography (CT), or magnetic resonance imaging (MRI). Involvement of the lymph nodes or other soft tissue was defined as appearance of new lesions on CT or MRI; equivocal cases were confirmed by a needle biopsy. We assessed the pattern of metastatic spread at initial manifestation, defining minimal disease as involvement of nodes or pelvic or axial skeleton and extensive disease as involvement of appendicular skeleton (with or without axial skeleton) or viscera (lung or liver) [10,15]. Metastatic disease was considered a definitive indication for initiating ADT, with a median time from diagnosis of metastases to initiation of hormonal therapy of 12 d (interquartile range [IQR], 3, 32). Secondary hormonal manipulations or treatment with chemotherapy on disease progression was instituted at the discretion of the treating physician. The median follow-up from entry into a noncastrate metastatic state was 3 yr (IQR, 2.2, 5.8). At last follow-up 33 (35%) had died; in 29 death was attributed to widespread progressive castrate metastatic prostate cancer.
PSA doubling time (PSA DT) was calculated by assuming first-order kinetics and based on all PSA measurements within 1 yr prior to development of noncastrate metastatic disease; we used a minimum of three measurements, each separated by a minimum of 6 wk [16,17]. Estimates of disease-specific survival were generated with the Kaplan-Meier method. Time was defined from the first evidence of metastasis to death or last follow-up. The four patients who died of causes other than prostate cancer were censored at the time of death. Univariate Cox regression analyses were used to test the association between cancer-related death and the following covariates as single explanatory variables: prostatectomy Gleason score (≤6 vs. 7 vs. ≥8), lymph node involvement at the time of RP (yes vs. no), interval from surgery to metastasis (continuous variable, years), initial metastatic extent (extensive vs. minimal disease), PSA DT (continuous variable, months), and PSA level at diagnosis of metastasis (continuous variable). Because hormonal therapy may introduce histologic artifacts and bias the assignment of tumour grade, we used biopsy rather than specimen Gleason score in the 34 patients who received neoadjuvant ADT prior to surgery. PSA DT was also analysed as a categorical variable (≤3 vs. >3 mo) based on evidence associating the 3-mo cut-off with prostate cancer-specific survival [17,18]. Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated. A multivariable Cox regression model was fit to test whether established predictors of survival in prostate cancer patients [1,15,16,18,19] retained their independent prediction ability in the setting of noncastrate metastatic disease after RP. All statistical analyses were performed using SPSS version 10.0 (SPSS, Chicago, IL) with p < 0.05 considered significant.
3. Results
Baseline clinical and pathologic characteristics for the 95 patients with noncastrate metastases after RP are outlined in Table 1. Organ-confined cancer was found in 32 patients (34%); however, 24 patients (25%) had high-grade tumours (Gleason score ≥8), 37 (39%) had seminal vesicle invasion, and 20 (21%) had lymph node involvement. Noncastrate status during the rising PSA state (serum testosterone range: 62–872 ng/ml) was confirmed in all of the 71 patients (75%) who had available measures, including all 34 patients who received neoadjuvant ADT prior to surgery. The mean and median intervals from surgery to first evidence of metastatic disease were 4.2 yr and 3.2 yr, respectively. The median PSA level at initial noncastrate metastasis was 13.5 ng/ml (IQR, 5.1, 42), and the median PSA DT was 3.4 mo (IQR, 2.2, 7.8).
Table 1.
Descriptive characteristics of the 95 study patients
| Characteristic | No. (%) |
|---|---|
| Median age at surgery, yr (range) | 62 (42–74) |
| Neoadjuvant androgen deprivation | 34 (36) |
| 1992 TNM clinical stage | |
| T1A-B | 3 (3) |
| T1C | 13 (14) |
| T2A | 14 (14) |
| T2B | 37 (39) |
| T2C | 15 (16) |
| T3 | 11 (12) |
| Unknown | 2 (2) |
| Median preoperative PSA (interquartile range) | 10.9 (7.3, 21) |
| Gleason score* | |
| ≤6 | 34 (36) |
| 7 | 37 (39) |
| 8–10 | 24 (25) |
| Extracapsular extension | 37 (39) |
| Positive surgical margins | 39 (41) |
| Seminal vesicle invasion | 37 (39) |
| Lymph node involvement | 20 (21) |
| Interval from surgery to metastases, yr | |
| Mean (range) | 4.2 (0.5–12.1) |
| Median (interquartile range) | 3.2 (1.7, 6) |
| PSA level at first documented metastasis, ng/ml | |
| Mean (range) | 46.4 (2.2–108.5) |
| Median (interquartile range) | 13.5 (5.1, 42) |
| PSA DT, mo | |
| Median (range) | 3.4 (0.4–49.5) |
| Interquartile range | 2.2, 7.8 |
| Serum testosterone level, ng/ml† | |
| Median (range) | 358 (61–872) |
| Interquartile range | 270, 529 |
PSA = prostate-specific-antigen; PSA DT = PSA doubling time.
Note: One patient lacked data for PSA DT, one for preoperative PSA, and 24 for testosterone level. Percentages may not add to 100% because of rounding.
Specimen Gleason score is reported for 61 patients and biopsy Gleason score is reported for 34 patients treated with neoadjuvant androgen deprivation therapy.
Refers to testosterone level in the rising PSA state.
At initial diagnosis of metastases, bone, lymph node, and viscera were involved in 60 (63%), 34 (36%), and 6 (6%) patients, respectively (Table 2). The median interval between the first radiographic evidence of metastatic disease and the preceding imaging study was 8.6 mo (IQR, 3.7, 10.3). Of the 95 patients, 56 (59%) had bone-only disease and in 8 patients (8%) the early metastatic manifestation included appendicular skeleton involvement. Six patients were found to have lung (4) or liver (2) metastases at first evidence of radiographic disease progression, four of which were confirmed by a needle biopsy. Overall, 13 patients (14%) had extensive disease at initial metastatic manifestation. Node-only metastases (excluding micrometastatic disease at the time of surgery) were identified in 30 patients (32%), the pelvic nodes being more commonly involved than retroperitoneal nodes. Of the 20 patients with lymph node involvement at prostatectomy, the site of initial metastatic manifestation was lymph nodes in 8 (40%) and bone in 12 (60%). Compared to patients who had negative nodes at RP, patients with positive nodes had no predilection for subsequent lymph node metastases over bone metastases ( p by χ2 = 0.66).
Table 2.
Primary site of noncastrate metastases in 95 patients following radical prostatectomy
| Primary metastatic site | No. (%) |
|---|---|
| Lymph nodes | 34 (36) |
| Pelvic | 25 |
| Retroperitoneal | 7 |
| Both | 2 |
| Bones | 60 (63) |
| Axial skeleton | 50 |
| Appendicular skeleton | 1 |
| Both | 7 |
| NA | 2 |
| Viscera | 6 (6) |
| Lung | 4 |
| Liver | 2 |
NA = bone scan not available for review.
Patients are included in more than one category if the initial metastatic manifestation involved more than one site.
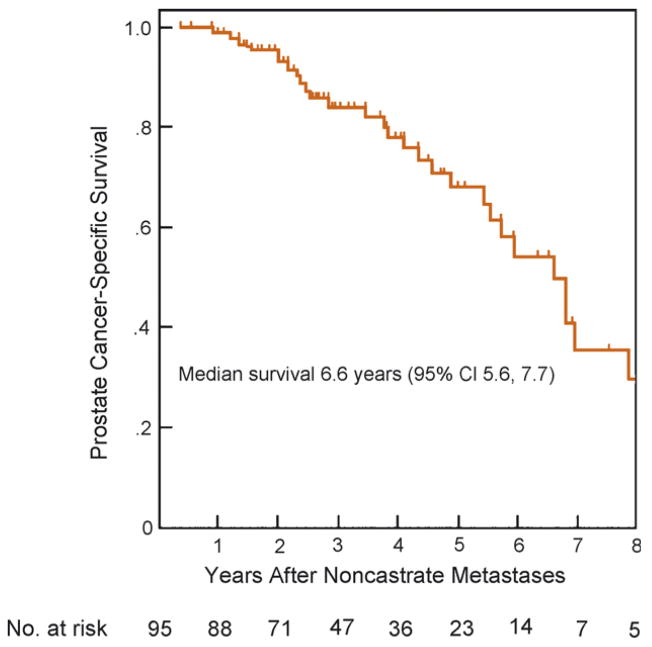
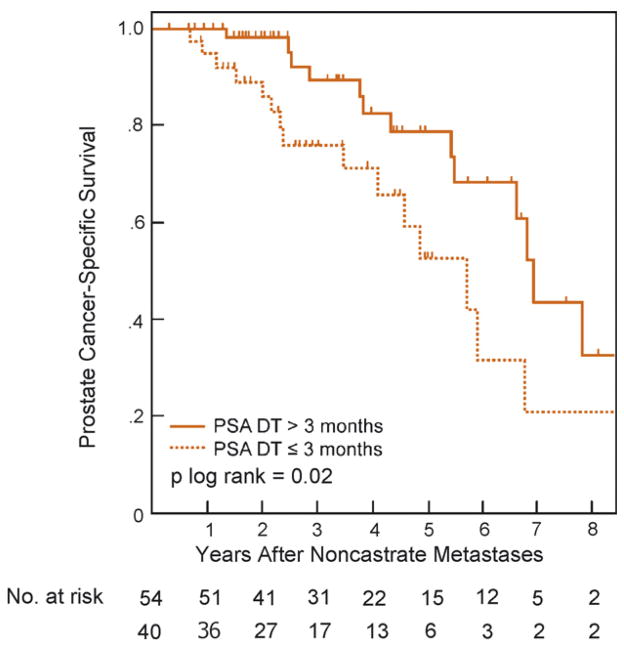
The median actuarial cancer-specific survival from diagnosis of noncastrate metastases was 6.6 yr (95%CI, 5.2–7.9 yr; Fig. 1) with 3-yr and 5-yr estimates of 84% (95%CI, 76–93%) and 68% (95%CI, 56–82), respectively. In univariate analyses, the initial metastatic phenotype (HR: 3, for extensive vs. minimal disease; 95%CI, 1.3–6.8; p = 0.003) and shorter PSA DT (HR: 0.78, for each month increase in PSA DT; 95%CI, 0.66–0.92; p = 0.03) were significantly associated with worse cancer-specific survival, whereas the Gleason score, lymph node status at surgery, PSA level, time to biochemical recurrence, and interval from surgery to diagnosis of metastases were not (Table 3). Initial disease extent and PSA DT remained significantly associated with survival outcome in multivariable analysis, carrying HRs of 2.9 (95%CI, 1.6–9.9; p = 0.009) and 0.8 (95%CI, 0.67–0.94; p = 0.04), respectively. As illustrated in Fig. 2, the PSA DT retained its ability to predict survival when dichotomized using a 3-mo cut-off ( p log rank = 0.02). Specifically, the 3-yr and 5-yr actuarial disease-specific survival probabilities were, respectively, 89% (95%CI, 79–99%) and 78% (95%CI, 63–92%) for a PSA DT of >3 mo, and 71% (95%CI, 54–87) and 42% (95%CI, 21–63) for a PSA DT of ≤3 mo.
Fig. 1.
Prostate cancer-specific survival after entry into the noncastrate metastatic disease state, among 95 patients who had undergone radical prostatectomy.
Table 3.
Univariate and multivariable Cox proportional hazards analyses of factors associated with prostate-cancer specific mortality in patients with noncastrate metastases following radical prostatectomy
| Covariate | Univariate analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Initial metastatic disease extent | ||||
| Minimal | 1.0* | – | ||
| Extensive | 3 (1.3–6.8) | 0.003 | 2.9 (1.6–9.9) | 0.009 |
| Premetastatic PSA DT, mo† | 0.78 (0.66–0.92) | 0.03 | 0.8 (0.67–0.94) | 0.04 |
| PSA level at primary metastasis, ng/ml | 1 (0.99–1.002) | 0.28 | ||
| Interval from surgery to PSA relapse, yr | 0.98 (0.91–1.09) | 0.23 | ||
| Interval from surgery to metastasis, yr | 1.06 (0.92–1.2) | 0.37 | 1.1 (0.95–1.24) | 0.18 |
| Specimen Gleason score | ||||
| ≥6 | 1.0* | – | ||
| 7 | 2 (0.6–6.9) | 0.27 | ||
| 8–10 | 1.2 (0.3–4.8) | 0.79 | ||
| Lymph node involvement at RP (yes vs. no) | 1.37 (0.6–3.1) | 0.45 | ||
HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; PSA DT = PSA doubling time.
All values in parentheses represent the 95%CIs.
Baseline reference for categorical variables.
Incorporated as a continuous variable and indicates the PSA DT during the year before the first radiographic evidence of metastatic disease. One patient lacked data and was excluded from analyses.
Fig. 2.
Prostate cancer-specific survival after development of noncastrate metastases, stratified by prostate-specific antigen doubling time.
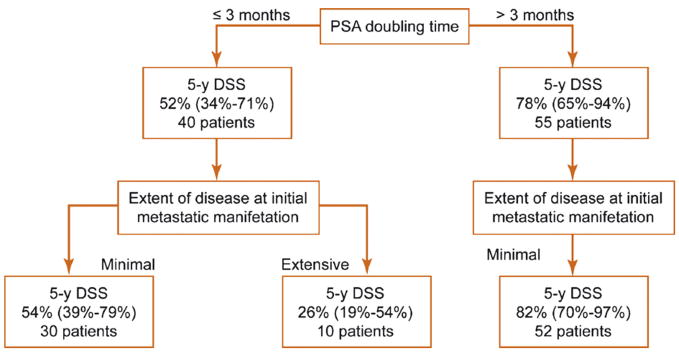
To identify patients who are most likely to experience rapid disease progression, we used the initial disease extent and PSA DT to stratify outcome (Fig. 3). Albeit the overall number of patients presenting with extensive disease was small, only 26% among those who presented with extensive disease and a PSA DT of <3 mo were alive 5 yr after being diagnosed with noncastrate metastases. Conversely, 83% of patients with minimal metastatic disease and a PSA DT of >3 mo survived 5 yr or longer from entering a noncastrate metastatic disease state.
Fig. 3.
Estimates of 5-yr disease-specific survival among patients developing noncastrate metastases after radical prostatectomy. Three patients presenting with extensive disease and a prostate-specific antigen doubling time >3 mo are excluded from the chart. DSS = disease-specific survival; PSA DT = prostate-specific antigen doubling time. Values in parentheses represent 95% confidence intervals (CIs).
4. Discussion
Clinicians may encounter men with metastatic prostate cancer in several different circumstances. Patients may be diagnosed with metastatic disease at presentation or develop it after failure of therapy for localised or locally advanced tumour. Because there is no known cure for metastatic prostate carcinoma, progression is inexorable and the vast majority of patients in this state are destined to die of the disease [15]. Yet data regarding clinical outcomes in this setting are confusing, particularly in an era in which many patients were first diagnosed with localised disease. Although the overall median survival for men presenting with metastatic prostate cancer reportedly ranges from 2 to 3 yr [8–10], the survival of patients with metastatic disease after definitive local therapy appears to be substantially longer [1,11,12]. In an earlier study, Pound and colleagues assessed the risk of metastatic disease and cancer-related death among 304 patients with PSA relapse after RP and no ADT [1]. Contrary to the recognised short survival in patients presenting with metastatic disease, median actuarial time from development of clinical noncastrate metastases to death was approximately 5 yr. Because ADT is often instituted when the only manifestation of disease recurrence is rising PSA [17], noncastrate metastases after RP are relatively uncommon, rendering validation of their intriguing finding difficult. Although the risk of prostate cancer-specific mortality in patients with rising PSA was reassessed by the same group in an updated analysis, using an expanded cohort with longer follow-up, survival was not estimated from the point of diagnosis of noncastrate metastases [19]. Thus, the intent of our study was to further evaluate the natural history of noncastrate metastatic prostate cancer after removal of the primary tumour.
Our results confirm those of Pound et al. and indicate that some patients developing noncastrate metastases after RP have a relatively protracted clinical course, reflected by a median survival that exceeds 6 yr. Involvement of the viscera or appendicular skeleton early in the course of metastatic disease and a rapidly increasing PSA prior to recognition of metastases appeared to be significant markers for reduced survival, whereas pathologic features of the primary tumour, PSA level at diagnosis of metastases, time to biochemical recurrence, and time from surgery to metastases had no apparent association with this outcome. Our study suggests that for patients in the postprostatectomy noncastrate metastatic state, the risk of dying from prostate cancer can be better stratified by the initial pattern of metastatic spread and PSA kinetics rather than time elapsing from prior disease states [1,19]. The identification of PSA DT as a risk factor for reduced survival in the noncastrate metastatic setting extends other evidence linking it to prostate cancer-specific mortality in earlier clinical states [18,19]. These findings may serve to characterise a subset of patients with aggressive cancers for whom early chemotherapy before development of hormone-refractory disease may be beneficial.
The impact of definitive local therapy on the natural history of metastatic prostate cancer remains unclear. Compared to prior series of men presenting with metastatic disease [8–10], patients in our cohort had a significantly prolonged survival. Furthermore, in a secondary retrospective analysis of a large randomised trial of 1286 men with noncastrate metastatic prostate cancer, Thompson et al. demonstrated a clinically and statistically significant 30% improvement in survival favoring men initially managed with RP compared to those whose primary tumour had not been treated (HR 0.70; 95%CI 0.49–0.99) [11]. Although the original study indicated that overall median survival for men presenting with metastatic prostate cancer is approximately 30 mo (and 51 mo for the subset of men with minimal metastatic disease at presentation) [10], the actual survival of patients who had previously undergone RP was not reported [11]. Because metastatic foci can develop not only from another metastasis, but also, potentially, from the primary tumour, the authors hypothesised that aggressive local control may aid in eradication of future metastatic clones. Along these lines, in a retrospective case-control study, Cadeddu et al. indicated that patients with lymph nodes metastases who underwent RP had improved 10-yr cancer-specific survival compared to patients who had pelvic lymph node dissection alone (56% vs. 34%) [13]. Similarly, Zagars and colleagues have shown that adding prostatic irradiation to hormonal therapy in patients with proven pelvic nodal metastases may confer a survival advantage [12]. Whether the natural history of noncastrate metastatic prostate cancer is truly interrupted by treating the primary tumour can be answered only through a prospective trial design. Nevertheless, it is likely that differences in survival between patients presenting with noncastrate metastases and those developing it after local therapy may result, at least in part, from differences in tumor burden at diagnosis of metastatic disease.
Given the retrospective nature of the present analysis, the relatively small cohort and limited number of death events, several caveats must be considered. Our estimate of actuarial survival assumes the study population is representative of all postprostatectomy patients with PSA recurrence who were followed long enough to have developed metastatic disease. Our cohort, however, could reflect a biased selection of patients with PSA relapse in whom ADT was deferred by virtue of favourable disease features (such as a slowly rising PSA or a low Gleason score), advanced age, or competing risks of death. Although the decision whether to withhold ADT was at the treating physician’s discretion, rather than set by predefined criteria, the proportion of high-grade cancers (Gleason score ≥8) and lymph node involvement in our study cohort is similar to or higher than the proportion in modern postprostatectomy or post-radiation series in which ADT was instituted early for a rising PSA [17]. Second, because follow-up was not dictated by a predefined protocol, it is conceivable that progression from minimal to extensive disease might occur before a first available imaging study. Thus, the primary objective evidence of metastasis may not reflect the actual pattern of initial disease spread. Although a lead-time bias cannot be definitively excluded, we consider this unlikely because 95% of our patients had a single metastatic site at initial manifestation and because the median interval between the first radiographic evidence of metastasis and the preceding imaging study was relatively short (8.6 mo). Third, with limited availability of actual images for review, we used the radionuclide scan and CT and MRI reports to characterise the pattern of metastatic spread (minimal vs. extensive). However, a more accurate stratification of the extent of osseous disease can be obtained from a bone scan index (BSI) [20]. This index reflects the total number of lesions, percentage of bone involved with tumour, and weight of the involved bone. Although the BSI does not account for visceral metastasis (6 of the 13 patients categorised with “extensive involvement” in the present analysis), it has been shown to more accurately quantify the extent of skeletal involvement and better correlate with survival in an androgen-independent setting [20]. Therefore, further prospective validation of our findings should include such tools that can better assess the baseline metastatic phenotype. Fourth, variability in secondary hormonal manipulations and chemotherapy regimens after diagnosis of noncastrate metastases may account for some difference in survival. Finally, we did not collect data on patients’ performance status at diagnosis of metastatic disease. Although this factor has been shown to be important in determining survival for patients with metastatic disease at presentation [8,15], minimal tumour burden in the early postprostatectomy metastatic setting is less likely to affect the overall well-being and performance status of the patients.
5. Conclusion
Men who develop noncastrate metastases following RP may have an indolent disease course, with a median survival of 6.6 yr. PSA DT preceding the diagnosis of metastases and the initial metastatic phenotype are independently associated with survival outcome in this setting.
Acknowledgments
Supported in part by funds from National Cancer Institute grant CA 92629-05 SPORE in prostate cancer.
Footnotes
Conflicts of interest
I hereby certify that all authors have made a substantial contribution to the information or material submitted for publication and have read and approved the final manuscript. The manuscript or portions thereof are not under consideration by another journal or electronic publication and have not been previously published.
References
- 1.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Loblaw DA, Mendelson DS, Talcott JA, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–41. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation inpatientswithlocally advancedprostatecancer(anEORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 4.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 5.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 8.Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991;265:618–21. [PubMed] [Google Scholar]
- 9.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168:1008–12. doi: 10.1016/S0022-5347(05)64562-4. [DOI] [PubMed] [Google Scholar]
- 12.Zagars GK, Pollack A, von Eschenbach AC. Addition of radiation therapy to androgen ablation improves outcome for subclinically node-positive prostate cancer. Urology. 2001;58:233–9. doi: 10.1016/s0090-4295(01)01168-2. [DOI] [PubMed] [Google Scholar]
- 13.Cadeddu JA, Partin AW, Epstein JI, Walsh PC. Stage D1. (T1-3, N1-3, M0) prostate cancer: a case-controlled comparison of conservative treatment versus radical prostatectomy. Urology. 1997;50:251–5. doi: 10.1016/S0090-4295(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 14.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6. [PubMed] [Google Scholar]
- 15.Tangen CM, Faulkner JR, Crawford ED, et al. Ten-year survival in patients with metastatic prostate cancer. Clin Prostate Cancer. 2003;2:41–5. doi: 10.3816/cgc.2003.n.011. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163–72. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 17.Stewart AJ, Scher HI, Chen MH, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005;23:6556–60. doi: 10.1200/JCO.2005.20.966. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–83. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 19.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 20.Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999;17:948–57. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]