Abstract
New predictive markers for managing prostate cancer are urgently required because of the highly variable natural history of this disease. At the time of diagnosis, Gleason score provides the gold standard for assessing the aggressiveness of prostate cancer. However, the recent discovery of TMPRSS2 fusions to the ERG gene in prostate cancer raises the possibility of using alterations at the ERG locus as additional mechanism-based prognostic indicators. Fluorescence in situ hybridization (FISH) assays were used to assess ERG gene status in a cohort of 445 prostate cancers from patients who had been conservatively managed. The FISH assays detected separation of 5′ (labelled green) and 3′ (labelled red) ERG sequences, which is a consequence of the TMPRSS2–ERG fusion, and additionally identify interstitial deletion of genomic sequences between the tandemly located TMPRSS2 and ERG gene sequences on chromosome 21. Cancers lacking ERG alterations exhibited favourable cause-specific survival (90% survival at 8 years). We identify a novel category of prostate cancers, characterized by duplication of the fusion of TMPRSS2 to ERG sequences together with interstitial deletion of sequences 5′ to ERG (called ‘2+Edel’), which by comparison exhibited extremely poor cause-specific survival (hazard ratio = 6.10, 95% confidence ratio = 3.33–11.15, P < 0.001, 25% survival at 8 years). In multivariate analysis, ‘2+Edel’ provided significant prognostic information (P = 0.003) in addition to that provided by Gleason score and prostate-specific antigen level at diagnosis. Other individual categories of ERG alteration were associated with intermediate or good prognosis. We conclude that determination of ERG gene status, including duplication of the fusion of TMPRSS2 to ERG sequences in 2+Edel, allows stratification of prostate cancer into distinct survival categories.
Keywords: prostate cancer, ERG gene, ERG gene break point, Gleason score, prognosis
Introduction
Gleason scoring is the sole grading system recommended by the World Health Organization for assessing the aggressiveness of prostate cancer. Although a useful prognostic indicator, as a system based on morphological appearance, it suffers from problems of inter-observer variability (Allsbrook et al., 2001a, b; Oyama et al., 2005; Melia et al., 2006; van der Kwast et al., 2006) and of changes in interpretation with time (Smith et al., 2002; Chism et al., 2003; Albertsen et al., 2005). Recently, fusion of the TMPRSS2 gene to the ETS transcription factor gene ERG has been reported as a common event in prostate cancer (Tomlins et al., 2005, 2006; Hermans et al., 2006; Iljin et al., 2006; Perner et al., 2006; Soller et al., 2006; Wang et al., 2006; Yoshimoto et al., 2006; Clark et al., 2007). Less frequently TMPRSS2 also becomes fused to ETV1 and ETV4 (Tomlins et al., 2005, 2006; Hermans et al., 2006). These discoveries raise the prospect that genetic alteration in ETS gene family members may provide an alternative or additional mechanism-based prognostic classification for prostate cancer. In support of this possibility, there are already several indications of correlations of ERG gene status with clinicopathological indicators (Perner et al., 2006; Wang et al., 2006; Demichelis et al., 2007; Nam et al., 2007). For example, Perner et al. (2006) found that the presence of ERG rearrangements accompanied by 5′-ERG deletion had a significant correlation with higher tumour stage with the presence of metastatic disease involving pelvic lymph nodes. In a watchful waiting cohort of 111 patients, Demichelis et al. (2007) reported both a significant association between the presence of a TMPRSS2–ERG fusion and prostate cancer-specific death, and a link between the presence of ERG alterations and higher Gleason score.
Because of the highly variable natural history of prostate cancer, additional new predictors of cancer aggressiveness are urgently required. Overtreatment of prostate cancers is a particular concern leading to substantial inappropriate morbidity (Yao and Lu-Yao, 2002). This is especially true for many prostrate-specific antigen (PSA) screen-detected cancers, which in the absence of treatment, may never become life threatening. Conversely more conservative approaches to disease detection and management can leave potentially aggressive cancers untreated. Therefore, improved biomarkers are required to allow radical therapies to be targeted to men with potentially lethal cancers, so that the remainder, with more benign-behaving indolent cancers, are spared inappropriate treatment.
To help identify and optimize markers that may be of use in the management of men with prostate cancer, we recently established a retrospective cohort of men whose cancers were conservatively managed (Cuzick et al., 2006). Improving on previous studies (Chodak et al., 1994; Albertsen et al., 1995; Adolfsson et al., 1997; Holmberg et al., 2002; Johansson et al., 2004; Albertsen et al., 2005; Bill-Axelson et al., 2005), our analyses included centrally assigned Gleason scores determined by modern grading criteria, and allowed comparisons with several additional clinical parameters. In agreement with Johansson et al. (2004) and Albertsen et al. (2005), we found Gleason score to be an important determinant of cancer-specific mortality, but baseline PSA and to a lesser extent stage of disease added further predictive value. The objective of the current study is to use our cohort of conservatively managed prostate cancer cases to assess the potential clinical significance and utility of different classes of ERG gene alteration that can be detected using fluorescence in situ hybridization (FISH).
Results
Fluorescence in situ hybridization detection of breaks at the ERG gene locus
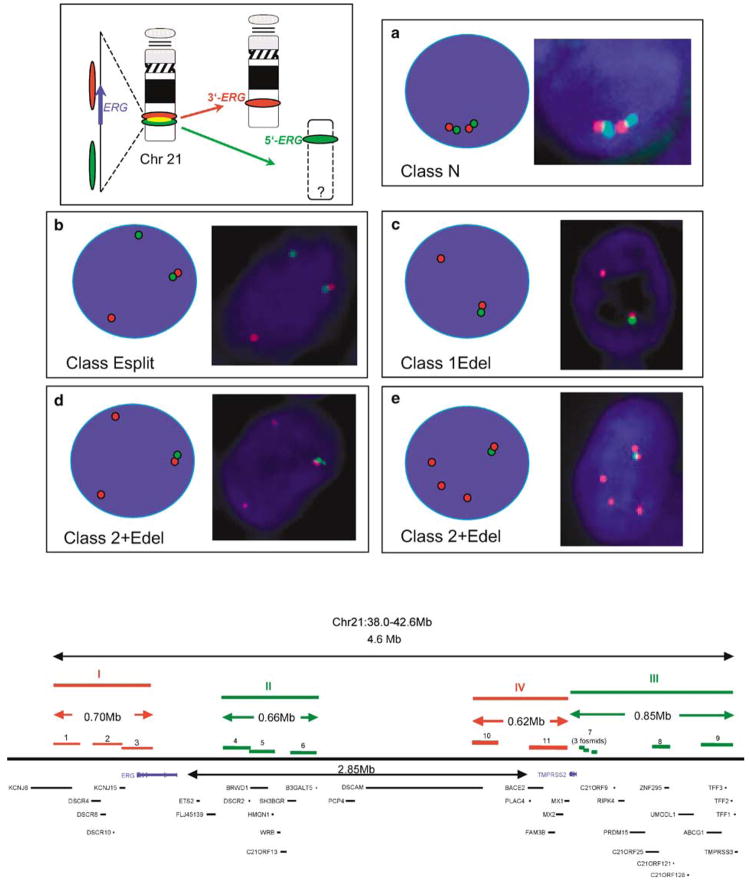
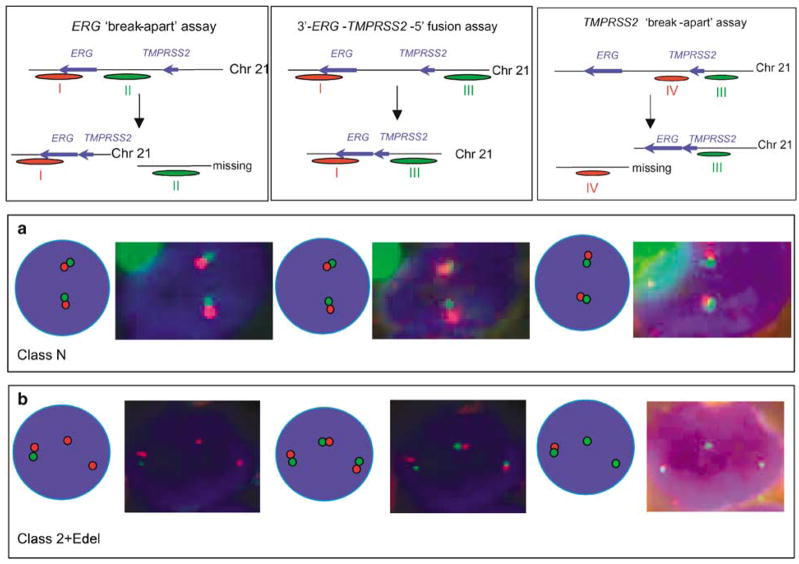
To assess the frequency of the TMPRSS2–ERG fusions in a set of 1062 cores from 445 patients, we used an ERG gene ‘break-apart’ assay similar to that described by Tomlins et al. (2005). To increase the sensitivity of this assay, we used three overlapping BAC probes at the centromeric 3′-end (red) and three BAC probes at the telomeric 5′-end (green). The positions of the probes are shown in Figure 1 (lower panel). Using this assay, unrearranged ERG loci are visualized in interphase nuclei as immediately adjacent green and red signals (Figure 1a). The TMPRSS2–ERG fusion results in the joining of TMPRSS2 exon 1 or 2 sequences usually to exon 2, 3 or 4 ERG sequences (Wang et al., 2006; Clark et al., 2007). When this fusion has occurred, the 3′-centromeric and 5′-telomeric ends of ERG occur as separated red and green signals (Figure 1b; Tomlins et al., 2005; Perner et al., 2006). Many cancers with alteration in the ERG gene FISH pattern also exhibit loss of the lone green 5′-ERG signal (Figures 1c–e), consistent with previous findings that the chromosomal region between TMPRSS2 and ERG on chromosome 21 is frequently deleted in cancers with TMPRSS–ERG fusions (Iljin et al., 2006; Perner et al., 2006).
Figure 1.
FISH detection of ERG gene breakpoints. Top right: Principle of detection of ERG gene status. Interphase nuclei are hybridized to probes that detect sequences 5′ to the ERG gene (green) and 3′ to the ERG gene (red). The red and green signals are separated when an ERG gene rearrangement occurs. (a) Signals from normal unrearranged ERG loci (class N). (b) Signals from rearranged ERG gene with retention of separated red (3′) and green (5′) probes (class Esplit). (c) Example of a ‘1Edel’ cancer: the rearrangement is associated with deletion of sequences 5′ to ERG (green) with retention of a single red 3′-ERG signal. (d and e) Examples of 2+Edel cancers: the rearrangement is associated with deletion of sequences 5′ to ERG (green) with retention of two or more red 3′-ERG signals. Bottom: Map of the ERG and TMPRSS2 genes showing the position of the BACs and fosmids used as probes in FISH assays. Probe I: 1:RP11-95G19, 2:RP11-720N21, 3:CTD-2511E13; probe II 4:RP11-372O18, 5:RP11-115E14, 6:RP11-729O4; probe III, 7:three pooled fosmids (G248P89444D12, G248P800876A1, G248P8239C5), 8:RP11-35C4, 9:RP11-282I20; probe IV, 10:RP11-114H1, 11:RP11-662D5. Probes I and II correspond, respectively, to sequences immediately 5′ (green) and 3′ (red) to the ERG gene. Probes III and IV correspond, respectively, to sequences immediately 5′ (green) and 3′ (red) to the TMPRSS2 gene.
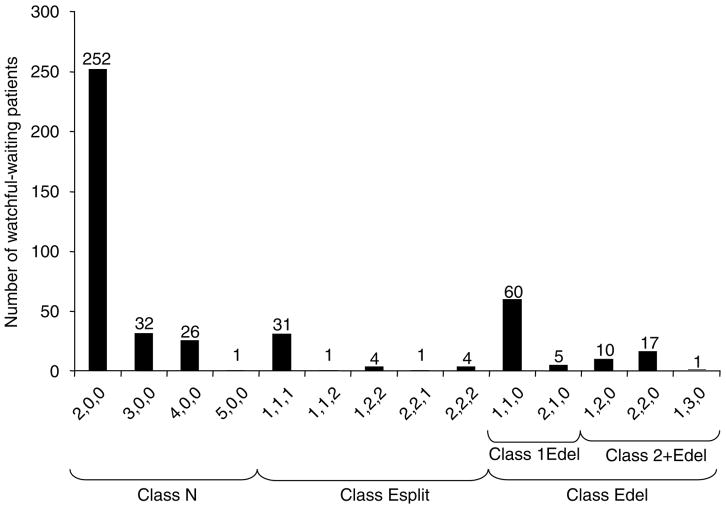
In view of the observed FISH patterns, cancers could be stratified according to whether they (i) had entirely normal ERG loci (class N, for Normal, 311 cancers, 70%), (ii) had rearranged ERG but had retained both 5′- and 3′-ERG sequences (class Esplit, for ERG signal split into separate 3′-red and 5′-green probes, 41 cancers, 9%) or (iii) had retained 3′-ERG but had no evidence for the presence of 5′-ERG sequences (class ‘Edel’ for ERG 5′ deletion, 93 cancers, 21%). Variation in the precise number of normal (n = 0–5), 3′-ERG (red, n = 0–3) and 5′-ERG (green n = 0–3) signals was observed leading to considerable diversity in the range of FISH patterns within each category. To record this variation, the interphase nuclei within each core were scored according to the number of each of the three FISH signals: twinned red and green; separate red 3′-ERG; separate green 5′-ERG. A cancer with the score ‘2,1,0’ (found for five cancers, Figure 2) is, for example, a class ‘Edel’ cancer, which contains two unrearranged ERG alleles, one separate red 3′-ERG signal and no green 5′-ERG signals. The variation in FISH patterns observed within class N, Esplit and Edel cancers is shown in Figure 2.
Figure 2.
Distribution of the pattern of ERG gene alterations in human prostate cancer. Each cancer is scored for three classes of FISH signals: normal unrearranged ERG loci (adjacent red and green signals); separate red signals that correspond to sequences 3′ to a rearranged ERG locus and separate green signals that correspond to sequences 5′ to a rearranged ERG locus. For example, ‘2,1,0’ means that the cancer contains two normal alleles, one separate red 3′-ERG signal and no green 5′-ERG signals. Cancers with only normal signals are designated as class N. When the ERG locus is split to form both separate 3′-ERG (red) and 5′-ERG (green) signals, the cancer is designated as class Esplit. Cancers containing separate red 3′ to ERG signals, but lacking separate green signals are designated ‘Edel’ cancers.
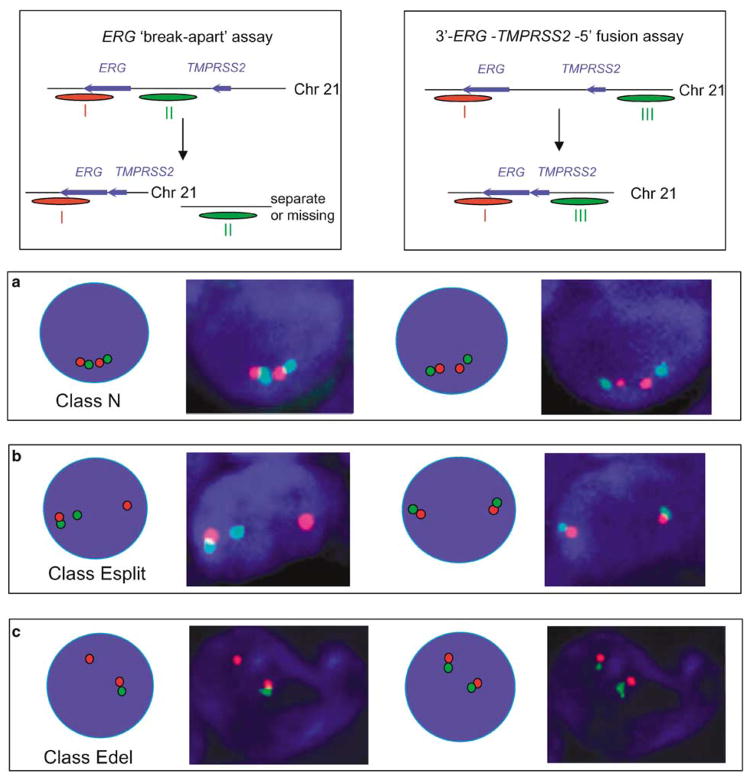
To confirm that class Esplit and class Edel ERG alterations detected by FISH do indeed correspond to 5′-TMPRSS2–ERG-3′ fusions as reported previously (Perner et al., 2006), selected cancers were rehybridized using 5′-TMPRSS2 (green) and 3′-ERG (red) FISH probes. These studies (Figure 3) demonstrated that each rearranged 3′-ERG signal retained a juxtapositioned 5′-TMPRSS2 signal, even though intervening sequences between the TMPRSS2 and ERG genes were either missing (class Edel) or located in a different region of the nucleus (class Esplit). Reverse transcription (RT)–PCR studies carried out as described previously (Clark et al., 2007) confirmed fusion of 5′-TMPRSS2 to 3′-ERG transcripts in each cancer category.
Figure 3.
FISH detection of TMPRSS2 and ERG gene status. (left-hand side): the status of the ERG gene was examined using the ERG ‘break-apart’ assay using probes that detected sequences immediately 5′ (green, probe II) and 3′ (red, probe I) to ERG. (Righthand side): the same representative cancer cell was re-hybridized with FISH probes that detected sequences 5′ to TMPRSS2 (green, probe III) and 3′ to ERG (red, probe I). The principle of detection is in each case shown at the top and the precise origin of the three separate FISH probes used in these studies (probes I–III) can be seen in Figure 1. Nuclei shown in this figure were from cancers with the following ERG status: class N (a), class Esplit (b) and class Edel (c). RT–PCR studies carried out as described previously (Clark et al., 2007) confirmed the presence of TMPRSS2–ERG fusion transcripts only in cancers that contained rearranged ERG (result not shown). A comparison of the left- and right-hand panels in this figure shows that rearranged 3′-ERG always remains linked to 5′-TMPRSS2, and that the sequences examined by FISH located between the TMPRSS2 and ERG loci (probe II) are either missing (class Edel cancers) or positioned elsewhere in the nucleus (class Esplit cancers).
Tumour demographics and characteristics for the cohort of 445 patients and correlations to clinicopathological parameters are shown in Table 1. There were significant associations between the presence of ERG gene alteration and Gleason score (P < 0.001), clinical stage (P = 0.001) and baseline PSA (P < 0.001), but no association with age (P = 0.765).
Table 1.
Relationship of ERG gene status with demographics and tumour characteristics
| Variable | Class N (n = 311) | Class Esplit (n = 41) | Edel (n = 93) | P |
|---|---|---|---|---|
| Mean age ±s.d. (years) | 69±5 | 69±5 | 69±5 | 0.765 |
| Gleason scorea | ||||
| <7 | 201 (65%) | 17 (41%) | 25 (27%) | <0.001 |
| = 7 | 66 (21%) | 15 (37%) | 32 (35%) | |
| >7 | 42 (14%) | 9 (22%) | 35 (38%) | |
| Clinical stageb | ||||
| T1 | 110 (60%) | 6 (27%) | 17 (35%) | 0.001 |
| T2 | 56 (31%) | 10 (46%) | 22 (45%) | |
| T3 | 17 (9%) | 6 (27%) | 10 (20%) | |
| Baseline PSA | ||||
| ≤4 | 144 (46%) | 7 (17%) | 22 (24%) | <0.001 |
| >4–10 | 73 (24%) | 12 (29%) | 17 (18%) | |
| >10–25 | 55 (18%) | 7 (17%) | 25 (27%) | |
| >25–50 | 25 (8%) | 9 (22%) | 24 (26%) | |
| >50–100 | 14 (5%) | 6 (17%) | 5 (5%) | |
Abbreviation: PSA, prostate-specific antigen.
Restricted to patients for whom Gleason score is available.
Restricted to patients for whom clinical stage is available.
Presence of ‘Edel’ rearrangements independently predicts poor survival
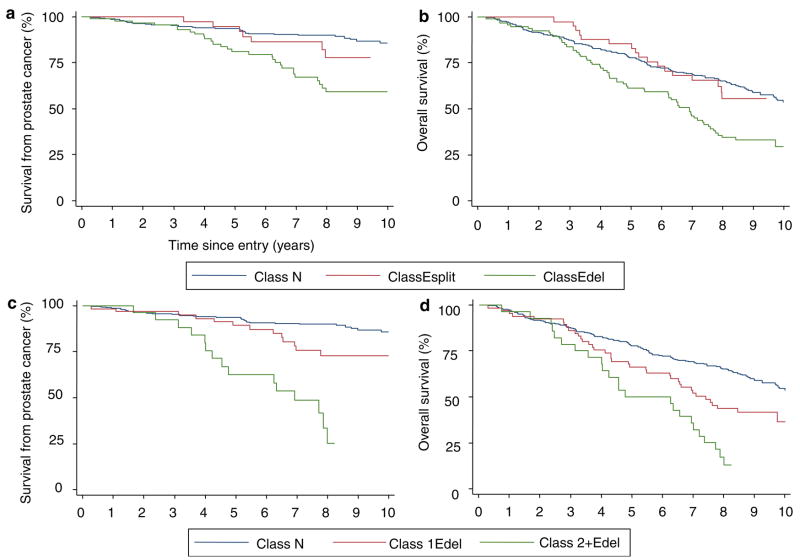
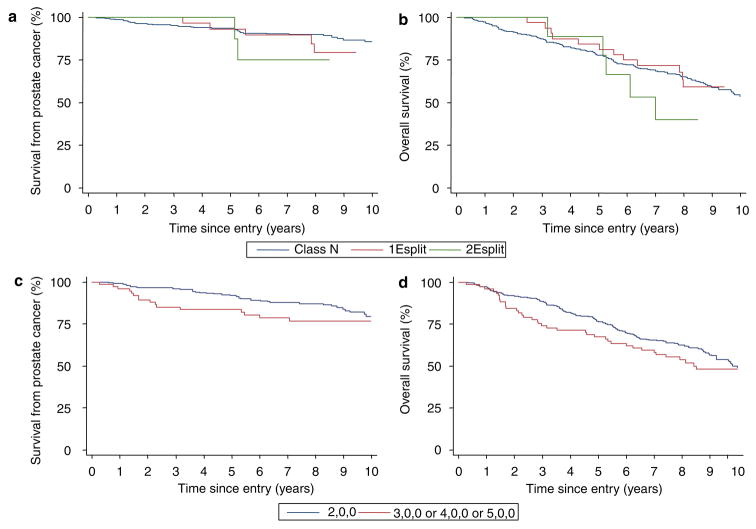
For the 445 patients, the cancer-specific survival and overall survival for the three categories of FISH pattern (N, Esplit and Edel) are shown in Figures 4a and b. Univariate Cox analysis demonstrates that, compared to cancers retaining only normal ERG FISH patterns (class N), Edel cancers had significantly worse cause-specific and overall survival (hazard ratio (HR) = 2.92, 95% confidence interval (CI) = 1.79–4.76, P < 0.001 and HR = 1.92, 95% CI = 1.42–2.59, P < 0.001, respectively). In contrast, class Esplit cancers did not exhibit significantly worse cause-specific (HR = 1.56, 95% CI = 0.73–3.34, P = 0.254) or overall survival (HR = 1.00, 95% CI = 0.61–1.63, P = 0.987) compared to class N cancers. A link between the ERG gene status and Gleason score in part explains the poorer survival of Edel cancers (Table 1, P < 0.001). However, in the multivariate analyses including age, Gleason score and baseline PSA, the presence of Edel still emerged as a significant independent marker of poor cause-specific and overall survival (HR = 1.72, 95% CI = 1.02–2.89, P = 0.042 and HR = 1.43, 95% CI = 1.04–1.97, P = 0.028, respectively).
Figure 4.
Kaplan–Meier analysis comparing prostate cancer outcomes for different categories of ERG gene alteration. (a and b) A comparison of class N, class Esplit and class Edel cancers. (c and d) Outcome stratified according to the copy number of 3′-ERG FISH signal in Edel cancers. The Kaplan–Meier curves compare class N cancer with Edel cancer containing a single copy of 3′-ERG sequences (1Edel), and with Edel cancer that contain two or more copies of 3′-ERG sequences (2+Edel). a and c are cause-specific survival. b and d are overall survival.
The importance of 3′-ERG FISH copy number in determining clinical outcome
We further stratified the 93 Edel cancers according to the number of retained copies of the FISH signal corresponding to 3′-ERG sequences. Kaplan–Meier plots are shown in Figures 4c and d. Univariate Cox analyses demonstrate that cancers with two or more copies of the 3′-ERG signal (called ‘2+Edel’, 6.6% of all cancers) had much worse cause-specific survival and overall survival (HR = 6.10, 95% CI = 3.33–11.15, P < 0.001 and HR = 2.89, 95% CI = 1.86–4.48, P < 0.001, respectively) when compared to class N cancers. Edel cancers with a single copy of 3′-ERG sequences exhibited intermediate survival, but the difference from class N cancers only reached significance for overall survival (HR = 1.81, 95% CI = 0.97–3.40, P = 0.064 and HR = 1.60, 95% CI = 1.12–2.27, P = 0.010, for cause-specific and overall survival, respectively), and the differences were not significant in multivariate analyses.
The distribution of Gleason scores for the poor prognosis 2+Edel category can be seen in Table 2. The cancers include a significant proportion of Gleason score 6 and 7 cancers that on the basis of Gleason score alone would have been considered to have a good or intermediate prognosis. Multivariate analyses formally demonstrated that knowledge of ERG gene status provides important prognostic information in addition to that provided by Gleason score, age and PSA level at diagnosis. Thus, in these analyses, 2+Edel cancers remained a significant predictor of poorer cause-specific and overall survival compared to class N cancers (HR = 2.66, 95% CI = 1.39–5.11, P = 0.003 and HR = 1.84, 95% CI = 1.15–2.94, P = 0.011, respectively). In analyses of cause-specific data, the survival of 2+Edel cancers was 25% at 8 years, compared to 90% survival in cancers that lacked ERG gene rearrangement. RT–PCR analyses (result not shown) confirmed fusion of 5′-TMPRSS2 to 3′-ERG transcripts in 2+Edel cancers. As expected, hybridization of 2+Edel cancers to a series of FISH probes that spanned the TMPRSS2 and ERG genes demonstrated that (i) both the TMPRSS2 and ERG loci are rearranged, (ii) intervening sequences between the rearranged TMPRSS2 and ERG loci are deleted and (iii) both copies of rearranged 3′-ERG sequences had immediately juxtapositioned 5′-TMPRSS2 sequences (Figure 5).
Table 2.
Relationship between ERG gene status and Gleason score
| Gleason score |
ERG gene statusa |
||||
|---|---|---|---|---|---|
| Class N | 1Esplit | 2Esplit | 1Edel | 2+Edel | |
| 4 | 3 | 0 | 0 | 0 | 0 |
| 5 | 18 | 1 | 0 | 1 | 0 |
| 6 | 180 | 14 | 2 | 22 | 2 |
| 7 | 66 | 9 | 6 | 20 | 12 |
| 8 | 28 | 4 | 0 | 12 | 6 |
| 9 | 13 | 4 | 1 | 8 | 7 |
| 10 | 1 | 0 | 0 | 0 | 0 |
| Unassigned | 2 | 0 | 0 | 1 | 0 |
Class N: unrearranged ERG. Esplit indicates that ERG gene is split to form both separate 3-ERG (red) and 5′-ERG (green) signals: 1Esplit, one copy of 3′-ERG signal; 2Esplit, two copies of 3′-ERG signal. Edel cancers contain separate red 3′-ERG signals in the absence of separate green 5′-ERG signals: 1Edel, one copy of 3′-ERG signal; 2+Edel, two or more copies of 3′-ERG signal.
Figure 5.
FISH analysis of the TMPRSS2 and ERG loci in 2+Edel cancers. The ‘left’ and ‘middle’ panels in this figure are the same as the ‘left’ and ‘right panels’ in Figure 3 showing, respectively, results from the ERG locus ‘break-apart’ assay (left), and from the FISH detection of sequences 5′ to TMPRSS2 and 3′ to ERG (middle). Additionally in the right-hand panel, the status of the TMPRSS2 gene has been examined using a TMPRSS2 ‘break-apart’ assay by re-hybridizing the same representative cancer cell with FISH probes that detected sequences immediately 5′ (green, probe III) and 3′ (red, probe IV) to TMPRSS2. The principle of detection is in each case shown at the top and the precise origin of the four separate FISH probes used in these studies (probes I–IV) can be seen in Figure 1. The nuclei shown in this figure were from a class N cancer (a) and a class 2+Edel cancer. (b) RT–PCR studies carried out as described previously (Clark et al., 2007) confirmed the presence of TMPRSS2–ERG fusion transcripts only in the 2+Edel cancer (result not shown). These analyses demonstrate that cancers with a rearranged ERG locus also have a rearranged TMPRSS2 locus, (ii) the rearranged 5′-TMPRSS2 and 3′-ERG sequences remain joined together and (iii) the rearrangement and joining of 5′-TMPRSS2 and 3′-ERG is accompanied by deletion of intervening sequences corresponding to both probes II and IV.
We also examined the effect of copy number of the 3′-ERG FISH signal for class Esplit patients (Figures 6a and b). Univariate Cox analyses demonstrated that class Esplit cancers with two or more FISH copies of 3′-ERG sequences (called ‘2Esplit’) cancers did not differ from class N cancers in terms of cause-specific survival (HR = 2.28, 95% CI = 0.55–9.49, P = 0.259) or overall survival (HR = 1.55, 95% CI = 0.63–3.79, P = 0.339).
Figure 6.
(a and b) Kaplan–Meier analysis stratifying prostate cancer outcomes according to copy number of 3′-ERG FISH signal: (a) class Esplit cancers, cause-specific survival; (b) class Esplit cancers, overall survival. (c and d) Kaplan–Meier analysis comparing prostate cancers outcomes depending on ERG ploidy status: (c) cause-specific survival and (d) overall survival.
We assessed the effect of ploidy status within class N cancers (Figures 6c and d). We failed to find any significant difference in outcome between cancers containing a different number of unrearranged ERG loci in agreement with the American College of Pathologists’ recommendations that the data on ploidy status is not compelling enough to warrant its routine use as a clinical marker (Bostwick and Foster, 1999).
Discussion
We have detected ERG rearrangements in 30% (134/445) of prostate cancers. This is higher than the value of 15% (17/111) reported by Demichelis et al. (2007) in a watchful waiting cohort, but lower than the value of 49% (58 of 118) found by Perner et al. (2006), who also assessed ERG gene alterations using a FISH break-apart assay. In turn all these estimates are lower than those of 55% (16 of 29) reported by Tomlins et al. (2006), 59% (35 of 59) reported by Wang et al. (2006) and 78% (14 of 18) reported by Soller et al. (2006) that were all obtained using PCR-based detection of TMPRSS2–ERG fusion transcripts. In view of our observation that incidence of ERG gene alterations are linked to Gleason score and to clinical stage, we suspect that these differences may be accounted for, at least in part, by the distinct clinical compositions of the cancer sets examined in each study. For example, the incidence of 15% found by Demichelis et al. (2007) in their series of stage T1 cancers is similar to the incidence of 19% in the stage T1 subset of cancers present in our study (Table 1). We found deletions between the ERG and TMPRSS2 genes in 69.5% of cancers containing ERG rearrangements, in broad agreement with the value of 60.3% found by Perner et al. (2006).
The objective of our study was to assess whether alteration at the ERG gene locus could be used as part of a clinically useful, mechanism-based prognostic classification system for prostate cancer. In this respect, we have identified an ERG gene alteration called 2+Edel defined by the presence of two or more lone FISH copies of 3′-ERG in the absence of sequences 5′ to ERG. This alteration, found in 6.6% of cancers in our study, is associated with very poor clinical outcome and provides information on potential cancer aggression in addition to that provided by Gleason score and PSA level at diagnosis. This observation now requires validation in a prospective cohort of patients managed by current protocols, but detection of 2+Edel could, in principle, provide a more robust marker of prognosis than Gleason score since its interpretation should not vary with observer or time. In contrast, the presence of 1Edel was of only borderline significance as a predictor of poor survival in univariate analysis. The ability of the Edel category of cancers (2+Edel Plus 1Edel) to predict clinical outcome in multivariate analyses was therefore mainly accounted for by the contribution of 2+Edel. We also examined the ability of class Esplit ERG alterations with two or more FISH copies of the 3′-ERG to predict clinical outcome. We failed to find any evidence of prognostic power, but because of the small number of such alterations it was not possible to formally exclude the importance of this cancer category.
The observation that prostate cancers with two or more FISH copies of 3′-ERG have worse clinical outcome than cancers that contain only a single copy of the rearranged 3′-ERG is consistent with the view that the high level of overexpression of ERG that results from the fusion of 5′-TMPRSS2 to 3′-ERG (Tomlins et al., 2005) is responsible for driving cancer progression. The failure to observe worse clinical outcome in class Esplit cancers indicates that the loss of sequences 5′ to ERG found in Edel cancers is also critical to cancer aggression. It has been shown by several groups (Hermans et al., 2006; Iljin et al., 2006; Perner et al., 2006) that fusion of the tandemly arranged TMPRSS2 and ERG fusions in prostate cancer is frequently accompanied by loss of the entire intervening chromosome 21 sequence, so a search of this region for genes with a tumour-suppressive effect may be a fruitful avenue of future investigation. Wang et al. (2006) have provided evidence that the presence of a particular fusion transcript between exon 2 of TMPRSS2 and exon 4 of ERG that encodes a TMPRSS2–ERG fusion protein may also be associated with aggressive disease. Our studies did not analyse the specific TMPRSS2–ERG fusions that are presumed to be encoded by the rearranged ERG loci. However, this observation raises the interesting possibility that cancers containing 2+Edel together with this particular fusion may have an especially poor clinical outcome.
Our study agrees with several other studies examining the relationship between ERG gene status and clinical indicators in prostate cancer patients. Correlations between the presence of the TMPRSS2–ERG fusion and higher Gleason grade and clinical stage (Perner et al., 2006; Demichelis et al., 2007) have been reported. In a watchful waiting cohort of 111 stage T1 patients, Demichelis et al. (2007) found a significant association between the presence of TMPRSS2–ERG fusions (which would have included both class Esplit and Edel alterations examined in our study) and prostate cancer-specific death, but multivariate analyses to test independence from Gleason score were not presented. In a series of 26 patients with Gleason 7 cancer who underwent prostatectomy, Nam et al. (2007) found that the presence of TMPRSS2–ERG fusions was associated with greater probability of biochemical disease relapse. However, many of these studies have limited statistical power and our analyses importantly show that in FISH analyses of ERG alterations, it is the presence of ‘2+Edel’ that provides the best predictor of poor clinical outcome, yielding prognostic information in addition to that provided by the most important prognostic factors, Gleason score and serum PSA levels at diagnosis.
New prognostic markers that can be used in the clinical management of prostate cancer are urgently required. The data presented here indicate that detection of 2+Edel could be useful as part of a new mechanism-based prognostic classification for assessing the potential future aggressiveness of every human prostate cancer at diagnosis. 2+Edel may be of particular utility in distinguishing the future clinical behaviour of the aggressive and indolent Gleason 6 and 7 cancers that are otherwise considered together to have a good or intermediate prognosis.
Materials and methods
Patient cohort
Tissue microarrays (TMAs) were constructed from 445 unselected transurethral resection of the prostate specimens taken from patients managed with no initial treatment in a cohort of conservatively managed men with prostate cancer (Cuzick et al., 2006). Patients from this cohort who were initially treated with hormone therapy (Cuzick et al., 2006) were excluded from this study, because the expression of the fusion gene under investigation of TMPRSS2–ERG is controlled by androgens (Tomlins et al., 2005). The median age of diagnosis was 70 years (49–76 years) and the median follow-up was 91 months (3–173 months). Most men were diagnosed after the age of 65 years. A competing risk analysis showed that after 10 years of follow-up, 50% of men had died: 17% from prostate cancer and 33% from other causes; only 27% were alive without progression (Cuzick et al., 2006). National approval for the collection of the cohort was obtained from the Northern Multi-Research Ethics Committee followed by local ethics committee approval at each of the collaborating hospital trusts. This work was approved by the Clinical Research and Ethics Committee at the Royal Marsden Hospital and Institute of Cancer Research.
Tissue microarrays
TMAs were constructed in 35×22×7mm blocks of Lamb paraffin wax using a manual tissue microarrayer (Beecher Instruments, Sun Prairie, WI, USA). Up to four cores of 600 μm diameter were taken from each tumour. Reassignment of areas of ‘cancer’ or ‘normal’ in each core was carried out on the basis of histopathological examination of haematoxylin and eosin and p63/AMACR-stained sections that flanked the TMA slice used for FISH studies. The morphological criteria for selection of ‘normal’ and ‘malignant’ prostatic epithelium conformed to previously published definitions (Foster, 2000; Foster et al., 2000, 2004). ‘Hyperplasia’, ‘dysplasia’ and ‘PIN’ were not scored in this study.
FISH studies
TMA sections (4 μm) were cut onto SuperFrostPlus glass slides (VWR International, Poole, UK). FISH studies were carried out by a similar method to that described by Lambros et al. (2006). TMA slices were dewaxed in xylene (55°C, 3 × 5 min), washed in ethanol and then boiled in ethanol for 30 s. The slices were then boiled in pre-treatment buffer (SPOT-light tissue pre-treatment kit, Zymed, South San Francisco, CA, USA) for 15 min, rinsed in water, digested with pepsin solution (Digest All-3, Invitrogen, Paisley, UK) at 28°C for 4.5 min, rinsed in water and drained before hybridization. For the ERG ‘break-apart’ assay, 100 ng of each BAC probe (Biotin-labelled BACs RP11-95G19, RP11-720N21 and CTD-2511E13 corresponding to 3′-ERG sequences and DIG-labelled BACs RP11-372O17, RP11-115E14 and RP11-729O4 corresponding to sequences immediately upstream of the ERG gene), 5 μg COT-1, 1 μg salmon sperm DNA (Invitrogen) combined in a total volume of 3.5 μl were added to 9.6 μl of hybridization buffer (60% (v/v) formamide, 12% (w/v) dextran sulphate, 2.4 × SSC, 0.14mM EDTA pH 8, 400 μg/ml salmon sperm DNA), mixed and pipetted onto a 22 × 22mm plastic coverslip (Sigma-Aldrich, Poole, UK). The TMA slide was inverted onto the coverslip and sealed in a metal hybridization chamber containing 200 μl 6 × 0.15M NaCl, 0.15M Na3 citrate; pH 7.0 (SSC). The chamber was heated to 98°C for 9 min and then transferred to 37°C overnight.
Following hybridization, the coverslips were removed and the TMA slices rinsed in 2 × SSC at 42°C, twice for 5 min in 50% (v/v) formamide/2 × SSC at 42°C, twice for 5 min in 2 × SSC at 42°C and for 3 min in SSCT (4 × SSC/0.5% (v/v) Tween-20 (Sigma-Aldrich)) at room temperature. Slices were drained, placed on a rack over a 37°C water bath and flooded with SSCTM (10 ml SSCT/0.5 g skimmed milk powder (Marvel Premier International Foods, Lincs, UK), 0.4 μm filtered) for 15 min and then washed for 3 min in SSCT. A total of 150 μl SSCTM containing 0.75 μl anti-DIG-FITC (Roche, Welwyn Garden City, UK) was pipetted onto the TMA slice under a 22 × 50mm (#1.5 Sigma-Aldrich) glass coverslip, incubated for 37°C for 10 min and then washed three times for 2 min in SSCT at room temperature. This procedure was then repeated with 0.75 μl Streptavidin-Cy3 conjugate (Sigma-Aldrich) in 150 μl SSCTM at 37°C for 10 min. Slides were washed for 2 min in SSCT at room temperature, then twice for 5 min in phosphate-buffered saline, rinsed in 70% (v/v) ethanol and air dried. The TMA slice was mounted in 10 μl Vectashield anti-fade containing 4′,6-diamidino-2-phenylindole (Vector labs, Burlingame, CA, USA) and scanned using an Ariol SL-50 (Applied Imaging, San Jose, CA, USA). Using this method the success rate in obtaining FISH results from TMA cores was greater than 90%. For re-hybridization DAPI and the old probe were removed by washing in 2 × SSC twice for 10 min at room temperature followed by 2 min in 2 × SSC and 4 min in 70% (v/v) formamide/2 × SSC at 68°C. Slides were then washed in water and re-hybridized as described above. The identities of the probes used in additional FISH assays are described in Figure 1.
Evaluation of the FISH results in each core that contained cancer was independently performed by two operators (GA and JCl) who, for each nucleus, scored the number of unrearranged ERG loci (twinned red and green signals), separate 5′ ERG sequences (labelled green) and separate 3′ ERG sequences (labelled red). At least 100 epithelial nuclei per case were evaluated and in each case the modal value, which was almost invariably also the maximum value and usually present in at least 50% of the nuclei, was taken as the score. For example, in cancers judged as having two copies of unrearranged ERG, two copies were always observed in at least 80% of nuclei. Lower numbers of signals were observed in a proportion of nuclei: this reflected the fact that some nuclei were sliced and had missing signals, and was not thought to reflect pattern heterogeneity.
Statistical analysis
The primary endpoints for this study were time to death from prostate cancer and time to death from any cause. Univariate and multivariate analysis were performed by proportional hazard (Cox) regression analysis (Cox and Oakes, 1984). All follow-up times commenced at the point of 6 months following diagnosis as in the previous report (Cuzick et al., 2006). Associations between categorical data were examined using the χ2 test and Fisher’s exact test when expected cell counts were less than 5. Associations between categorical and numerical variables were assessed using analysis of variance. All P-values were two-sided. The following variables, determined as described previously (Cuzick et al., 2006), were included in the multivariate analyses: centrally reviewed Gleason score, baseline PSA (last PSA value within 6 months of diagnosis) and age at diagnosis. Clinical stage data, only available for up to 60% of patients and found to be of minimal significance in our previous analysis (Table 1), were not included.
Acknowledgments
This work was funded by Cancer Research UK, National Cancer Research Institute, a specialized program of Research Excellence grant from US National Cancer Institute, Grand Charity of Freemasons, Rosetrees Trust, The Bob Champion Cancer Trust, The Orchid Appeal and David Koch Foundation. Funding bodies had no involvement in the design and conduct of the study, or in collection management, analysis and interpretation of the data or in preparation, review or approval of the manuscript. We thank Christine Bell and Sandra Edwards for help in typing the manuscript and preparing figures and tables.
References
- Adolfsson J, Steineck G, Hedlund PO. Deferred treatment of clinically localized low-grade prostate cancer: actual 10 year and projected 15 year follow up of the karolinska series. Urology. 1997;50:722–726. doi: 10.1016/S0090-4295(97)00320-8. [DOI] [PubMed] [Google Scholar]
- Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. Long term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274:626–631. [PubMed] [Google Scholar]
- Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowakczyk PD, Sanders MM, et al. Cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Amin MB, et al. Interobserver reproducibility of gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001a;32:74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of gleason grading of prostatic carcinoma: general pathologist. Hum Pathol. 2001b;32:81–88. doi: 10.1053/hupa.2001.21135. [DOI] [PubMed] [Google Scholar]
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Anderson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Foster CS. Predictive factors in prostate cancer: current concepts from the 1999 college of American pathologists conference on solid tumor prognostic factors and the 1999 world health organization second international consultation on prostate cancer. Semin Urol Oncol. 1999;17:222–272. [PubMed] [Google Scholar]
- Chism DB, Hanlon AL, Troncoso P, Al Saleem T, Horwitz EM, Pollack A. The gleason score shift: score four and seven years ago. Int J Radiat Oncol Biol Phys. 2003;56:1241–1247. doi: 10.1016/s0360-3016(03)00268-2. [DOI] [PubMed] [Google Scholar]
- Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- Clark J, Merson S, Jhavar S, Flohr P, Edwards S, Foster CS, et al. Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene. 2007;26:2667–2673. doi: 10.1038/sj.onc.1210070. [DOI] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of Survival Data. Chapman & Hall; London, New York: 1984. [Google Scholar]
- Cuzick J, Fisher G, Kattan MW, Berney D, Oliver T, Foster CS, et al. Long-term outcome among men with conservatively treated localised prostate cancer. Br J Cancer. 2006;95:1186–1194. doi: 10.1038/sj.bjc.6603411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007 doi: 10.1038/sj.onc.1210237. epub ahead of publication, 22 Janurary. [DOI] [PubMed] [Google Scholar]
- Foster CS. Pathology of benign prostatic hyperplasia. Prostate Suppl. 2000;9:4–14. doi: 10.1002/1097-0045(2000)45:9+<4::aid-pros3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Foster CS, Bostwick DG, Bonkhoff H, Damber JE, van der KT, Montironi R, et al. Cellular and molecular pathology of prostate cancer precursors. Scand J Urol Nephrol Suppl. 2000;205:19–43. doi: 10.1080/003655900750169284. [DOI] [PubMed] [Google Scholar]
- Foster CS, Falconer A, Dodson AR, Norman AR, Dennis N, Fletcher A, et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871–5979. doi: 10.1038/sj.onc.1207800. [DOI] [PubMed] [Google Scholar]
- Hermans KG, van Marion R, van Dekken H, Jenster G, van Weerden WM, Trapman J. TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer. Cancer Res. 2006;66:10658–10663. doi: 10.1158/0008-5472.CAN-06-1871. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Haggman M, et al. A randomised trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI, et al. TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res. 2006;66:10242–10246. doi: 10.1158/0008-5472.CAN-06-1986. [DOI] [PubMed] [Google Scholar]
- Johansson JE, Andren O, Anderson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early localised prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- Lambros MB, Simpson PT, Jones C, Natrjan R, Westbury C, Steele D, et al. Unlocking pathology archives for molecular genetic studies: a reliable method to generate probes for chromogenic and fluorescent in situ hybridisation. Lab Invest. 2006;86:398–408. doi: 10.1038/labinvest.3700390. [DOI] [PubMed] [Google Scholar]
- Melia J, Moseley R, Ball RY, Griffiths DF, Grigor K, Harnden P, et al. A UK-based investigation of inter- and intra-observer reproducibility of gleason grading of prostatic biopsies. Histopathology. 2006;48:644–654. doi: 10.1111/j.1365-2559.2006.02393.x. [DOI] [PubMed] [Google Scholar]
- Nam RK, Sugar L, Wang Z, Yang W, Kitching R, Klotz LH, et al. Expression of TMPRSS2 ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progression. Cancer Biol Ther. 2007 doi: 10.4161/cbt.6.1.3489. epub ahead of publication, 2 January. [DOI] [PubMed] [Google Scholar]
- Oyama T, Allsbrook WC, Jr, Kurokawa K, Matsuda H, Segawa A, Sano T, et al. A comparison of interobserver reproducibility of gleason grading of prostatic carcinoma in Japan and the United States. Arch Pathol Lab Med. 2005;129:1004–1010. doi: 10.5858/2005-129-1004-ACOIRO. [DOI] [PubMed] [Google Scholar]
- Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- Smith EB, Frierson HF, Jr, Mills SE, Boyd JC, Theodorescu D. Gleason scores of prostate biopsy and radical prostatectomy specimens over the past 10 years: is there evidence for systematic upgrading? Cancer. 2002;94:2282–2287. doi: 10.1002/cncr.10457. [DOI] [PubMed] [Google Scholar]
- Soller MJ, Isaksson M, Elfving P, Soller W, Lundgren R, Panagopoulos I. Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes Chromosomes Cancer. 2006;45:717–719. doi: 10.1002/gcc.20329. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–3400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- van der Kwast TH, Ciatto S, Martikainen PM, Hoedemaeker R, Laurila M, Pihl CG, et al. Detection rates of high-grade prostate cancer during subsequent screening visits. Results of the european randomized screening study for prostate cancer. Int J Cancer. 2006;118:2538–2542. doi: 10.1002/ijc.21667. [DOI] [PubMed] [Google Scholar]
- Wang J, Cai Y, Ren C, Ittmann M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 2006;66:8347–8351. doi: 10.1158/0008-5472.CAN-06-1966. [DOI] [PubMed] [Google Scholar]
- Yao SL, Lu-Yao G. Understanding and appreciating over-diagnosis in the PSA era. J Natl Cancer Inst. 2002;94:958–960. doi: 10.1093/jnci/94.13.958. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Joshua AM, Chilton-Macneill S, Bayani J, Selvarajah S, Evans AJ, et al. Three-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates that genomic microdeletion of chromosome 21 is associated with rearrangement. Neoplasia. 2006;8:465–469. doi: 10.1593/neo.06283. [DOI] [PMC free article] [PubMed] [Google Scholar]