Abstract
A hybrid quadrupole orthogonal time-of-flight mass spectrometer optimized for MALDI and electrospray ionization has been equipped with a C60 cluster ion source. This configuration is shown to exhibit a number of characteristics that improve the performance of traditional time-of-flight secondary ion mass spectrometry (SIMS) experiments for the analysis of complex organic materials, and potentially, for chemical imaging. Specifically, the primary ion beam is operated as a continuous rather than a pulsed beam, resulting in up to 4 orders of magnitude greater ion fluence on the target. The secondary ions are extracted at very low voltage into 8 millitorr of N2 gas introduced for collisional focusing and cooling purposes. This extraction configuration is shown to yield secondary ions that rapidly lose memory of the mechanism of their birth, yielding tandem mass spectra that are identical for SIMS and MALDI. With implementation of ion trapping, the extraction efficiency is shown to be equivalent to that found in traditional TOF-SIMS machines. Examples are given, for a variety of substrates that illustrate mass resolution of 12,000–15,600 with mass range for inorganic compounds to m/z 40,000. Preliminary chemical mapping experiments show that with added sensitivity, imaging in the MS/MS mode of operation is straightforward. In general, the combination of MALDI and SIMS is shown to add capabilities to each technique, providing a robust platform for TOF-SIMS experiments that already exists in a large number of laboratories.
1. Introduction
The unique properties of cluster ion bombardment in secondary ion mass spectrometry (SIMS) experiments suggest that alternative instrumental approaches to existing time-of-flight technology could provide more efficient molecular analysis capability. These properties include increased sensitivity to inorganic and organic materials, extended mass range, reduced sample damage, and the ability to perform chemical imaging experiments at submicron lateral resolution.1–7 Moreover, an important new application of cluster SIMS, molecular depth profiling, is not well suited to a pulsed incident beam since the experiment is carried out using a continuous beam for erosion purposes.4, 5, 7, 8 Current instrumentation does not allow for detection of material removed during sputtering. And finally, since cluster SIMS experiments are now widely applied to the characterization of more complex organic samples, the availability of ms/ms is essential, yet it is unavailable in commercial TOF-SIMS instrumentation, and has only been reported in specialized applications.9 Hence, a number of groups are in the process of examining new instrumental configurations.10–12
With the expanded capabilities of cluster projectiles, there is interest in combining cluster SIMS with MALDI mass spectrometry to acquire complementary information. From a fundamental point of view, the physics behind both methods is still quite different, but closer than when employing atomic bombardment. For larger clusters such as C60, for example, computer simulations suggest that the interaction between the cluster and the surface creates correlated energy flow on a mesoscopic scale of several nm, a scale larger than seen for collision cascades associated with atomic projectiles, but smaller than the micron scale associated with laser ablation or MALDI experiments.13, 14 An experimental comparison has been tested in a preliminary fashion only recently. A high performance TOF-SIMS was modified to allow matrix enhanced imaging on similar samples using both approaches.15, 16 Others workers have also developed a strategy for using a chemical initiator to desorb molecules either with a cluster beam or with a laser beam, providing great flexibility.17
One of the most important modes of operation of both of these methods involves chemical mapping. In this mode, a probe is focused to a well-defined spot on the sample causing the stimulated desorption of target molecules in an ionized state. Initial imaging experiments were employed with SIMS which utilizes an energetic tightly focused ion beam as the desorption probe. For example, lateral resolution of less than 100 nm has been achieved18, 19 for many years with liquid metal ion gun technology. Images are constructed by acquiring thousands of mass spectra at different locations which are then sorted according to specific m/z values to produce molecule-specific pictures. In these experiments, the imaging procedure consists of acquiring secondary ions under low dose conditions since extensive chemical damage occurs during and after the desorption event. More recently, with cluster ion beams damage accumulation is greatly reduced, allowing imaging to be combined with molecular depth profiling to achieve 3-dimensional information.5, 6
Laser desorption based imaging methods have also become popular. With this type of probe, the sample is usually treated with a thin coating of a MALDI matrix.20, 21 This procedure has been found to enhance ionization, greatly reduce fragmentation, and allow molecules of virtually any molecular weight to be desorbed. Although the laser can, in principle, be focused to a submicron spot, lateral resolution has been limited to 20–50 microns,16, 22 due either to fundamental physical limitations23 and/or to disturbances induced by the application of the matrix.24 Moreover, chemical noise at low mass and long acquisition times have limited some applications. Similar strategies are being developed at atmospheric pressure using desorption electrospray ionization (DESI)25 where samples can be analyzed directly on the laboratory bench, but the best lateral resolution achieved to date is about 150 micron.26 Hence, cluster SIMS offers cleaner spectra than MALDI at low mass (<m/z 1000) without the use of matrix and submicron lateral resolution, while other methods provide improved spectra at higher mass but at lower lateral resolution. A significant driver for both methods is the notion that biomolecule mapping directly in biological tissue yields fundamental and clinical opportunities.
In this paper, we explore the combination of cluster SIMS and MALDI by modifying commercially available MALDI instrumentation to allow use of both modalities on the same platform. Specifically, we have chosen to implement this technology using a high performance hybrid quadrupole orthogonal (QqTOF) design.27 This instrumentation provides mass resolution of greater than 1 part in 10,000 and tandem mass spectrometry capability.27, 28 Moreover, the orthogonal orientation of the QqTOF permits the use of a continuous primary ion beam during analysis, resulting in up to 4 orders of magnitude higher secondary ion flux than a pulsed instrument. There is also a large existing imaging MALDI user base available to embrace this strategy. The work presented here has been motivated in part by collaboration with the Vickerman group at the University of Manchester where a radical new TOF platform with comparable performance characteristics is under a parallel development path.10 Our results show that modification of the QqTOF can be completed in a straightforward fashion and that the quality of the SIMS and MALDI spectra are directly comparable in the lower mass range. After the desorption process, the secondary ion beam is shown to be thermalized before entering the quadrupole mass analyzer via collisions with several millitorr of N2 gas. Hence, this beam loses all memory of whether it was created via laser desorption or ion beam-induced desorption. Because of this property, the collision-induced dissociation (CID) mass spectra are found to be essentially identical for both methods. Moreover and perhaps most importantly, this platform allows the complementary properties of cluster SIMS and MALDI to be readily exploited.
2. Experimental
Materials and Sample Preparation
Acetone, methanol, indium foil, digitonin, and gramicidin S were purchased from Sigma Aldrich (St. Louis, MO). The digitonin solution was prepared by dissolving 200 mg digitonin in 10 ml 50:50 water/methanol. For matrix-enhanced SIMS and MALDI measurements, an equal amount of a 50:50 mixture of a saturated methanol solution of sinnapic acid and α-cyano-4-hydroxycinnamic acid was added to the analyte. This mixture was found to produce more consistent results for a variety of compounds than those produced using the pure matrices. The gramicidin S solution was prepared by dissolving 250 mg of gramicidin S in 10 ml methanol. Both samples were prepared by drying a 50 μl aliquot onto a stainless steel MALDI sample plate. The In sample was prepared by attaching a 1 cm2 of 0.1 mm thick In foil to the MALDI sample plate with double sided copper tape. The sample was conditioned to a reproducible state by bombarding with C60 projectiles until the In+ secondary ion signal remained constant with time, corresponding to a fluence of about 1014 ions/cm2.
For high mass range analysis, CsI in powder form was purchased from mpbio.com and gallium arsenide was purchased from Alpha. The CsI was first pelletized and then adhered to a stainless steel MALDI plate with copper tape. The GaAs was analyzed by placing a small shard onto a MALDI plate with copper tape. Both were sputtered with C60+ before being analyzed with approximately 100 pA C60+ beam current.
Mass Spectrometer Design Considerations
Experiments employing the QqTOF mass spectrometer were performed on a modified QSTAR® XL manufactured by Applied Biosystems/MDS Analytical Technologies. Details of the standard configuration of this system have been described extensively in a previous publications.27 For the experiments reported here, this instrument was modified by the addition of a specially designed extension chamber that allowed mounting of a 20 keV C60 cluster ion source from Ionoptika Ltd, UK. A schematic design is shown in Figure 1, where the C60 beam is shown to strike the sample at an incident angle of 45°. The length of the Q0 quadrupole rods was increased by 10 cm to transfer the analyte ions through the extension chamber. The entrance of the extension chamber was made to be compatible with the QSTAR® XL sample inlet system, allowing MALDI, electrospray or SIMS analysis to be performed.
Figure 1.
Schematic diagram of the modified QSTAR® XL hybrid QqTOF mass spectrometer. Note the differential pumping column surrounding the C60 source and the conical needle needed to inject ions within 3 mm of the sample surface (see text for details). Primary beam currents reaching the sample plate varied between several pA and 100 pA depending upon the nose cone aperture size. The secondary ions are transported via quadrupole rods Q0, mass selection is possible using Q1, and collision induced dissociation at impact energies up to 200 eV occurs in the differentially pumped collision cell, Q2. For a small pre-selected mass range, ion storage is possible in Q2 before pulsing a section of the continuous beam into the orthogonal TOF ion mirror. See text for details.
As previously reported and as shown in the inset of Figure 1, secondary ion extraction occurs via a small electric field.29 These ions enter the Q0 ion guide where the ion beam is compressed using collisional focusing. The pressure in the Q0 region is typically set to about 8 millitorr of N2.27 Simulations and experimental data show that C60+ ions with 20 keV of kinetic energy will fragment into smaller carbon clusters down to C11 upon a single collision with buffer gas molecules. 30, 31 To avoid these collisions, the C60 gun column was modified to include a differential pumping shield to allow the ions to be formed and transported close to the surface at a pressure of 1 × 10−6 torr or lower.29 A conical shaped needle (nose cone) was fitted to the end of the column as shown in Figure 1. The tip of the nose cone was positioned in close proximity (~ 3 mm) to the sample surface. Such close spacing is essential to minimize the probability of fragmentation of C60+ ions when they exit the nose cone and fly through the region with 8 millitorr of N2 above the sample. In this configuration it is possible to deliver intact C60+ ions to the sample without significant degradation or fragmentation using the native operating pressures of the QSTAR® XL spectrometer.
Due to limitations of this prototype source, focusing of the C60+ beam to a submicron spot size on the target is problematic, although such focusing has been demonstrated using more advanced methods on other instruments.32 In our experiments, the beam width is defined by the size of the opening of the conical needle. The size of the opening could be chosen to be between 150 μm providing a beam current of up to 100 pA and approximately 15 μm providing a beam current of 5 pA. The lateral resolution achievable during imaging was found to be close to the size of the opening associated with the conical needle.
The sample stage attachment in the SIMS/oMALDI™ source is equipped with a hinged door that opens to atmosphere allowing for quick sample transfer. The stage is movable in either an automated fashion or manually, controlled by oMALDI™ Server software, and is capable of movements as small as 10 μm with a maximum movement of 50 mm in the X and Y directions. The presence of the C60+ ion gun does not interfere with normal operation of the MALDI source, allowing concurrent SIMS and MALDI experiments to be performed with no alterations of the system. Use of the electrospray source requires removal of the cluster source, which can be accomplished in about one hour.
Traditional axial TOF-SIMS experiments were performed using a previously described system constructed in-house.33 These measurements were performed using a focused beam generated by the IOG40 C60 ion source from Ionoptika. All spectra were recorded at pressures of 10−9 torr or less.
3. Results and Discussion
To establish the efficacy of this type of instrument for cluster SIMS experiments, a number of performance features need to be examined in detail. Here, we first evaluate the relative efficiency of the QqTOF design, primarily with respect to the higher pressure operation. Next, we examine the prospects of utilizing the MS/MS capability of this instrument and compare these results directly with MALDI experiments. The utility of high mass resolution, Q2 trapping and imaging will also be addressed.
Transmission Efficiency
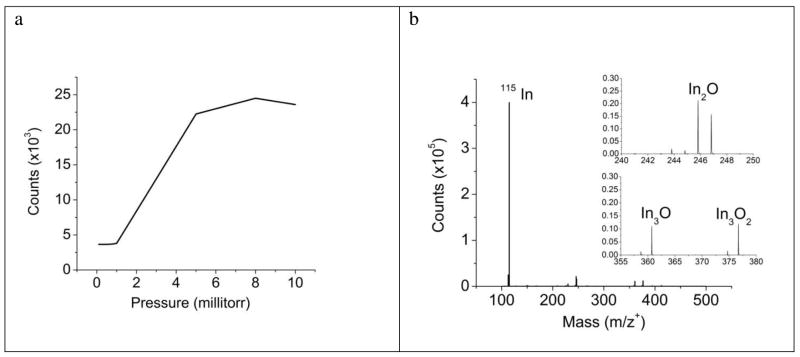
To determine the ability of the QqTOF configuration to extract secondary ions generated during C60+ bombardment, it is useful to compare the performance of this instrument to traditional TOF-SIMS instrumentation with axial geometry where secondary ion extraction occurs at 5–10 keV. To avoid issues of fragmentation and ionization, an initial set of measurements were performed on sputtered In foil, a simple system where the major ion signal occurs at 113In ( 4%) and 115In (96 %). In the QqTOF instrument, the intensity of the 115 In ions strongly depends upon the N2 pressure in the Q0 region due to collisional focusing34 as shown in Figure 2a. Note that the signal reaches a maximum value when the pressure reaches about 8 millitorr. The slight decline in signal at high pressure presumably arises due to fragmentation of some portion of the C60+ projectile ions as a consequence of collisions with the N2 gas. We observe this behavior for both organic and inorganic samples, regardless of molecular weight. A typical spectrum acquired from In foil is shown in Fig. 2b. The dominant peak is found to be 115In with various oxide peaks following at relatively low intensities.
Figure 2.
(a) A graph of the transmission dependence on sample N2 pressure introduced at the Q0 inlet. As pressure increases, collisional focusing facilitates transmission of the secondary ions through the Q0 quadrupole rods. A maximum efficiency is reached at ~8 millitorr, and further increases in pressure reduces the secondary ion counts, presumably due to primary ion collisions with the gas. (b) An In spectrum acquired with a typical operating sample pressure of 8 millitorr N2. The spectrum consists almost entirely of In and various In-oxide peaks.
Secondary ion yield, defined as the number of secondary ions per incident C60 molecule, is critically important for SIMS experiments. Hence, it is useful to compare the performance of the QqTOF design to that of traditional TOF-SIMS instrument. In the QqTOF instrument, a continuous beam of primary ions generates a continuous beam of secondary ions that is in turn periodically sampled in the orthogonal TOF mass analyzer. In a traditional TOF-SIMS design, a pulsed primary ion beam and a coaxial mass analyzer are used. Transmission efficiency of the QqTOF mass analyzer operating in continuous flow mode is limited by the duty cycle as described by the following formula:28, 35
| Eqn. 1 |
Here, m/zmax corresponds to the upper m/z limit for a given spectrum, l is the length of the TOF entrance window and b is the midpoint distance between the entrance window and the detector,35 30 mm/120 mm in our case. This approximation results in a duty cycle of about 8% at m/z 100, and 25% at m/z 1000, for m/zmax = 1000. From the data shown in Figure 2b, it is possible to calculate the efficiency for 115In detection with results shown in Table 1. The number is obtained by integrating the number of counts under the mass peak of interest, and dividing by the number of C60+ ions required to produce the peak. Similar measurements for gramicidin S at m/z+ 1141 are also reported in the Table.
Table 1.
A comparison of efficiencies for the prototype QSTAR® XL instrument and a traditional high performance coaxial ToF-SIMS instrument for In (m/z+115) and gramicidin S (m/z+ 1141).
| Instrument Geometry | 115In secondary ion yield | Gramicidin S secondary ion yield |
|---|---|---|
| QSTAR® XL | 1.5 × 10−3 | 5 × 10−5 |
| Coaxial TOF | 1.6 × 10−2 | 1.8 × 10−4 |
Although the numbers reported in Table 1 for the QqTOF are smaller than those reported for the coaxial TOF, it is clear that this difference arises almost entirely from duty cycle considerations discussed above. If these effects are taken into consideration, both designs have about the same capabilities of extracting secondary ions from the sample. This secondary ion yield difference must be balanced against the fact that the number of primary ions available for desorbing sample molecules is ~10,000 higher for a continuous primary ion beam than for pulsed beam systems. Moreover, as shown below, ion trapping combined with pulsed injection into the orthogonal TOF35 can recover a significant fraction of this loss, if the mass spectrum is limited to a small mass range.
Mass spectral performance and MS/MS measurements
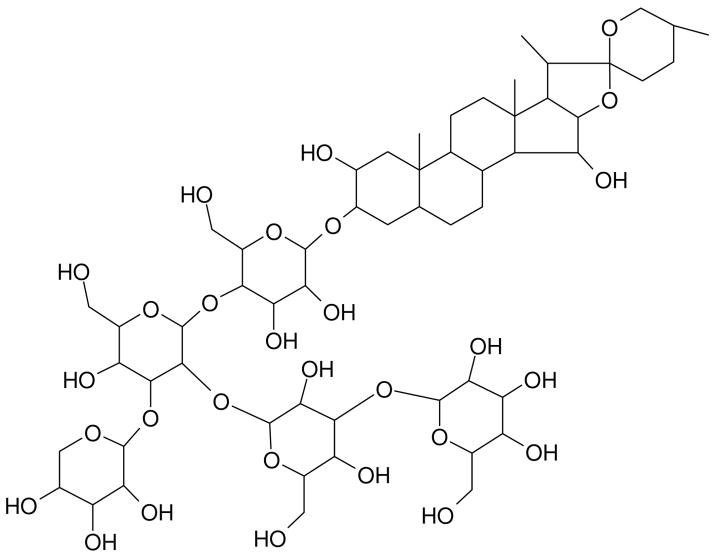
Although cluster ion sources such as C60 have certainly extended the measurement capabilities of SIMS experiments through reduced fragmentation and higher ion yields at high mass, spectra are still characterized by the presence of intense fragment ion peaks at lower mass. This spectral congestion leads to data interpretation issues, particularly when dealing with complex biological samples. The MS/MS capability of the QqTOF configuration provides an enormous opportunity to deal with these problems in a straightforward fashion. Digitonin, the structure of which is shown in Figure 3, is a detergent utilized to solubilize membrane proteins, precipitate cholesterol, and permeabilize cell membranes and is used here to illustrate the strategy under development.36–38
Figure 3.
The molecular structure of digitonin. Loss of successive glycosyl ring units results in intense fragment ion peaks that can be easily identified during MS/MS analysis.
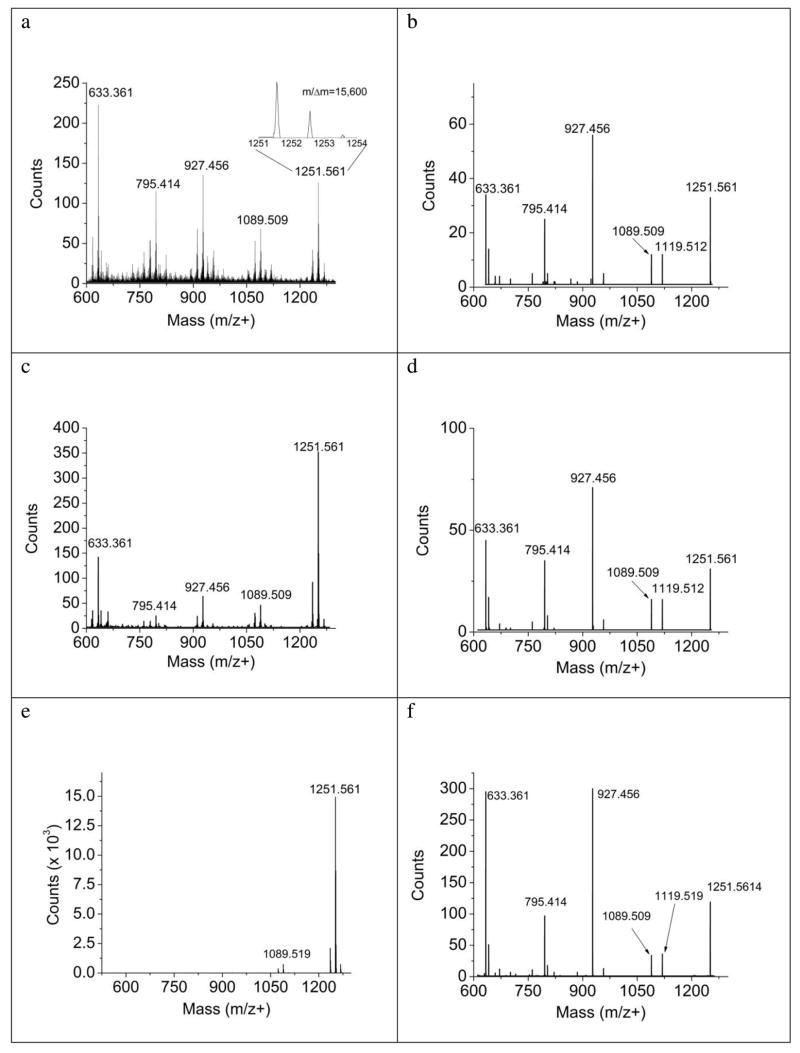
The SIMS spectrum of a film of digitonin deposited onto the sample holder from a methanol/water solution is shown in Figure 4a. The mass resolution achieved with the QqTOF, m/Δm of 15,600, is considerably improved over that generally achievable with a coaxial TOF. For coaxial detection using a pulsed beam, the presence of 13C isotopes in some C60+ ions causes an intrinsic time broadening of the incident ion beam pulse, degrading resolution to some degree. Since the QqTOF used in these experiments employs a DC beam, the 13C contribution has no influence on the ultimate mass resolution. Note the presence of the cationized (Na) molecular ion peak at m/z 1251.561, as well as the presence of several prominent fragment ions. These ions arise from sequential loss of the glycosyl units as indicated from the structure of digitonin. The spectra were acquired using a dose of 1.2 × 1011 primary ions and required about 4 minutes using an 80 pA incident ion beam. Matrix enhanced SIMS is performed by adding a MALDI matrix consisting of a mixture of a saturated solution of sinnapic acid/methanol and a saturated α-cyano-4-hydroxycinnamic acid/methanol to the digitonin solution and preparing the film as before. In these experiments, the fragmentation in the spectrum is greatly reduced, and the intensity of the cationized molecular ion increases by nearly a factor of 3 as shown in Figure 4c. When this film is interrogated by MALDI, virtually all of the fragmentation is gone, and the cationized molecular ion peak exhibits considerably higher intensity. This intensity difference is expected since each laser pulse is thought to induce the desorption of about 1012 molecules, while each C60 molecule desorbs about 200 molecules.13
Figure 4.
Comparison of a thin film of digitonin acquired with SIMS (a, b), Matrix-enhanced SIMS (c, d), and MALDI (e, f). TOF-MS results are shown in the left column and MS/MS results are shown in the right column. SIMS spectra were acquired with a dose of approximately 2 × 1015 ions/cm2. MALDI spectra were obtained with a 337 nm N2 laser at 14 μJ power at a repetition rate of 30Hz. The MALDI MS spectrum was generated from 130s accumulation time and the corresponding MS/MS spectrum from 15s accumulation time. For the MS/MS data, collision energy was 120 eV and residual collision cell N2 pressure was 3.5 × 10−5 Torr. Loss of successive glycosyl units resulted in the characteristic fragmentation pattern shown in the MS/MS spectra as well as, to a lesser extent, the SIMS spectra. Regardless of the method of secondary ion generation, ions passing through the mass filter are identical resulting in nearly identical MS/MS information.
Although the information content of the three spectra shown in Figure 4 is comparable, the spectral congestion of the neat digitonin film associated with the SIMS spectrum in Figure 4a can complicate characterization goals when dealing with more complex samples. In these cases, the MS/MS capability of the QqTOF design can be extremely helpful. CID spectra (or MS/MS spectra) resulting from the three sampling techniques discussed above are shown in Figures 4b, d, and f. There are two points of considerable interest. First, the C60-SIMS and corresponding CID spectrum are very similar, showing the same general fragmentation pattern. This resemblance may be indicative of some similarity of the fragmentation processes occurring in CID and during SIMS sputtering. The second point is that the same fragments appear in all three CID spectra with relative intensities that are very similar. This result has proven to be a general one, and provides an important link between information acquired by SIMS and MALDI, allowing the use of common spectral databases.
One advantage of using MS/MS is that these fragment ions can be used to provide higher intensity and contrast in chemical mapping than can be obtained when using the parent ion.39 Additionally, use of the fragment ions provides more confidence into assignment of the identity of the analyte and more specificity and contrast when performing chemical mapping.
Q2 Enhancement
The issue of instrument transmission is particularly important for SIMS experiments, since in some cases, sample damage reduces the allowable incident beam fluence, and in other cases, particularly with monolayers and thin films, the amount of sample is limited. As noted above, the overall transmission is slightly lower for the QqTOF design than for the coaxial TOF-SIMS instrumentation, primarily due to reduced efficiency of sampling of the dc beam into the orthogonal TOF. It is feasible, however, when dealing with a limited mass range, to adjust the voltages in the Q2 section so as to temporarily store a portion of the traveling beam. At the appropriate time, these ions are then gated into the pusher plate of the reflectron and analyzed with no signal loss. Details associated with this procedure have been discussed in detail elsewhere,35 but this technique is a valuable tool for the SIMS experiments where it is often important to maximize detection efficiency.
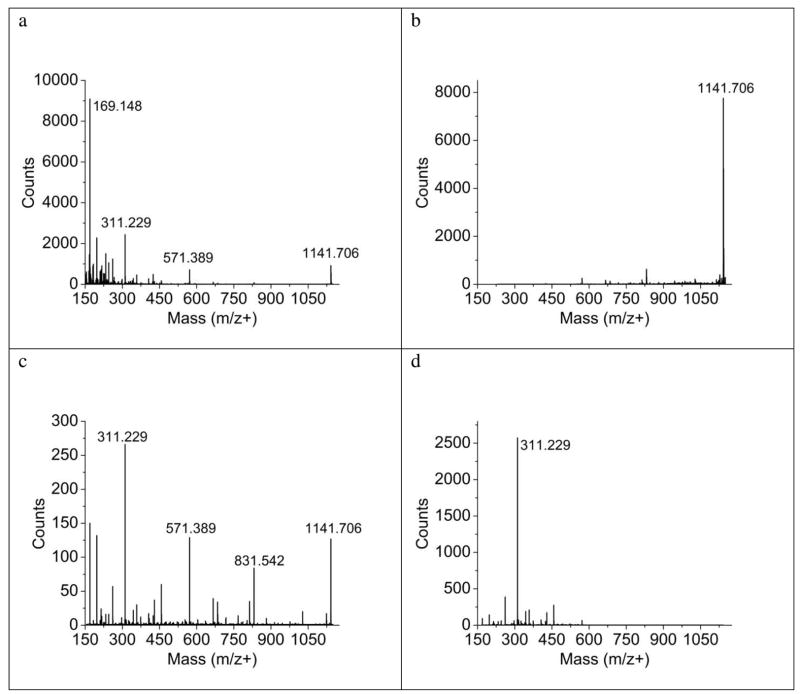
Two examples of the use of Q2 trapping are illustrated in Figure 5. In the first instance, the SIMS spectra of a thin film of gramicidin S is shown in Fig. 5a, acquired up to m/z 1150. By adjusting the trapping mass range in Q2 to enhance m/z 1141 as shown in Fig. 5b, the signal increases by about a factor of 10, a value that yields a detection efficiency greater than obtained for the coaxial TOF. This increase is not precisely the same as predicted by Eqn. 1 and is likely due to slightly different steering and focusing voltages in the non-enhancing mode vs. the pulsing mode. Note also that, as expected, the fragment ions at low mass are lost due to inefficient trapping. In a second experiment, illustrated in Fig. 5c and 5d, lower mass fragments associated with the MS/MS spectrum of gramicidin S can also be enhanced by trapping. In this instance, the ions near m/z 311 are stored, again yielding a significant increase in signal. Hence it is feasible to use this aspect of the instrument to regain lost instrument sensitivity, albeit with a restriction on the mass range available for analysis.
Figure 5.
The QqTOF SIMS instrument allows enhancement of ions by Q2 trapping during either TOF-MS mode (panels a, b) or MS/MS mode (panels c, d) shown here with a thin film of gramicidin. In TOF-MS mode, the protonated molecular ion at m/z+ 1141.706 is increased by 9X shown in panel B over the non-enhanced experiment (a), while in MS/MS mode, a characteristic fragment at m/z+ 311 is increased by a factor of 9 (panel d) over the non-enhanced CID experiment (c). The peaks outside of the enhancement window are attenuated or absent during Q2 trapping. All spectra were acquired with a dose of ~4 × 1014 ion/cm2 and the collision energy for ms/ms data was 65 eV.
Mass range
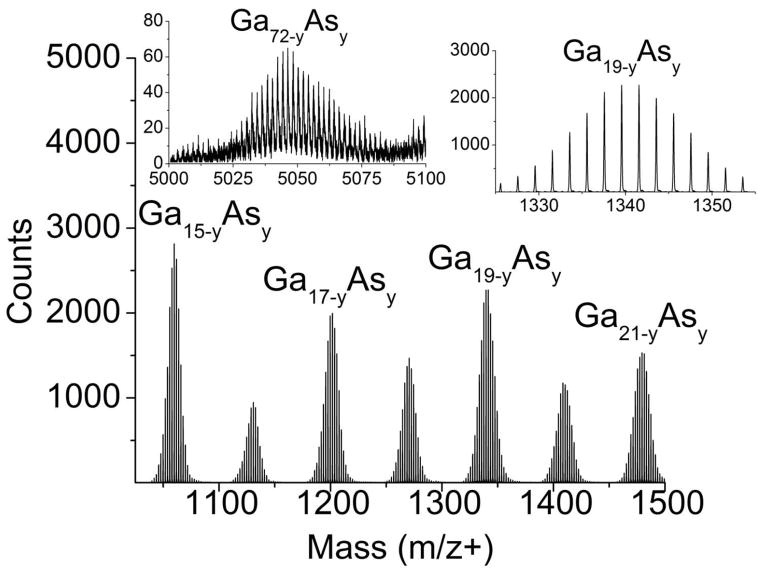
The mass range of secondary ions produced during SIMS experiments generally extends to a few thousand Dalton while the mass range associated with MALDI experiments is virtually unlimited. Many studies with cluster ion beams suggest that the SIMS mass range may be extended for some systems. It is of interest to determine whether an effect such as metastable decay or the kinetic energy distribution of secondary ions influences the performance of the QqTOF. Two samples have been tested. In the first instance, a GaAs crystal was bombarded with C60+ as shown in Figure 6. A series of GaxAsy cluster ions is observed to occur to m/z 5000 and beyond. Each cluster of peaks consists of overlapping peaks associated with Gax-yAsy, with y ranging from zero to approximately 5. These results are in good agreement with the distribution of high mass cluster ions reported recently using an coaxial TOF.40 In that work, however, metastable ions associated with clusters near m/z 1500 and above were seen to complicate the spectrum, a result not seen in the data shown in Figure 6. Apparently, the presence of the N2 background gas of the QqTOF quenches metastable decay for this system.
Figure 6.
C60-SIMS spectrum of GaAs. Repeating GaAs clusters separated by the mass of Ga were detected up to ~m/z 6000. Each cluster consisted of overlapping peaks of the form Gax-yAsy where x is the number of Ga atoms and y is the number of As atoms which varied from 0–5. The upper inset shows the clusters centered on m/z+ 1967 and m/z+ 2036. Higher mass clusters appear to have a larger contribution of As as shown in the lower inset (Ga72 would be centered on m/z+ 5020). Mass resolution was approximately 11,000–13,000 for the mass range shown here.
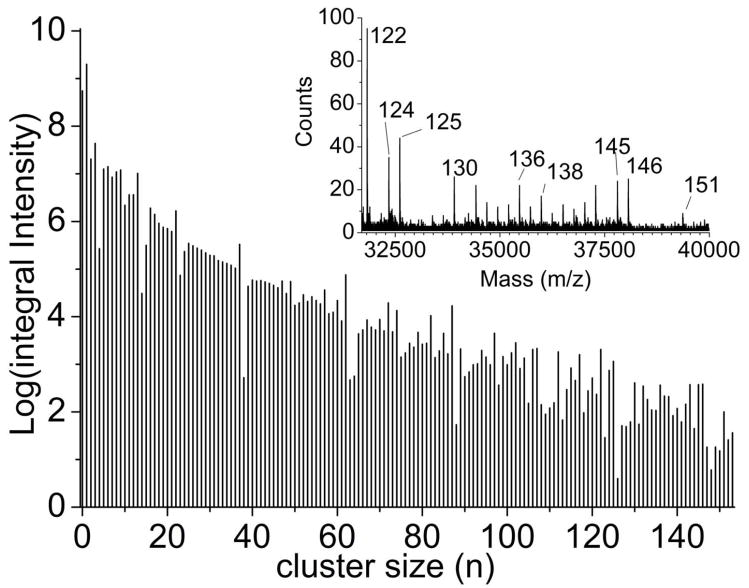
In our instrument, the properties of the quadrupole ion guides are optimized for efficient transfer of ions up to m/z of ~6000 and the use of a TOF accelerating voltage of only 4 keV significantly dampens detection efficiency at high m/z. Despite these limitations, using (CsI)nCs+ cluster ions desorbed from a CsI pellet, significant intensity could be observed to m/z 40,000 with n = 151 as shown in Figure 7. These results, at least to m/z 18,000, are very similar to those reported earlier,41 except that the signal to noise ratio and mass range are greatly improved. Hence, it is clear that there are no obvious performance issues when attempting to utilize the SIMS-QqTOF instrumentation over a broad mass range.
Figure 7.
C60-SIMS spectrum of CsI. Analysis of a CsI pellet revealed clusters of the form [Cs(CsI)n]+ up to ~ m/z+ 40,000, the limit of this instrument. Panel B shows a log plot of the intensity of these clusters vs. cluster size, with the inset showing the mass spectrum from m/z+ 30,000–40,000. These peaks are labeled with cluster number. Mass resolution is ~ 9000 within the mass range shown in the inset.
Chemical Imaging
The primary motivation for creating hybrid SIMS/MALDI instrumentation is to provide additional flexibility for molecule-specific imaging experiments. At this stage of development, only preliminary studies are presented here. The usual mode of imaging for TOF-SIMS is to raster a focused primary ion beam across the sample and to collect spectra at each point. Here, rastering of the primary ion beam is not possible due to geometric constraints associated with the nose cone detailed in Figure 1. As a test of feasibility, however, imaging experiments were attempted using an ion probe size of about 10 μm and by moving the sample stage at 10 μm increments.
A spin-cast film of gramicidin S overlaid with a Cu grid is used as an example, with spectra and images shown in Figure 8. Note that the color-coded molecular ion signal of the peptide and Cu are easily distinguished with good contrast. The lateral resolution is about 25–30 μm as expected. The sensitivity of the instrument is sufficient to perform MS/MS experiments in parallel with imaging. The gramicidin S MS/MS spectrum is shown in Figure 8c. By choosing a single ion in this spectrum, it is possible to monitor its intensity as a function of beam position and to record an image, shown in Figure 8d. This mode of operation will be important for more complex samples where isobaric interferences may degrade the image contrast. This procedure allows imaging to be performed using either SIMS or MALDI on the same sample. A next step in these experiments is to improve the focusing properties of the C60 source, add a set of raster plates on the high pressure side of the nose cone, and improve the accuracy of the stage positioning. Implementation of these steps should ultimately allow imaging with submicron lateral resolution to be achieved.
Figure 8.
TOF-MS and MS/MS imaging analysis of a copper grid positioned over a gramicidin S thin film. TOF-MS spectrum (a) and image (b) of a 100 mesh (250 μm pitch, 45μm bar width) Cu grid over a thin film of gramicidin acquired with 30 μm step size, 3s/pixel, ~10 pA C60+ beam current and 2 mm field of view. The ions at m/z+ 1141.706 are shown in blue, and those at m/z+ 62.929 are shown in green. (c) MS/MS spectrum of the underlying gramicidin S film, and the corresponding selected ion image (d) showing all gramicidin S CID fragments combined in blue. Field of view for the MS/MS image is 1 mm.
4. Conclusions
Cluster SIMS experiments are characterized by increased sensitivity, extended mass range and reduced chemical damage to the sample when compared to SIMS experiments employing atomic ion beams. This change has prompted new thinking in the design of TOF-SIMS instrumentation. Here we have performed prototype studies aimed toward testing the efficacy of using a hybrid quadrupole orthogonal TOF (QqTOF) platform retrofitted with a C60+ primary ion beam as a means to significantly improve the capabilities of the SIMS technique. A major advantage of this configuration is that it allows the use of a DC primary ion beam, rather than a pulsed beam, producing as much as 4 orders of magnitude increase in incident ion beam current. The results show that the instrument transmission is nearly equivalent to high performance axial TOF-SIMS instruments, and that the signal to noise ratio is improved by several orders of magnitude. The use of orthogonal extraction provides improved mass resolution since the peak widths of the secondary ions are not influenced by the time-width of the incident ion pulse. This configuration also allows routine use of tandem mass spectrometry for unraveling spectra acquired from complex samples, either in spectral acquisition mode or in imaging mode.
Of special interest here is the opportunity to use this configuration for complementary MALDI and SIMS studies of the same sample. This idea is intriguing since SIMS probes yield higher lateral resolution than MALDI during mass spectrometry imaging studies, and can be performed on biological samples with or without the use of a MALDI matrix. Although cluster SIMS has not yet shown the sensitivity to peptides normally seen with MALDI, sensitivity to smaller molecules is generally quite high and the resultant spectra are less complicated because of the lack of matrix ions. Our results show that desorbed ions that have been mass selected by the quadrupole mass filter are the same composition for both MALDI and SIMS, providing an additional link between the two methodologies.
Finally, straightforward steps can be taken to improve spatial resolution of this platform. These steps include installation of a more advanced C60 source with submicron focusing of the primary ion beam, addition of rastering plates compatible with the nose cone arrangement and/or replacement of the positioning stage to enable sub-micron sample movement. Implementation of these steps should ultimately allow imaging with submicron lateral resolution.
Acknowledgments
The authors acknowledge Applied Biosystems/MDS Analytical Technologies for use of the QSTAR® XL Orthogonal TOF instrument. Financial support was provided by The National Institute of Health under grant #EB002016-15, and the National Science Foundation under grant #CHE-0555314. NW also acknowledges many stimulating discussions with John Vickerman.
References
- 1.Davies N, Weibel DE, Blenkinsopp P, Lockyer N, Hill R, Vickerman JC. Appl Surf Sci. 2003;203:223–227. [Google Scholar]
- 2.Winograd N. Anal Chem. 2005;77:142A–149A. doi: 10.1021/ac051263k. [DOI] [PubMed] [Google Scholar]
- 3.Wong SCC, Hill R, Blenkinsopp P, Lockyer NP, Weibel DE, Vickerman JC. Appl Surf Sci. 2003;203:219–222. [Google Scholar]
- 4.Cheng J, Winograd N. Anal Chem. 2005;77:3651–3659. doi: 10.1021/ac048131w. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JS, Lockyer NP, Vaidyanathan S, Vickerman JC. Anal Chem. 2007;79:2199–2206. doi: 10.1021/ac061370u. [DOI] [PubMed] [Google Scholar]
- 6.Wucher A, Cheng J, Winograd N. Anal Chem. 2007;79:5529–5539. doi: 10.1021/ac070692a. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney CM, Roberson SV, Gillen G. Anal Chem. 2004;76:3199–3207. doi: 10.1021/ac035532n. [DOI] [PubMed] [Google Scholar]
- 8.Shard AG, Brewer PJ, Green FM, Gilmore IS. Surf Interface Anal. 2007;39:294–298. [Google Scholar]
- 9.Leggett GJ, Chilkoti A, Ratner BD, Vickerman JC. Surf Interface Anal. 1992;18:210–216. [Google Scholar]
- 10.Fletcher JS, Henderson A, Rabbani S, Blenkinsopp P, Thompson S, Lockyer N, Vickerman JC. Anal Chem. doi: 10.1021/ac8015278. in press. [DOI] [PubMed] [Google Scholar]
- 11.Tempez A, Schultz JA, Della-Negra S, Depauw J, Jacquet D, Novikov A, Lebeyec Y, Pautrat M, Caroff M, Ugarov M, Bensaoula H, Gonin M, Fuhrer K, Woods A. Rapid Commun Mass Sp. 2004;18:371–376. doi: 10.1002/rcm.1342. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka K, Mori K, Asakawa D. J Mass Spectrom. 2006;41:894–902. doi: 10.1002/jms.1048. [DOI] [PubMed] [Google Scholar]
- 13.Garrison BJ, Postawa Z. Mass Spectrom Rev. 2008;27:289–315. doi: 10.1002/mas.20165. [DOI] [PubMed] [Google Scholar]
- 14.Postawa Z, Czerwinski B, Winograd N, Garrison BJ. J Phys Chem B. 2005;109:11973–11979. doi: 10.1021/jp050821w. [DOI] [PubMed] [Google Scholar]
- 15.Heeren RMA, McDonnell LA, Amstalden E, Luxembourg SL, Altelaar AFM, Piersma SR. Appl Surf Sci. 2006;252:6827–6835. [Google Scholar]
- 16.Heeren RMA, Sweedler JV. Int J Mass Spectrom. 2007;260:89–89. [Google Scholar]
- 17.Northen TR, Yanes O, Northen MT, Marrinucci D, Uritboonthai W, Apon J, Golledge SL, Nordstrom A, Siuzdak G. Nature. 2007;449:1033–U1033. doi: 10.1038/nature06195. [DOI] [PubMed] [Google Scholar]
- 18.Levi-Setti R, Chabala JM, Li J, Gavrilov KL, Mogilevsky R, Soni KK. Scanning Microscopy. 1993;7:1161–1172. [Google Scholar]
- 19.Benninghoven A. Surf Sci. 1994;300:246–260. [Google Scholar]
- 20.Hillenkamp F. Molecular & Cellular Proteomics. 2005;4:S7–S7. [Google Scholar]
- 21.Hillenkamp F, Karas M. Int J Mass Spectrom. 2000;200:71–77. [Google Scholar]
- 22.Chaurand P, Schriver KE, Caprioli RM. J Mass Spectrom. 2007;42:476–489. doi: 10.1002/jms.1180. [DOI] [PubMed] [Google Scholar]
- 23.Dreisewerd K, Schurenberg M, Karas M, Hillenkamp F. International Journal of Mass Spectrometry and Ion Processes. 1995;141:127–148. [Google Scholar]
- 24.Bouschen W, Spengler B. Int J Mass Spectrom. 2007;266:129–137. [Google Scholar]
- 25.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman JM, Ifa DR, Song QY, Cooks RG. Angew Chem Int Ed. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 27.Loboda AV, Krutchinsky AN, Bromirski M, Ens W, Standing KG. Rapid Commun Mass Sp. 2000;14:1047–1057. doi: 10.1002/1097-0231(20000630)14:12<1047::AID-RCM990>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Chernushevich IV, Loboda AV, Thomson BA. J Mass Spectrom. 2001;36:849–865. doi: 10.1002/jms.207. [DOI] [PubMed] [Google Scholar]
- 29.Carado AJ, Kozole J, Passerelli M, Winograd N, Loboda A, Wingate J. Appl Surf Sci. 2008 in press. [Google Scholar]
- 30.Ilchenko S, Cotter RJ. Int J Mass Spectrom. 2007;265:372–381. doi: 10.1016/j.ijms.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young AB, Cousins LM, Harrison AG. Rapid Commun Mass Sp. 1991;5:226–229. [Google Scholar]
- 32.Jones EA, Lockyer NP, Vickerman JC. Anal Chem. 2008;80:2125–2132. doi: 10.1021/ac702127q. [DOI] [PubMed] [Google Scholar]
- 33.Braun RM, Blenkinsopp P, Mullock SJ, Corlett C, Willey KF, Vickerman JC, Winograd N. Rapid Commun Mass Sp. 1998;12:1246. doi: 10.1002/(SICI)1097-0231(19980930)12:18<1246::AID-RCM316>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Krutchinsky AN, Chernushevich IV, Spicer VL, Ens W, Standing KG. J Am Soc Mass Spectr. 1998;9:569–579. [Google Scholar]
- 35.Chernushevich IV. Eur J Mass Spectrom. 2000;6:471–479. [Google Scholar]
- 36.Adam SA, Marr RS, Gerace L. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam SA, Sternemarr R, Gerace L. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- 38.Liu JM, Xiao NQ, DeFranco DB. Methods-a Companion to Methods in Enzymology. 1999;19:403–409. doi: 10.1006/meth.1999.0876. [DOI] [PubMed] [Google Scholar]
- 39.Piehowski P, Carado A, Kurczy M, Ostrowski S, Heine M, Winograd N, Ewing A. Anal Chem. doi: 10.1021/ac801591r. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goacher RE, Luo H, Gardella JA. Anal Chem. 2008;80:3261–3269. doi: 10.1021/ac7024656. [DOI] [PubMed] [Google Scholar]
- 41.Campana JE, Barlak TM, Colton RJ, Decorpo JJ, Wyatt JR, Dunlap BI. Phys Rev Lett. 1981;47:1046–1049. [Google Scholar]